Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) is present in capsaicin-sensitive sensory neurons and inflammatory/immune cells, therefore it is suggested to play a role in neuro-immune interactions. Our aim was to investigate the role of PACAP in oxazolone-induced delayed-type hypersensitivity reaction in the skin using deficient mice (PACAP-/-). Sensitization was induced by 2% oxazolone application on the shaved abdomen on two consecutive days; inflammation was elicited by oxazolone smearing on the ears 6 days later. Ear thickness was measured by micrometry. Histological examination, cytokine profile [IL-2, IL-4, IL-5, and monocyte chemoattractant protein-1: MCP-1, IFN-γ, tumor necrosis factor alpha (TNF-α)] and myeloperoxidase activity correlating with the number of neutrophils/macrophages were determined 24 and 48 h later. Oxazolone induced a 110–130% swelling after 24–48 h in wild-type mice, which was significantly greater in PACAP-deficient mice. Histological analysis confirmed markedly increased edema in PACAP-/- mice, but the moderately enhanced inflammatory cell accumulation was not statistically significant compared with the wild-types. There was no difference in myeloperoxidase activity of the ear homogenates. Elevation of MCP-1, but not the levels of the other cytokines, was significantly higher in the samples of the PACAP-deficient mice. These results suggest that PACAP exerts anti-inflammatory, particularly edema-inhibiting effects in allergic contact dermatitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of immune cells, nerve endings, and their interactions in cutaneous inflammation has been intensively investigated during the last decades (Roosterman et al. 2006). Capsaicin-sensitive sensory nerve endings are able to release neuropeptides, such as substance P and calcitonin gene-related peptide (CGRP) with pro-inflammatory actions, as well as somatostatin having anti-inflammatory roles. These sensory neuropeptides are also able to positively or negatively modulate the activities of immune cells. CGRP inhibits the antigen presentation of Langerhans cells in vitro and influences their cytokine production. Substance P enhances the induction of contact hypersensitivity and augments the synthesis of pro-inflammatory cytokines (Streilein et al. 1999).
Pituitary adenylate cyclase-activating polypeptide (PACAP) is present in 27 and 38 amino acid-containing biologically active forms; it was first isolated from ovine hypothalamus (Arimura 2007). It is the most highly conserved member of the vasoactive intestinal peptide (VIP)/secretin/glucagon peptide superfamily (Vaudry et al. 2009). Molecular cloning of PACAP receptors has shown the existence of three distinct receptor subtypes: the PACAP-specific PAC1-R, which is coupled to several transduction systems, and the PACAP/VIP-indifferent VPAC1-R and VPAC2-R, which are primarily coupled to adenylyl cyclase (Vaudry et al. 2009). PACAP and its receptors are widely distributed in the central nervous system and the periphery, such as the gastrointestinal, endocrine, immune, and cardiovascular systems (Vaudry et al. 2009). PACAP plays a role in a great variety of physiological and pathological processes, including vascular integrity, neuronal development and regeneration, circadian rhythm, food intake, behavior, and reproduction (Ohtaki et al. 2008; Racz et al. 2008; Reichenstein et al. 2008; Shimakura et al. 2008; Lenti et al. 2009). The connection between the immune system and growth factors is considered to be an intricate innate interaction (Gozes, 2008). In the immune system, PACAP was first described as a potent anti-inflammatory peptide, together with its structural homologue, VIP (Ganea and Delgado 2002; Gomariz et al. 2006). Although a few studies indicate some pro-inflammatory properties, VIP and PACAP are mainly considered as effective inhibitory mediators, particularly on cellular inflammatory responses (Abad et al. 2006). The therapeutic effects of PACAP in animal models of inflammatory diseases are associated with the down-regulation of pro-inflammatory cytokines, chemokines, and receptors (Gomariz et al. 2006; Nemetz et al. 2008).
In the skin, PACAP has been described predominantly in dermal nerve fibers close to the dermal–epidermal junction, hair follicles, blood vessels, and sweat glands. The cutaneous expression of the high-affinity, specific PAC1 receptor has also been shown in these localizations (Steinhoff et al. 1999). Furthermore, a remarkable up-regulation was observed in psoriasis patients indicating a role of PACAP in inflammatory processes of the skin (Steinhoff et al. 1999). Although evidence has accumulated for the anti-inflammatory role of PACAP, particularly in vitro, a few studies focused on its actions in cutaneous inflammatory processes (Kodali et al. 2003; Peters et al. 2006; Ding et al. 2007). Recently, we have shown that systemically administered PACAP inhibits acute neurogenic plasma protein extravasation and edema formation in rat and mouse models by inhibiting the release of pro-inflammatory sensory neuropeptides (Németh et al. 2006; Helyes et al. 2007).
Studies with gene-deficient mice help to elucidate the endogenous role of PACAP in various physiological and pathophysiological processes. PACAP-deficient mice have been shown to display several abnormalities, such as increased temperature-sensitivity (Gray et al. 2002), decreased fertility (Isaac and Sherwood 2008), early neonatal death (Niewiadomski et al. 2008), and behavioral and metabolic alterations (Nakata et al. 2004; Tomimoto et al. 2008; Hashimoto et al. 2009). A few studies have demonstrated increased inflammatory reactions in PACAP knockout animals in models of colitis, autoimmune encephalomyelitis, and axotomy-induced neuroinflammation (Armstrong et al. 2008; Azuma et al. 2008; Nemetz et al. 2008; Tan et al. 2009). Therefore, the aim of the present study was to investigate the delayed-type hypersensitivity reaction in the skin of PACAP-deficient and wild-type mice using the oxazolone-induced allergic contact dermatitis model.
Materials and Methods
Animals
Experiments were performed on PACAP gene-deficient mice (PACAP-/-) and their wild-type (PACAP+/+) counterparts. The generation and maintenance of the PACAP-deficient mice on the CD1 background have been described previously in detail (Hashimoto et al. 2001); they were backcrossed for ten generations with the CD1 strain. Offsprings within the first three generations were used for the experiments. Animals were bred and kept in the Laboratory Animal House of the Department of Pharmacology and Pharmacotherapy of the University of Pécs at 24–25°C and provided with standard chow and water ad libitum. Experiments were performed in accordance with the ethical guidelines approved by the University of Pécs (BA02/2000-20/2006).
Experimental Protocol
Anesthesia was induced by ketamine (100 mg/kg i.p.; Richter Gedeon Plc., Hungary) with xylazine (5 mg/kg IM; Lavet Ltd., Hungary). Animals were sensitized on two consecutive days by smearing 2% oxazolone dissolved in 96% ethanol (50–50 μl) on the shaved abdomen. Six days later, the inflammation on the right ears was elicited by smearing 2% oxazolone on both the inner and outer surfaces (15–15 μl). The left ears were treated the same way with 96% ethanol, and these served as controls. The mice were sacrificed by cervical dislocation in deep anesthesia at the end of the experimental period. The ears were dissected for histological processing, myeloperoxidase assay and cytokine analysis with cytometric bead array.
Ear Edema Measurement
Ear thickness was measured with an engineer's micrometer (Moore and Wright, Sheffield, UK) with 0.1 mm accuracy, before the induction of the inflammation and after the challenge at different time points. Data were expressed as percent increase of the ear thickness compared with the initial values.
Determination of Myeloperoxidase Activity
Myeloperoxidase (MPO) activity correlating with the number of accumulated neutrophil cells and macrophages was determined from the ear samples frozen at −80°C until processing. The ears were thawed and chopped into small pieces, then homogenized in phosphate buffer containing 0.5% hexadecyl trimethylammonium bromide detergent (1 ml buffer per ear). The homogenate was centrifuged at 10,000g at 4°C for 10 min, and 0.5-ml aliquots of the supernatants were placed in Eppendorf tubes. The spectrophotometric reactions were performed in 96-well microtiter plates at room temperature; the MPO activity was assayed using H2O2-3,3′5,5′-tetramethyl-benzidine (TMB/H2O2; Sigma-Aldrich Ltd, Hungary). The optical density (OD) at 620 nm was measured at 5-min intervals for 30 min using a microplate reader (Labsystems) and plotted. The reaction rate (Δ OD/time) was derived from an initial slope of the curve. A calibration curve was then produced, with the rate of reaction plotted against the standard human MPO preparation (Sigma-Aldrich Ltd, Hungary).
Histological Studies
Dissected ears were fixed in 4% buffered formaldehyde. Cross-sections (6 µm) were cut at the base of the ears after paraffin embedding and stained with hematoxylin-eosin. For semiquantitative evaluation of the severity of the inflammation, three histopathological characteristics, such as the extent of the edema, formation of microabscess after necrosis of hair follicles/sebaceous glands, and the number of accumulated mononuclear and polymorphonuclear cells were scored according to the following: edema was measured with Analysis software (Soft Imaging System), expressed in micrometers and scored from 0–5 (below 300 = 0; 301–400 = 1; 401–500 = 2; 501–600 = 3; 601–700 = 4; and above 700 μm = 5). The number of microabscesses per ear was counted and scored from 0–5 (0–5 = 1; 6–10 = 2; 11–15 = 3; 16–20 = 4; and 21–25 = 5). The cellular infiltrate was evaluated by viewing the number of accumulated mononuclear and polymorphonuclear cells per field scored from 0–5 at ×200 magnification.
Flow Cytometric Studies
The cytokine profile during DTH reaction was determined by cytometric bead array (CBA, BD Biosciences, Belgium). Ears were homogenized in 1 ml RPMI-1640 medium (Sigma-Aldrich Ltd, Hungary) and 1% phenylmethanesulfonyl-fluoride (Sigma-Aldrich Ltd, Hungary) mixture at 4°C. Samples were centrifuged at 10,000g for 10 min at 4°C. The CBA kit contains six bead populations with distinct fluorescent intensities that have been coated with capture antibodies specific for IL-6, IL-10, monocyte chemoattractant protein-1 (MCP-1), IFN-γ, TNF-α, and IL-12 proteins. The six bead populations were mixed together in the CBA kit. The ear homogenate (50 μl) was mixed with the same volume of phycoerythrin-labeled detection antibody solution and the capture beads in an assay tube and analyzed by flow cytometry (Partec CyFlow Space). Data were collected by the FloMax Software, and the results were calculated by the FCS Express Software.
Statistics
Results are expressed as mean ± SEM of n = 20 mice per group. Comparisons between the different groups were made by one-way ANOVA followed by Dunnett's post-test for the edema and MPO data. The concentrations of respective cytokine pairs were analyzed with Student's t test for unpaired comparison. Kruskall–Wallis followed by Dunn's post-test was used to evaluate the semiquantitative histopathological scores. Probability values P < 0.05 were regarded as significant in all cases.
Results
Oxazolone-Induced Ear Edema in PACAP+/+ and PACAP-/- Mice
In wild-type mice, oxazolone induced about a 130% (from 290 to 690 μm diameter) and 110% (from 290 to 600 μm diameter) ear edema 24 and 48 h after eliciting the inflammatory reaction, respectively. This was moderately, but significantly greater in PACAP-deficient mice at both time points (Fig. 1).
Percentage increase of the ear thickness induced by 2% oxazolone treatment 6 days after sensitization in wild-type (PACAP+/+) and PACAP-deficient (PACAP-/-) mice. The contralateral ears of the respective mice treated with the solvent of oxazolone (96% ethanol) served as controls; n = 20 mice per group; *p < 0.05 vs. PACAP+/+ (one-way ANOVA+ Dunnett's post hoc test)
Myeloperoxidase Activity in the Ear Homogenates
Oxazolone evoked a remarkable MPO activity increase in the homogenized ear samples 24 h after its topical application compared with the vehicle (96% ethanol)-treated, contralateral control ears. This decreased to about its 50% 48 h following the induction of the inflammatory process. However, there was no difference between the PACAP+/+ and PACAP-/- groups (Fig. 2).
Histopathological Changes in Response to Oxazolone in PACAP+/+ and PACAP-/- Mice
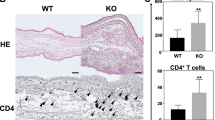
Compared with the ethanol-treated non-inflamed ears (Fig. 3a), in the PACAP-/- group, oxazolone induced greater edema, as well as tendencies for more pronounced inflammatory cell accumulation and microabscess formation (Fig. 3c) than in their wild-type counterparts (Fig. 3b). The difference between these characteristic histopathological alterations in the two groups were clearly seen by the composite semiquantitative inflammation scores (Fig. 4b), but only the extent of edema formation proved to be statistically significant (Fig. 4a).
Representative light micrographs showing the cross-sections of a ethanol-treated PACAP+/+, b oxazolone-treated PACAP+/+, and c oxazolone-treated PACAP-/- mouse ears. The slides were stained with hematoxylin-eosin, and the pictures were taken at ×100 magnification. Vertical two-headed arrows indicate ear diameters in each part and show the extent of edema in sections b and c, the other arrows point to b accumulation of granulocytes and macrophages as well as c microabscess formation
Semiquantitative evaluation and scoring of the ear sections obtained from oxazolone-treated PACAP+/+ and PACAP-/- mice. b Represents the composite scores obtained by adding the values of the individual parameters shown in a (MA number of microabscesses, CA extent of cell accumulation). Boxes represent the upper and lower quartiles with the medians (line in the boxes) and the whiskers show the smallest and the largest values in the respective groups of n = 4–5 mice; *p < 0.05 vs. solvent-treated group (Kruskal–Wallis+Dunn's post hoc test)
Cytokine Analysis of Ear Homogenates
Among the six measured cytokines, the concentrations of only three (MCP-1, IL-6, and IL-10) elevated in the homogenized ear samples both 24 and 48 h after oxazolone administration. The levels of IL-6 and MCP-1 decreased to 50% by 48 h compared with the 24-h values; IL-10 concentrations were similar. The lack of PACAP did not influence IL-6 and IL-10 concentrations, but significantly increased the level of MCP-1 at 24 h. However, this difference disappeared at 48 h (Fig. 5a, b).
Discussion
The present results provide in vivo functional, morphological, and biochemical evidence for a protective role of PACAP in delayed-type hypersensitivity reaction in the skin with the help of genetically manipulated mice. The anti-edema action of PACAP was clearly demonstrated by ear thickness and histological measurements. The production of MCP-1, which is the most important inflammatory cytokine in this response as shown by its much higher concentration, is also inhibited by PACAP. In contrast, PACAP does not seem to play a significant role in the accumulation of inflammatory cells, particularly neutrophils, in this model.
The observed anti-edema action of PACAP is supported by our previous results showing that it decreases acute neurogenic inflammatory reactions, such as mustard oil-, capsaicin-, and resiniferatoxin-induced plasma protein extravasation in the rat paw skin and ear swelling of the mouse (Németh et al. 2006; Helyes et al. 2007). These peripheral inhibitory actions are supported by the ability of PACAP to diminish the release of pro-inflammatory sensory neuropeptides (SP and CGRP) from the peripheral terminals of capsaicin-sensitive sensory fibers in vitro which mediate neurogenic vasodilatation and plasma extravasation (Németh et al. 2006). The observed inhibitory effect of PACAP on sensory neuropeptide release cannot be explained by the classical Gs and Gq protein-coupled signal transduction mechanisms related to PAC1 and VPAC receptors. They all increase intracellular cAMP and Ca2+ levels and therefore stimulate neuronal functions (Laburthe et al. 2007). Meanwhile, elevation of intracellular cAMP attenuates the release of inflammatory mediators (bradykinin, prostaglandins, leukotriens, etc.) from mast cells and granulocytes (Weston and Peachell 1998). The peripheral anti-edema effect of PACAP might be due to the decreased release of these pro-inflammatory mediators from cellular sources through the classical receptors. However, the existence of a presently unknown inhibitory receptor for PACAP on sensory nerve terminals or an overlapping action on other inhibitory receptors such as cannabinoid, opioid, or somatostatin receptors are also possible (Németh et al. 2006; Helyes et al. 2007; Muller et al. 2007). Since several data show a prominent direct vasodilating effect of PACAP in the skin through VPAC receptor activation on the vascular smooth muscle cells (Steinhoff et al. 1999), the presently described anti-edema effect in this model is most likely to be an indirect action.
PACAP has been shown to down-regulate several pro-inflammatory cytokines and chemokines and their receptors (Gomariz et al. 2006; Nemetz et al. 2008). The synthesis of interleukins, such as IL-1β, IL-2, IL-6, IL-10, IL-11, IL-15, IL-17, IL-21, IL-25, and TNF-α was decreased in different inflammation models, such as septic shock, rheumatoid arthritis, and inflammatory bowel disease. Chemokines, such as MCP-1 and RANTES (or chemokine (C-C motif) ligand 5), as well as several other macrophage-derived similar small molecules, were also shown to be suppressed in these models (Delgado and Ganea 2001).
Our finding that higher MCP-1 levels were measured in PACAP-deficient mice well correlates with these results. Stimulated MCP-1 mRNA has been reported to be inhibited by PACAP in retinal pigment epithelial cells, further supporting our data, although in an entirely different system (Zhang et al. 2005).
Increased inflammatory reactions have been described earlier in PACAP-deficient mice. PACAP knockouts exhibit more severe clinical symptoms of colitis and have higher colonic inflammation in dextran sulfate sodium-induced colitis (Azuma et al. 2008; Nemetz et al. 2008). These data are in agreement with the increased inflammatory responses observed in VIP knockout animals, such as exaggerated inflammatory response in cyclophosphamide-induced cystitis (Girard et al. 2008). In experimental autoimmune encephalomyelitis, PACAP-deficient mice exhibit increased sensitivity accompanied by enhanced mRNA expression of pro-inflammatory cytokines, including MCP-1 (Tan et al. 2009).
In conclusion, these data provided evidence for an anti-inflammatory, particularly edema-inhibiting effects of PACAP in allergic contact dermatitis. Although in this model PACAP does not play a predominant role in cellular inflammatory responses, its inhibitory effect on the synthesis of an important inflammatory cytokine, MCP-1, was clearly shown.
References
Abad C, Gomariz RP, Waschek JA (2006) Neuropeptide mimetics and antagonists in the treatment of inflammatory disease: focus on VIP and PACAP. Curr Top Med Chem 6:151–163
Arimura A (2007) PACAP: the road to discovery. Peptides 28:1617–1619
Armstrong BD, Abad C, Chhith S et al (2008) Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience 151:63–73
Azuma YT, Hagi K, Shintani N et al (2008) PACAP provides colonic protection against dextran sodium sulfate induced colitis. J Cell Physiol 216:111–119
Delgado M, Ganea D (2001) Inhibition of endotoxin-induced macrophage chemokine production by vasoactive intestinal peptide and pituitary adenylate cyclase activating polypeptide in vitro and in vivo. J Immunol 167:966–975
Ding W, Wagner JA, Granstein RD (2007) CGRP, PACAP, and VIP modulate Langerhans cell function by inhibiting NF-kappaB activation. J Invest Dermatol 127:2357–2367
Ganea D, Delgado M (2002) Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) as modulators of both innate and adaptive immunity. Crit Rev Oral Biol Med 13:229–237
Girard BM, Malley SE, Braas KM, Waschek JA, May V, Vizzard MA (2008) Exaggerated expression of inflammatory mediators in vasoactive intestinal polypeptide knockout (VIP-/-) mice with cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci 36:188–199
Gomariz RP, Juarranz Y, Abad C, Arranz A, Leceta J, Martinez C (2006) VIP-PACAP system in immunity. New insights for multitarget therapy. Ann N Y Acad Sci 1070:51–74
Gozes I (2008) VIP, from gene to behavior and back: summarizing my 25 years of research. J Mol Neurosci 36:115–124
Gray SL, Yamaguchi N, Vencova P, Sherwood NM (2002) Temperature-sensitive phenotype in mice lacking pituitary adenylate cyclase activating polypeptide. Endocrinology 143:3946–3954
Hashimoto H, Shintani N, Tanaka K et al (2001) Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase activating polypeptide (PACAP). Proc Natl Acad Sci USA 98:13355–13360
Hashimoto H, Hashimoto R, Shintani N et al (2009) Depression-like behavior in the forced swimming test in PACAP-deficient mice: amelioration by the atypical antipsychotic risperidone. J Neurochem 110:595–602
Helyes Zs, Pozsgai G, Borzsei R et al (2007) Inhibitory effect of PACAP-38 on acute neurogenic and non-neurogenic inflammatory processes in the rat. Peptides 28:1847–1855
Isaac ER, Sherwood NM (2008) Pituitary adenylate cyclase-activating polypeptide (PACAP) is important for embryo implantation in mice. Mol Cell Endocrinol 280:13–19
Kodali S, Friedman I, Ding W, Seiffert K, Wagner JA, Granstein RD (2003) Pituitary adenylate cyclase activating polypeptide inhibits cutaneous immune function. Eur J Immunol 33:3070–3079
Laburthe M, Couvineau A, Tan V (2007) Class II G protein-coupled receptors for VIP and PACAP: structure, models of activation and pharmacology. Peptides 28:1631–1639
Lenti L, Zimmermann A, Kis D et al (2009) PACAP and VIP differentially preserve neurovascular reactivity after global cerebral ischemia in newborn pigs. Brain Res 1283:50–57
Muller JM, Debaigt C, Goursaud S et al (2007) Unconventional binding sites and receptors for VIP and related peptides PACAP and PHI/PHM: an update. Peptides 28:1655–1666
Nakata M, Kohno D, Shintani N et al (2004) PACAP deficient mice display reduced carbohydrate intake and PACAP activates NPY-containing neurons in the rat hypothalamic arcuate nucleus. Neurosci Lett 370:252–256
Németh J, Reglodi D, Pozsgai G et al (2006) Effect of pituitary adenylate cyclase activating polypeptide-38 on sensory neuropeptide release and neurogenic inflammation in rats and mice. Neuroscience 143:223–230
Nemetz N, Abad C, Lawson G et al (2008) Induction of colitis and rapid development of colorectal tumors in mice deficient in the neuropeptide PACAP. Int J Cancer 122:1803–1809
Niewiadomski P, Coute-Monvoisin AC, Abad C, Ngo D, Menezes A, Waschek JA (2008) Mice deficient in both pituitary adenylyl cyclase-activating polypeptide and vasoactive intestinal peptide survive, but display growth retardation and sex-dependent early death. J Mol Neurosci 36:200–207
Ohtaki H, Nakamachi T, Dohi K, Shioda S (2008) Role of PACAP in ischemic neural death. J Mol Neurosci 36:16–25
Peters EM, Ericson ME, Hosoi J et al (2006) Neuropeptide control mechanisms in cutaneous biology: physiological and clinical significance. J Invest Dermatol 126:1937–1947
Racz B, Horvath G, Faluhelyi N et al (2008) Effects of PACAP on the circadian changes of signaling pathways in chicken pinealocytes. J Mol Neurosci 36:220–226
Reichenstein M, Rehavi M, Pinhasov A (2008) Involvement of pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors in the mechanism of antidepressant action. J Mol Neurosci 36:330–338
Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M (2006) Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev 86:1309–1379
Shimakura S, Kojima K, Nakamachi T et al (2008) Neuronal interaction between melanin-concentrating hormone- and alpha-melanocyte-stimulating hormone-containing neurons in the goldfish hypothalamus. Peptides 29:1432–1440
Steinhoff M, McGregor GP, Radleff-Schlimme A, Steinhoff A, Jarry H, Schmidt WE (1999) Identification of pituitary adenylate cyclase activating polypeptide (PACAP) and PACAP type I receptor in human skin: expression of PACAP-38 is increased in patients with psoriasis. Regul Pept 80:49–55
Streilein JW, Alard P, Niizeki H (1999) Neural influences on induction of contact hypersensitivity. Ann N Y Acad Sci 885:196–208
Tan YV, Abad C, Lopez R et al (2009) Targeted gene deletion reveals that pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance in mice and plays a protective role in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 106:2012–2017
Tomimoto S, Ojika T, Shintani N et al (2008) Markedly reduced white adipose tissue and increased insulin sensitivity in adcyap1-deficient mice. J Pharmacol Sci 107:41–48
Vaudry D, Falluel-Morel A, Bourgault S et al (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61:283–357
Weston MC, Peachell PT (1998) Regulation of human mast cell and basophil function by cAMP. Gen Pharmacol 31:715–719
Zhang XY, Hayasaka S, Chi ZL, Cui HS, Hayasaka Y (2005) Effect of pituitary adenylate cyclase activating polypeptide (PACAP) on IL-6, IL-8, and MCP-1 expression in human retinal pigment epithelial cell line. Curr Eye Res 30:1105–1111
Acknowledgements
This work was sponsored by Hungarian Grants OTKA K72592, K73044, NK78059, CNK78480, the “Science, Please! Research Teams on Innovation” program (SROP-4.2.2/08/1/2008-0011), ETT 03-380/2009, ETT 04-364/2009, ETT 278-04/2009, and Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science. Zs. Helyes and D. Reglodi were supported by the János Bolyai Postdoctoral Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Pinter and Zs. Helyes made equal contributions to the present work.
Rights and permissions
About this article
Cite this article
Kemény, Á., Reglődi, D., Cseharovszky, R. et al. Pituitary Adenylate Cyclase-Activating Polypeptide Deficiency Enhances Oxazolone-Induced Allergic Contact Dermatitis in Mice. J Mol Neurosci 42, 443–449 (2010). https://doi.org/10.1007/s12031-010-9368-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-010-9368-3