Abstract
Oligodendrocytes (OLG) are the cells resident in the CNS responsible for myelination. OLG undergo a succession of morphological and molecular changes along several maturational stages. Galectin-3 (Gal-3) is a 25- to 35-KDa protein belonging to the family of carbohydrate-binding galectins, which bind to glycoconjugates containing β-galactosides. Gal-3 lacks a specific receptor and its binding is thus rather unspecific, as it depends on the cellular environment and the repertoire of glycomolecules at the time when Gal-3 is present. Our previous work revealed that recombinant Gal-3 (rGal-3)–treated OLG showed accelerated differentiation, evidenced by an increase in the number of mature cells to the detriment of immature ones and accelerated actin cytoskeleton dynamics. These changes were a consequence of rGal-3 influence on Akt, Erk 1/2, and β-catenin signaling pathways. Considering this previous evidence, the aim of this study was to identify the temporal window of rGal-3 action on the OLG lineage to induce OLG maturation by using specific single pulses of rGal-3 over the different maturational stages of OLG, and to unravel its main direct targets promoting OLG differentiation by mass spectrometry analysis. Our results reveal a key temporal window spanning between OPC and pre-OLG states in which rGal-3 action promotes OLG differentiation, and identify several targets for rGal-3 binding including proteins related to the cytoskeleton, signaling pathways, metabolism and intracellular trafficking, among others. These results highlight the relevance of Gal-3 in signaling pathways regulating oligodendroglial differentiation and support a potential therapeutic role for rGal-3 in demyelinating diseases such as multiple sclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oligodendrocytes (OLG) are the cells resident in the CNS responsible for myelination, the physiological process of ensheathing axons with myelin, a specialized membrane rich in lipids and proteins which provides metabolic and trophic support to axons [1]. OLG undergo a succession of morphological and molecular changes along several maturational stages. First, they constitute bipolar cells called oligodendrocyte progenitor cells (OPC) which have proliferative and migratory capacity and express molecular markers such as PDGFRα and NG2. In an intermediate stage represented by pre-OLG, these cells become more ramified and express CNPase, Olig 1, and O4, among others. Subsequently, pre-OLG eventually develop the ability of forming myelin membranes and express MBP, APC, and PLP, thus becoming fully mature OLG [2, 3]. Recent work has proposed a two-step model for actin dynamics in OLG: first, pro-polymerizing actin cytoskeleton dynamics favor OPC branching until OLG fully mature; second, the actin cytoskeleton shifts to pro-depolymerizing dynamics, which triggers myelination [4, 5]. These mechanisms are controlled in part by the relation between MBP and actin disassembly proteins such as cofilin-1 and gelsolin, which are normally sequestered and inactivated by phosphatidylinositol 4, 5-bisphosphate (PIP2) present in the plasma membrane. Being expressed in mature OLG, MBP competes for binding to PIP2 with gelsolin and cofilin-1, displacing and activating them to trigger the disassembly of actin filaments [5].
Galectin-3 (Gal-3) is a 25- to 35-KDa protein belonging to the evolutionarily conserved family of carbohydrate-binding lectins called galectins. These proteins share a carbohydrate recognition domain (CDR), which allows their binding to glycoconjugates containing β-galactosides. Extracellular Gal-3 recognizes oligosaccharide terminals of poly-N-acetyllactosamine in major components of the extracellular matrix (ECM) or in membrane receptors. This capacity allows it both to lay a bridge between plasma membrane receptors and the ECM and cross-link receptors to form lattices, both of which can promote or inhibit intracellular events. Gal-3 can also be endocyted and, together with intracellular Gal-3, modulate diverse functions binding to intracellular molecules [6]. Gal-3 lacks a specific receptor and its binding is thus rather unspecific, as it depends on the cellular environment and the repertoire of glycomolecules at the time when Gal-3 is present.
Our previous work revealed that recombinant Gal-3 (rGal-3)–treated OLG showed accelerated differentiation, evidenced by an increase in the number of mature cells to the detriment of immature ones and accelerated actin cytoskeleton dynamics. These changes resulted from rGal-3 induction of an increase in the activation of Akt and the levels of β-catenin, MBP, and gelsolin, and a decrease in the activation of Erk 1/2 [7]. Although the participation and precise time of action of the Akt/mTORC and Erk1/2 pathways in OLG differentiation and myelination have long generated controversy [8], a recent report has shown them to play independent and cooperative roles along development and adulthood both in vitro and in vivo [9]. In addition, Erk 1/2 inhibition has been recently demonstrated to favor OLG generation and recovery of demyelinating diseases [10]. Regarding β-catenin, some authors describe it as an OLG differentiation inhibitor [11,12,13], while others describe it as a promoter [14,15,16]. Considering this evidence, the aim of this study was to identify the temporal window of rGal-3 action on the OLG lineage and unravel its main direct targets promoting OLG differentiation.
Materials and Methods
Materials
Bovine insulin, progesterone, putrescine, sodium selenite, T3, penicillin, Hoechst 33342, streptomycin, kanamycin, and cocktail of phosphatase inhibitors were obtained from Sigma Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) was purchased from CRIPION (Buenos Aires, Argentina). Protease inhibitor cocktail was obtained from Roche Applied Science (Mannheim, Germany). DMEM/F12 was purchased from Life Technologies (Carlsbad, CA, USA). Recombinant human PDGF-AA was obtained from PeproTech (Veracruz, Mexico). Human recombinant bFGF was a gift from Dr. Baldi (IBYME, Buenos Aires, Argentina). Anti-MBP was donated by Dr. Pablo Paez (University of California, Los Angeles, CA, USA). Anti-PDGFRα was obtained from Neuromics (Edina, MN, USA). Anti-Akt, phosphorylated anti-Akt, anti-Erk 1/2, phosphorylated anti-Erk 1/2, anti-gelsolin, anti-GAPDH, anti-phosphorylated 4EB-P1, and anti-human Gal-3 for immunoprecipitation were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-beta-catenin was obtained from Abcam (Cambridge, UK). The sir700-actin kit was purchased from Cytoskeleton Inc. (Denver, CO, USA). Secondary antibodies were obtained from Jackson Immuno Research Co. Laboratories (West Grove, PA, USA). All the other reagents used were of analytical quality and of the highest possible purity.
rGal-3 Production and Purification
rGal-3 was obtained using Escherichia coli BL21DE3 star transformed with pET28b+/LGALS3 plasmid based on Salomonsson et al. (2010) [17]. Briefly, preculture of bacteria in Terrific broth (TB; yeast extract 24 g/l, tryptone 20 g/l, glycerol 4 ml/l, KH2PO4 0.017 M, K2HPO4 0.072 M) supplemented with 2 mM of glucose and 50 μg/mL kanamycin reached stationary phase within 18–20 h at 37 °C. Afterwards, the preculture was diluted in TB with kanamycin and cultured at 37 °C until 0.8 optical density (OD) at 600 nm was reached. Induction with 1 mM IPTG for 3 h was performed at 28 °C. After induction, bacteria were pelleted and resuspended in Tris buffer with protease inhibitor and sonicated through 14 pulses of 30 s in a Branson Sonifier 250, with an output power of 35 W and a duty cycle of 0.5%. An aliquot was plated in TB-agar for colony forming unit (CFU) counting. The remaining material was centrifuged and an aliquot of the supernatant (SN) was tested for hemagglutination and analyzed by Western blot to identify the presence of rGal-3. The rGal-3 present in the SN after bacterial lysis and centrifugation was purified from other bacterial proteins by lactose affinity chromatography and from lipopolysaccharide (LPS) by polymyxin B affinity chromatography and an aliquot was analyzed by SDS-PAGE and Western blot to identify the presence of rGal-3. Further confirmation of rGal-3 presence and integrity in the eluate was performed by mass spectrometry [7].
Primary Rat OLG Culture
The primary OLG cultures of Wistar strain rats were performed as described by McCarthy and de Vellis (1980). The cerebral hemispheres were dissected from newborn rats (P0-2), the meninges were removed and the hemispheres were dissociated by mechanical disintegration in DMEM/F12 containing 5 mg/ml of streptomycin and 5 U/ml of penicillin, supplemented with 10% FBS. Cell suspensions were seeded in 75-cm2 tissue culture flasks coated with poly-l-lysine. After 10 days in culture, differential agitations began to yield each cell type of purified glia. First, microglia were separated by differential agitation of the culture flasks for 1 h in an orbital shaker, and then OLG were isolated from astrocytes by continuous agitation for 24 h. The cell suspension obtained from OLG was filtered through a 15-μm mesh filter and then centrifuged at 300 g for 10 min. The OLG were seeded on poly-l-lysine-coated Petri dishes for Western blot studies, or on poly-l-lysine–coated coverslips placed in multi-well plates for morphological and immunocytochemical studies. The purity of the OLG was greater than 95%, evaluated by cell count by immunocytochemistry of glial fibrillary acidic protein (GFAP), ionized calcium-binding adapter molecule 1 (Iba1), and OLG transcription factor 2 (Olig2), for astrocytes, microglia, and OLG, respectively (data not shown). The purified OLG were maintained in glial defined medium (GDM, DMEM/F12 supplemented with glucose 4 g/l, NaHCO3 2.4 g/l, insulin 25 mg/l, putrescine 8 mg/l, transferrin 50 mg/l, T3 9.8 mg/l, 20 nM progesterone, sodium selenite 8 mg/l, biotin 10 mg/l) supplemented with growth factors (PDGFAA 10 ng/ml and bFGF 10 ng/ml) and 0.5% FBS for 48 h. Subsequently, OPC were incubated in GDM without FBS and without growth factors for3 h and treated during the corresponding times described in each test with GDM with 0.5% FBS, without growth factors, with or without 20 μg/ml of rGal-3. The optimal rGal-3 concentration was determined by MTT assay in our previous work so that OLG viability was not compromised [7]. Briefly, cell cultures were administered rGal-3 at treatment days (TD) 0, TD2, and TD4, corresponding to OPC, pre-OLG, and OLG, respectively. rGal-3 effects were evaluated 1 day after treatment in each case.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde (PFA) for 20 min, rinsed with PBS, permeabilized with 1% Triton X-100 for 30 min (only for cytosolic antigens), and blocked with 5% FBS for 2 h. Incubation with primary antibodies was performed overnight (ON) at 4 °C. Cells were incubated with secondary antibodies anti-rabbit, anti-mouse, or anti-goat conjugated with fluorophores (Cy3, Alexa Fluor 488, 549 or 647) diluted 1/600 or SirActin700 diluted 1/1000 for 90 min. Nuclei were stained with the fluorescent dye Hoechst 33342 (5 mg/ml DMSO at 1%). The preparation was mounted with Mowiol and analyzed by ultraviolet light microscopy or confocal microscopy with an Olympus BX50 microscope (Olympus, Tokyo, Japan). Photographs were taken with a CoolSnap digital camera and analyzed with ImageJ software. Confocal images were obtained by capturing several sequential images at regular intervals on the z-axis of an area of interest. Subsequently, 3D reconstruction was performed using the IMARIS 6.3.1 program (BitplaneSci Software). Total PDGFRα and MBP+ cells per field were counted in 20 fields selected at random and normalized to Hoechst-positive cells. ImageJ software was also used to quantify reactive or immunoreactive areas and integrated optical density (IOD). Briefly, a threshold for each photograph was determined so that all positive cells were included and the background excluded. The immunoreactive area or IOD for positive cells was calculated by normalizing the immunoreactive area or IOD to the count of positive cells for each antigen. The reactive area or IOD for SirActin700 was normalized to Hoechst-positive nuclei.
Western Blot Analysis
The samples were lysed with RIPA extraction buffer (150 mM NaCl, 5 mM EDTA, 50 mM Tris, 1% NP-40, 0.1% SDS) supplemented with protease and phosphatase inhibitors for 5 min on ice. In order to eliminate cell debris, the lysate was centrifuged for 10 min at 3650 g. An aliquot of the supernatant was used to determine protein concentration by the Lowry method. Then, samples were resolved by electrophoretic run of SDS-PAGE gels seeding similar amounts of protein and, subsequently, they were transferred to PVDF membranes. The membranes were incubated with the primary antibodies indicated in each case ON at 4 °C, followed by washes with TBS or TBS-0.1% Tween and subsequent incubation with secondary antibodies conjugated with peroxidase diluted 1/15000. The signal was detected by chemiluminescence through ImageQuant500 equipment. Quantification was carried out using the Gel Pro Analyzer 4.0 program (Media Cybernetics Gel Pro Analyzer, Bethesda, MD, USA).
Immunoprecipitation Assay
OPC or pre-OLG seeded on poly-l-lysine–coated 60-mm plates were treated with 20 μg/ml of rGal-3 for 15 min in GDM with 0.5% FBS and subsequently washed with PBS. Cells were then lysed in a non-denaturing buffer (1861603 Thermo Scientific) supplemented with protease and phosphatase inhibitors to be frozen at − 20 °C. The thawed samples were centrifuged at 3650 g to remove cell debris without lysis. The supernatant was separated into aliquots every 2 mg of protein per condition quantified by Nanodrop at 280 nm. The control aliquot was treated with 2 μg/ml of non-immunized rabbit serum, while the aliquot sample was treated with the anti-human Gal-3 monoclonal antibody at a dilution of 1/100. Both conditions were treated with agarose spheres conjugated to protein A/G and incubated for 2 h at room temperature. Then, the samples were centrifuged at 300 g for 30 s and the pellet was washed with PBS four times centrifuging each time. The pellets obtained after the washings were resuspended in Laemmli buffer and subsequently boiled for 5 min. Then, the bands were resolved by SDS-page in a 10% acrylamide-bisacrylamide gel. The gel was stained with colloidal Coomassie blue G: the proteins in the gel were fixed with 30% (v/v) EtOH-2% (v/v) phosphoric acid ON at room temperature, the gel was washed three times for 30 min with distilled water, then added the staining solution (18% (v/v) MeOH, 17% (w/v) NH42SO4 2% (v/v) phosphoric acid) under stirring at room temperature during 1 h and finally added 0.5 g/l of Coomassie blue G-250 powder. The gel was washed with distilled water and each lane was cut into three parts: from 0 kDa to 25 kDa, from 25 to 50 kDa, and from 50 kDa to 250 kDa. These parts were sent to the mass spectrometry service of CEQUIBIEM (School of Exact and Natural Sciences, University of Buenos Aires) for analysis. Briefly, bands were submitted to washing, reduction and alkylation, digestion with trypsin, and extraction of peptides. Peptides were solved in a Q exative mass spectrometer (Thermo Fisher, Waltham, MA, USA). The results were analyzed first in Microsoft Office Excel to determine rGal-3–specific binding proteins present only in the sample and not in the control. The proteins thus identified were classified by ontological genetics in the FunRich program, and the interaction maps were generated using the Cytoscape software [18].
Statistical Analysis
The Graph-Pad Prism software was used for statistical data analysis. The results are presented as the mean of at least three independent experiments ± standard error of the mean (SEM). Comparisons were made using two-way ANOVA followed by Bonferroni’s post hoc test. A p value less than 0.05 was considered statistically significant. Data distribution was assumed to be normal, but this was not formally tested.
Results
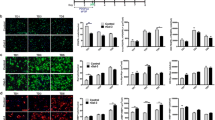
In order to evaluate the time window of action of rGal-3, three experimental protocols (Fig. 1a) were designed limiting treatment to a single rGal-3 pulse on OPC (TD0), pre-OLG (TD2), or OLG (TD4). First, we evaluated the effect of rGal-3 pulses on MBP, PDGFRα, and F-actin by immunocytochemistry (Fig. 1b). Following rGal-3 pulse at TD0 (Fig. 1c), confocal images and their quantification revealed an increase in the area of MBP at TD5 and in MBP IOD at all times evaluated. In addition, no significant changes were observed in the area, IOD or number of cells positive for PDGFRα, although an increasing trend in area at TD1 hinted at the presence of more ramified cells. Regarding F-actin, treatment with rGal-3 at TD0 produced a significant increase in F-actin at TD3 followed by depolymerization at TD5. These results are in line with accelerated differentiation, pointing at OPC as Gal-3 targets.
a. Experimental protocols. After treatment of purified rat OPC with growth factors for 48 h, followed by 3 h of FBS and growth factor deprivation, i. OPC were treated with a rGal-3 pulse at TD0; ii. OPC were changed to differentiating medium for 2 days (TD2) and treated with a rGal-3 pulse at TD2; iii. OPC were changed to differentiating medium for 4 days (TD4) and treated with a rGal-3 pulse at TD4. b. Confocal microscopy images of OLG treated with or without rGal-3 according to the experimental protocols described in a. Cells were fixed and immunostained for PDGFRα and MBP, in addition to F-actin staining with sirActin700 probe. Graphs represent image quantification for rGal-3 treatment in c. TD0. d. TD2 and e. TD4. Graphs for MBP show immunoreactive area over Hoechst+ nuclei (TD0: interaction: yes, factor ratio 13.25, degrees of freedom 2; TD2: interaction: yes, factor ratio 5.848, degrees of freedom 2; TD4: interaction: no, factor ratio 0.1683, degrees of freedom 2) and IOD over immunoreactive area (TD0: interaction: no, factor ratio 2.936, degrees of freedom 2; TD2: interaction: yes, factor ratio 35.71, degrees of freedom 2; TD4: interaction: no, factor ratio 2.052, degrees of freedom 2). Graphs for PDGFRα show the number of immunoreactive cells over the number of Hoechst+ nuclei (TD0: interaction: no, factor ratio 0.4963, degrees of freedom 2; TD2: interaction 0.3631, factor ratio 5.848, degrees of freedom 2; TD4: interaction: no, factor ratio 0.1184, degrees of freedom 2), immunoreactive area over the number of immunoreactive cells (TD0: interaction: no, factor ratio 2.374, degrees of freedom 2; TD2: interaction: no, factor ratio 1.352, degrees of freedom 2; TD4: interaction: no, factor ratio 0.5260, degrees of freedom 2), and IOD over immunoreactive area (TD0: interaction: no, factor ratio 2.072, degrees of freedom 2; TD2: interaction: no, factor ratio 1.201, degrees of freedom 2; TD4: interaction: no, factor ratio 1.046, degrees of freedom 2). Graphs for F-actin show IOD over immunoreactive area (TD0: interaction: yes, factor ratio 10.25, degrees of freedom 2; TD2: interaction: yes, factor ratio 4.903, degrees of freedom 2; TD4: interaction: no, factor ratio 1.046, degrees of freedom 2). Comparisons were made using two-way ANOVA followed by Bonferroni post hoc test (* p < 0.05; ** p < 0.01; *** p < 0.001; * regarding control). Scale: 100 μm
Regarding rGal-3 treatment at TD2 (Fig. 1d), results showed an increase in MBP area at TD5 and in MBP IOD at TD3 and TD5. No changes were observed in any of the parameters evaluated for PDGFRα. Furthermore, F-actin dynamics behaved similarly to TD0 treatment, peaking at TD3 and decreasing at TD5. These findings indicate that pre-OLG are also targets of rGal-3 to drive accelerated differentiation.
As for rGal-3 treatment at TD4 (Fig. 1e), no significant changes were observed in MBP, PDGFRα, or F-actin, which indicates that OLG are not targets for rGal-3 action in promoting differentiation.
Afterwards, we evaluated the effect of rGal-3 pulses on the levels of Erk 1/2, Akt, β-catenin, eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), gelsolin, and MBP through Western blot analysis of cell lysates (Fig. 2a). These signaling pathways have been previously described as mediators of rGal-3–induced OLG differentiation, as rGal-3 activates Akt, decreases Erk-1/2 activation, and increases β-catenin levels, leading to MBP expression [7]. In the current work, TD0 rGal-3 treatment (Fig. 2b) induced a decrease in Erk 1/2 activation at TD1 and TD3, concomitant with an increase in Akt activation. β-Catenin levels were increased with TD0 rGal-3 treatment both at TD1 and TD3, whereas 4EB-P1, an mTORC1 substrate, was activated at TD3 and TD5. In turn, actin depolymerization protein gelsolin showed increased expression at all times evaluated with TD0 rGal-3 treatment, while MBP showed higher expression at TD3 and TD5, which confirms the results obtained by immunocytochemistry both in terms of MBP IOD and area.
a. Representative immunoblots for β-catenin, gelsolin, pAkt, pErk 1/2, MBP, p-4 EB-P1, and GAPDH, from OLG treated with or without rGal-3 according to the experimental protocols described in Fig. 1a. Graphs show Western blot IOD quantification for rGal-3 treatment relativized to GAPDH and expressed as fold increases over TD1 control in b. TD0; c. TD2 and d. TD4 (β-catenin – TD0: interaction: yes, factor ratio 44.99, degrees of freedom 2; TD2: interaction: no, factor ratio 0.5182, degrees of freedom 2; TD4: interaction: no, factor ratio 0.1918, degrees of freedom 2 – gelsolin – TD0: interaction: no, factor ratio 2.323, degrees of freedom 2; TD2: interaction: yes, factor ratio 38.24, degrees of freedom: 2; TD4: interaction: yes, factor ratio 19.04, degrees of freedom 2 – pAkt – TD0: interaction: yes, factor ratio 4.324, degrees of freedom 2; TD2: interaction: no, factor ratio 3.482, degrees of freedom 2; TD4: interaction: no, factor ratio 0.04736, degrees of freedom 2 – pErk 1/2 – TD0: interaction: yes, factor ratio 15.22, degrees of freedom 2; TD2: interaction: yes, factor ratio 4.525, degrees of freedom 2; TD4: interaction: yes, factor ratio 18.35, degrees of freedom 2 – MBP – TD0: interaction: yes, factor ratio 19.15, degrees of freedom 2; TD2: interaction: yes, factor ratio 15.74, degrees of freedom 2; TD4: interaction: no, factor ratio 0.3134, degrees of freedom 2 – p-4EB-P1 – TD0: interaction: no, factor ratio 1.181, degrees of freedom 2; TD2: interaction: yes, factor ratio 92.86, degrees of freedom 2; TD4: interaction: yes, factor ratio 28.35, degrees of freedom 2). Comparisons were made using two-way ANOVA followed by Bonferroni post hoc test (* p < 0.05; ** p < 0.01; *** p < 0.001; * regarding control)
In addition, rGal-3 treatment at TD2 (Fig. 2c) inactivated Erk 1/2 at TD3 and activated Akt, leaving β-catenin levels unchanged. Furthermore, an increase was observed in the phosphorylation of 4EB-P1 at TD3 to later decrease at TD5. This treatment also produced an increase in the expression of both gelsolin and MBP at TD3 and TD5.
Regarding rGal-3 treatment at TD4 (Fig. 2d), a decrease was observed in the phosphorylation of Erk 1/2 and 4EB-P1 at TD5, accompanied by an increase in the expression of gelsolin and without changes in the other molecules. These results indicate that rGal-3 interacts with mature OLG but this interaction does not promote higher MBP expression.
Taken together, these results indicate that rGal-3 impacts on the OLG lineage, causing different effects according to the moment in which the treatment is performed. The major changes were perceived with the pulse of rGal-3 at TD0 and TD2, especially in MBP, the mature OLG marker, which points at rGal-3 interaction mainly with OPC and pre-OLG to accelerate oligodendroglial differentiation.
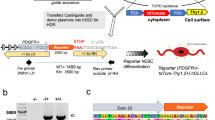
In order to evaluate which molecules could bind rGal-3 at TD0 and TD2 to exert a pro-differentiating effect, we performed co-immunoprecipitation assays with OPC and pre-OLG treated with one pulse of rGal-3 at TD0 or TD2 for 15 min and then lysed in a non-denaturing form (Fig. 3a), as our previous work had shown that a 15-min rGal-3 pulse exerted a pro-polymerizing effect on OPC [7]. The co-immunoprecipitation was performed with a human anti-Gal-3 monoclonal antibody in order to rule out possible molecules bound to endogenous Gal-3. In addition, non-immune serum was used as non-specific precipitation control, and precipitation specificity evaluated by Western blot (Fig. 3b) showed heavy and light chain bands corresponding to the antibodies and a specific band for rGal-3 only in the lysate precipitated with anti-Gal-3. These results confirmed co-immunoprecipitation specificity.
a. Experimental protocols. After treatment of purified rat OPC with growth factors for 48 h, followed by 3 h of FBS and growth factor deprivation, i. OPC were treated with a rGal-3 pulse at TD0 for 15 min followed by co-immunoprecipitation; ii. OPC were changed to differentiating medium for 2 days (TD2) and treated with a rGal-3 pulse at TD2 for 15 min followed by co-immunoprecipitation. b. Immunoblots show Gal-3 in OPC lysates previously treated with rGal-3 for 15 min followed by co-immunoprecipitation using a specific anti-Gal-3 antibody (Ac Gal-3) or non-specific serum (Serum). The presence of rGal-3 is observed only in the lane immunoprecipitated with anti-rGal-3 antibody. c. Venn diagram of the number of proteins found at TD0, TD2, and their intersection using FunRich software
Having determined binding specificity, we proceeded to evaluate the immune-precipitates by mass spectrometry. Of the proteins identified, we selected those that only appeared in the lysate precipitated with specific antibody, discarding those that precipitated with the control serum. The proteins that co-precipitated with rGal-3 at TD0 are presented in Table S1, those that co-precipitated with rGal-3 at TD2 are shown in Table S2 and the proteins common to both times are displayed in Table S3. The amount of proteins that interacted with rGal-3 at TD0 and at TD2 is shown in Fig. 3c; 34 proteins were found to interact with rGal-3 at TD0 and 92 at TD2, among which, six were common to both groups.
With the extracted data, we conducted an ontological genetics study using the FunRich program to classify the proteins by cellular component, biological process, and molecular function (Figs. 4a–c, respectively). Afterwards, we generated interaction maps for the same categories using the Cytoscape program (Figs. 5a–c, respectively). In the classification according to cellular component (Figs. 4a and 5a), we observed that both at TD0 and TD2 rGal-3 interacted with many cytoplasmic and nuclear proteins, most of them belonging to the cytoskeleton, the proteasome, and vesicle trafficking. At TD0, we noticed an enrichment in the interaction with proteins of the myelin sheath. Regarding the classification according to biological process (Figs. 4b and 5b), rGal-3 interacted with proteins involved in oxide reduction, RNA translation, cytoskeleton dynamics, and signal transduction. Finally, in the classification according to molecular function (Figs. 4c and 5c), we observed proteins involved in protein binding, cytoskeleton ubiquitin binding, ATPases, GTPases, and kinases.
a. Ontological classification of proteins by cellular component for TD0 (outer circle) and TD2 (inner circle). b. Ontological classification of proteins by biological process for TD0 (outer circle) and TD2 (inner circle). c. Ontological classification of protein by molecular function for TD0 (outer circle) and TD2 (inner circle). Analyses were carried out using FunRich software
a. Ontological interaction map of proteins classified by cellular component for TD0 (yellow circles), TD2 (blue circles) and both TD0 and TD2 (gray circles). b. Ontological interaction map of proteins classified by biological process for TD0 (yellow circles), TD2 (blue circles) and both TD0 and TD2 (gray circles). c. Ontological interaction map of proteins classified by molecular function for TD0 (yellow circles), TD2 (blue circles) and both TD0 and TD2 (gray circles). Names of the genes found in each group are specified. Analyses were carried out using Cytoscape software
Taking into account the results of our previous studies [7], the identification at TD0 of rGal-3 interaction with cytoskeleton proteins gelsolin, Arp3, and Rac1 is of particular interest, as gelsolin is responsible for F-actin depolymerization, Arp3 is in charge of actin monomer nucleation in lamellipodia and Rac1 is the monomeric GTPase controlling lamellipodia formation. Moreover, rGal-3 also interacted with calmodulin, in turn involved in MBP cross-talk with the actin cytoskeleton, and with proteins implicated in cell metabolism and ATP production which play a key role in myelination by generating the energy required. Finally, rGal-3 was found to interact with proteins of the Rab family, which are part of endocytic trafficking and endosome escape path. This interaction may be regarded as a possible cause for the presence of rGal-3 in the cytoplasm.
At TD2, rGal-3 was also observed to interact largely with ribosomal proteins, lipid metabolism proteins such as fatty acid binding protein, vitamin transport proteins such as retinol-binding protein 1 (Rbp1), proteins of energy metabolism and yet again with actin cytoskeleton proteins such as Arp3 and Rac1 and microtubule cytoskeleton proteins such as microtubule-actin cross-linking factor 1 (Macf1). Furthermore, we found endosomal escape proteins such as rab10 and rab4A.
Of note, Arp3 and Rac1 were among the proteins interacting both at TD0 and TD2, which highlights the importance of the cytoskeleton as a mediator of the pro-differentiating effect of rGal-3. Also, the results obtained with Rac1 are in line with the increase in its active form previously reported upon rGal-3 treatment [7].
Discussion
In our previous work [7], we added rGal-3 to purified OPC once every 2 days and observed that rGal-3 promoted accelerated differentiation evidenced by an early increase in mature OLG markers and a decrease in immature OLG ones. These results were accompanied by accelerated F-actin dynamics, as rGal-3 treatment rendered an earlier polymerization peak and subsequent depolymerization. Being a carbohydrate-binding protein, Gal-3 lacks a specific receptor and binds to different glycoproteins or glycolipids present in the cell along the differentiation process. Therefore, the purpose of this study was to elucidate the main targets of rGal-3 over the OLG lineage to promote their differentiation, using different treatment protocols with a single pulse of rGal-3 applied to different stages of OLG maturation, in addition to mass spectrum analysis.
A single rGal-3 treatment at both TD0 and TD2 led to increased MBP expression and accelerated actin dynamics, indicating that the main temporal window of rGal-3 action to promote OLG differentiation is at OPC and pre-OLG states. These findings are in accordance with previous results of our group showing a permissive glycophenotype for rGal-3 binding in OPC [19]. Additionally, rGal-3 treatment at TD0 and TD2 generated an increase in pAkt, β-catenin, and F-actin, also in line with our previous evidence that rGal-3 increased Akt activation and β-catenin levels and promoted pro-polymerization actin dynamics in pre-OLG [7]. Regarding the mTORC1 pathway, we evaluated its activation through the phosphorylation of substrate p4EB-P1, observing that it was active at TD1 and TD3 with TD0 treatment, and at TD3 with TD2 treatment. These results indicate that mTORC1 is activated by rGal-3 treatment, which contributes to MBP expression and OLG maturation, as previously established [20].
However, a single rGal-3 treatment at TD4 failed to increase MBP expression, F-actin and β-catenin levels, or Akt activation. We also observed lesser mTORC1 signaling activation, probably corresponding to rGal-3 binding to different molecules present at that stage of OLG maturation.
Furthermore, an increase in gelsolin concomitant with a decrease in pErk 1/2 was found with all treatments. Current knowledge implicates that gelsolin inhibits Erk 1/2 activation in Jurkat T cells [21], besides an additionally possible direct interaction between rGal-3 and Erk 1/2, as also observed in our current studies. These events may be associated to increased MBP expression, as higher gelsolin levels positively correlate with OLG differentiation [22]. Moreover, we did not observe changes in PDGFRα+ cells with any of the treatments.
Taken together, these results indicate that, although the action window of rGal-3 is mainly at OPC and pre-OLG stages, there are interactions occurring throughout OLG maturation, which reinforces the notion that the action of rGal-3 depends on the repertoire of glycolipids and glycoproteins present at the time of the treatment.
Having confirmed that rGal-3 pro-differentiation action is mainly limited to its interaction with OPC and pre-OLG to promote MBP expression, direct rGal-3 interaction with molecules was evaluated in OPC (TD0) and pre-OLG (TD2) through their co-immunoprecipitation with rGal-3 and their subsequent identification by mass spectrometry.
Most interestingly, the proteins interacting with rGal-3 identified at TD0 treatment included proteins related to cell proliferation, cytoskeleton, signaling cascades, lipids, and those that could mediate rGal-3 cellular internalization.
Regarding proliferation, we identified casein kinase II subunit alpha (CKII), which phosphorylates Olig2 to promote OPC division [23]. The inhibition of this enzyme increases Akt signaling [24] and its interaction with rGal-3 could then also promote an increase in Akt and limit OLG proliferation to induce their differentiation. rGal-3 also interacted with proliferating cell nuclear antigen (PCNA), which is widely used as a marker of cell proliferation and whose interaction with rGal-3 help decrease the proliferation of OLG. Likewise, rGal-3 was found to interact with voltage-dependent anion-selective channel protein 1 (VDAC1), whose inhibition prevents the proliferation of OLG [25].
The finding that rGal-3 interacted with cytoskeletal proteins sparked considerable interest, revealing molecules such as ras-related C3 botulinum toxin substrate 1 (Rac1) and its inhibitor, rho GDP-dissociation inhibitor 1 (RhoGDI), which possibly mediate the rGal-3-dependent activation of Rac1 observed in our previous work [7]. Most importantly, rGal-3 was observed to interact with gelsolin, an F-actin depolymerizing protein which is necessary for the final stage of OLG maturation, and with actin-related protein 3 (Arp3), crucial for the early OLG maturation [5]; upon binding to rGal-3, these proteins could promote nucleation of actin and pro-polymerization to later promote depolymerization. rGal-3 interaction was also found with junction plakoglobin (JUP), a protein localized in desmosomes and adherent junctions whose role in OLG has not yet been fully described but which could mediate rGal-3 effects on the cytoskeleton.
In terms of energy metabolism and lipids, our assays also provided evidence of rGal-3 interaction with transaldolase (TAL), which participates in the pentose phosphate pathway and is selectively expressed by OLG to produce the large amounts of lipids necessary for myelin synthesis and protection from the deleterious effects of free radicals [26]. In addition, rGal-3 interacted with ATP-citrate synthase, an important enzyme implicated in the synthesis of cytosolic acetyl-CoA in many tissues, with a central role in de novo lipid synthesis.
Regarding signaling pathways, we found that rGal-3 interacted with carboxypeptidase E (CPE), which is known to modulate the Wnt-β-catenin pathway in neurons in order to decrease β-catenin levels by inhibiting the secretion of Wnt3a; in addition, rGal-3 bound to several receptors to increase the activation of Akt and Erk 1/2 [27] and with poly-ADP-ribose-polymerase 1 (PARP1), whose inhibition in the cuprizone model and in type III lesions of multiple sclerosis leads to a reduction in demyelination by increasing Akt activity [28] and favoring the OLG lineage in the subventricular zone [29].
On the subject of intracellular trafficking, rGal-3 interacted with Rab-5A, involved in multiple molecule internalization and trafficking in OLG [30]. This interaction was of great importance to establish a link between extracellular rGal-3 and cytosolic proteins, since rGal-3 could be internalized and redistributed in different subcellular compartments to exert its function.
Certain important proteins were identified at TD2 treatment related to the cytoskeleton, signaling cascades, lipids, and cellular internalization. In contrast to TD0, no proteins were found to be involved in proliferation. Among cytoskeletal proteins, rGal-3 was again found to interact with JUP, Rac1, Arp3, and gelsolin, adding importance to actin-related proteins in the action of rGal-3 and possibly leading to the events observed at TD3. rGal-3 also bound to F-actin-capping protein subunit alpha-1 (CAPZA1), actin depolymerizing protein, and to septin-11 (SEPT11) and septin-7 (SEPT7), cytoskeletal GTPases promoting the formation of actin filaments. Although these GTPases have not yet been studied in OLG, SEPT7 deprivation in Schwann cells is known to cause defects in myelination by preventing the correct organization of the actin cytoskeleton [31]. rGal-3 also interacted with proteins related to microtubules, such as cytoplasmic dynein 1 light intermediate chain 2 (DYNC1LI2), which contributes to vesicle transport along microtubules, and with microtubule-actin cross-linking factor 1 (MACF1), which relates and articulates actin microfilaments with microtubules. MACF1 also plays an important role in the regulation of signaling cascades, as it acts as a positive regulator of the Wnt signaling pathway participating in the translocation of AXIN1 and its associated complex (composed of APC, β-catenin and GSK3B) from the cytoplasm to the cell membrane, increasing β-catenin levels. MACF1 may also partly mediate the increase observed in β-catenin upon rGal-3 treatment. Likewise, rGal-3 interacted with calmodulin (CAM), involved in the interaction of MBP with actin [32], and to stathmin (STMN1), which promotes OLG branching [33]. Taken together, these interactions emphasize the key role played by rGal-3 in the regulation of actin filament dynamics.
Concerning signaling pathways, interaction was found with nucleoside diphosphate kinase A (NME1), whose function in OPC is to inhibit the differentiation of OLG [34]. rGal-3 also interacted with many subunits of the proteasome, which is known to degrade β-catenin in several cell types, profilin 1 in breast cancer [35], gelsolin in pancreatic cancer [36], and interestingly, MBP in OLG [37]. Furthermore, previous studies from our laboratory indicate that inhibition of the proteasome leads to OLG differentiation and promotes remyelination in the cuprizone model [38, 39]. Worth highlighting, an inhibitory relationship between rGal-3 and the proteasome may explain the increase in the levels of several proteins found upon rGal-3 treatment in our previous work [7], which may in future be corroborated through mRNA analyses. rGal-3 also bound AP-2 complex subunit mu (AP-2), which is involved in the selection of vesicle cargo and their transport and regulates the endocytosis of frizzled receptors, again indicating the importance of rGal- 3 in the β-catenin pathway. Interestingly, rGal-3 also interacted with inositol monophosphatase 1 (IMPA1), responsible for the supply of inositol required for the synthesis of phosphoinositides, which play a crucial role in the dynamics of MBP-gelsolin-F-actin. Also, of note, rGal-3 was found to interact with mitogen-activated protein kinase 1 (Erk2), possibly involved in the decrease in Erk activation following rGal-3 treatment, perhaps by blocking the phosphorylation site or generating conformational changes that prevent its activation. Interaction was also found with protein quaking, which regulates the splicing of pre-mRNA, export of mRNA, stability of mRNA and protein translation, and is necessary for MBP stability and correct actin polymerization in OLG [40].
As for intracellular traffic, rGal-3 again interacted with Rab proteins such as Rab-8A, involved in vesicular trafficking and insulin-induced transport to the plasma membrane of GLUT4 glucose transporter, Rab-10, involved mainly in the transport of proteins from the Golgi to the plasma membrane, and Rab-4A and Rab-18, whose functions have not been elucidated yet. These interactions suggest that extracellular rGal-3 is internalized and then redistributed together with target molecules to the corresponding subcellular compartments to mediate changes in the cytoskeleton, proliferation, lipid synthesis, and signaling pathways necessary to achieve OLG differentiation. Figure 6 shows the main proteins found to interact with rGal-3.
The results obtained in the present work will allow to open doors from a mechanistic point of view to identify new signaling pathways regulated by Gal-3 to control OLG proliferation and differentiation. This knowledge will also provide support to evaluate the therapeutic potential of rGal-3 in demyelinating diseases such as multiple sclerosis.
References
Bercury KK, Macklin WB (2015) Dynamics and mechanisms of CNS myelination. Dev Cell 32:447–458. https://doi.org/10.1016/j.devcel.2015.01.016
Nave KA, Werner HB (2014) Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol 30:503–533. https://doi.org/10.1146/annurev-cellbio-100913-013101
Snaidero N, Simons M (2017) The logistics of myelin biogenesis in the central nervous system. Glia 65:1021–1031. https://doi.org/10.1002/glia.23116
Nawaz S, Sánchez P, Schmitt S, Snaidero N, Mitkovski M, Velte C, Brückner BR, Alexopoulos I et al (2015) Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Dev Cell 34(2):139–151. https://doi.org/10.1016/j.devcel.2015.05.013
Zuchero JB, Fu MM, Sloan SA, Ibrahim A, Olson A, Zaremba A, Dugas JC, Wienbar S et al (2015) CNS myelin wrapping is driven by actin disassembly. Dev Cell 34(2):152–167. https://doi.org/10.1016/j.devcel.2015.06.011
Nabi IR, Shankar J, Dennis JW (2015) The galectin lattice at a glance. J Cell Sci 128(13):2213–2219. https://doi.org/10.1242/jcs.151159
Thomas L, Pasquini LA (2018) Extracellular Galectin-3 induces accelerated oligodendroglial differentiation through changes in signaling pathways and cytoskeleton dynamics. Mol Neurobiol 56:336–349. https://doi.org/10.1007/s12035-018-1089-6
Gaesser JM, Fyffe-Maricich SL (2016) Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp Neurol 283(PtB):501–511. https://doi.org/10.1016/j.expneurol.2016.03.008
Ishii A, Furusho M, Macklin W, Bansal R (2019) Independent and cooperative roles of the Mek/ERK1/2-MAPK and PI3K/Akt/mTOR pathways during developmental myelination and in adulthood. Glia 67(7):1277–1295. https://doi.org/10.1002/glia.23602
Suo N, Guo YE, He B, Gu H, Xie X (2019) Inhibition of MAPK/ERK pathway promotes oligodendrocytes generation and recovery of demyelinating diseases. Glia. 67(7):1320–1332. https://doi.org/10.1002/glia.23606
Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ et al (2009) Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev 23:1571–1585
Feigenson K, Reid M, See J, Crenshaw EB 3rd, Grinspan JB (2009) Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci 42:255–265
Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM et al (2009) HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci 12:829–838
Kalani MY, Cheshier SH, Cord BJ, Bababeygy SR, Vogel H, Weissman IL, Palmer TD, Nusse R (2008) Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci 105:16970–16975
Ortega F, Gascón S, Masserdotti G, Deshpande A, SimonC FJ, Dimou L, Chichung Lie D et al (2013) Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat Cell Biol 15:602–613
Tawk M, Makoukji J, Belle M, Fonte C, Trousson A, Hawkins T, Li H, Ghandour S et al (2011) Wnt/beta-catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis. J Neurosci 31:3729–3742
Salomonsson E, Carlsson MC, Osla V, Hendus-Altenburger R, Kahl-Knutson B, Oberg CT, Sundin A, Nilsson R et al (2010) Mutational tuning of galectin-3 specificity and biological function. J Biol Chem 285(45):35079–35091. https://doi.org/10.1074/jbc.M109.098160
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B et al (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13(11):2498–2504
Pasquini LA, Millet V, Hoyos HC, Giannoni JP, Croci DO, Marder M, Liu FT, Rabinovich GA et al (2011) Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ 18(11):1746–1756. https://doi.org/10.1038/cdd.2011.40
Figlia G, Gerber D, Suter U (2018) Myelination and mTOR. Glia 6 6(4):693–707. https://doi.org/10.1002/glia.23273
Morley SC, Sung J, Sun GP, Martelli MP, Bunnell SC, Bierer BE (2007) Gelsolin overexpression alters actin dynamics and tyrosine phosphorylation of lipid raft-associated proteins in Jurkat T cells. Mol Immunol 44:2469–2480
Shao Z, Lee X, Huang G, Sheng G, Henderson CE, Louvard D, Sohn J, Pepinsky B et al (2017) LINGO-1 regulates oligodendrocyte differentiation through the cytoplasmic gelsolin signaling pathway. J Neurosci 37(12):3127–3137. https://doi.org/10.1523/JNEUROSCI.3722-16.2017
Zhou J, Tien AC, Alberta JA, Ficarro SB, Griveau A, Sun Y, Deshpande JS, Card JD et al (2017) A sequentially priming phosphorylation cascade activates the gliomagenic transcription factor Olig2. Cell Rep 18(13):3167–3177. https://doi.org/10.1016/j.celrep.2017.03.003
Bastian C, Quinn J, Tripathi A, Aquila D, McCray A, Dutta R, Baltan S, Brunet S (2018) CK2 inhibition confers functional protection to young and aging axons against ischemia by differentially regulating the CDK5 and AKT signaling pathways. Neurobiol Dis 126:S0969–9961(18)30149-9. https://doi.org/10.1016/j.nbd.2018.05.011
Imada S, Yamamoto M, Tanaka K, Seiwa C, Watanabe K, Kamei Y, Kozuma S, Taketani Y et al (2010) Hypothermia-induced increase of oligodendrocyte precursor cells: Possible involvement of plasmalemmal voltage-dependent anion channel 1. J Neurosci Res 88(16):3457–3466. https://doi.org/10.1002/jnr.22520
Banki K, Colombo E, Sia F, Halladay D, Mattson DH, Tatum AH, Massa PT, Phillips PE et al (1994) Oligodendrocyte-specific expression and autoantigenicity of transaldolase in multiple sclerosis. J Exp Med 180(5):1649–1663
Ji L, Wu HT, Qin XY, Lan R (2017) Dissecting carboxypeptidase E: Properties, functions and pathophysiological roles in disease. Endocr Connect 6(4):R18–R38. https://doi.org/10.1530/EC-17-0020
Veto S, Acs P, Bauer J, Lassmann H, Berente Z, Setalo G Jr, Borgulya G, Sumegi B et al (2010) Inhibiting poly(ADP-ribose) polymerase: a potential therapy against oligodendrocyte death. Brain. 133(Pt 3):822–834. https://doi.org/10.1093/brain/awp337
Plane JM, Grossenbacher SK, Deng W (2012) PARP-1 deletion promotes subventricular zone neural stem cells toward a glial fate. J Neurosci Res 90(8):1489–1506. https://doi.org/10.1002/jnr.23040
Bouverat BP, Krueger WH, Coetzee T, Bansal R, Pfeiffer SE (2000) Expression of rab GTP-binding proteins during oligodendrocyte differentiation in culture. J Neurosci Res 59(3):446–453
Roth AD, Liazoghli D, Perez De Arce F, Colman DR (2013) Septin 7: actin cross-organization is required for axonal association of Schwann cells. Biol Res 46(3):243–249. https://doi.org/10.4067/S0716-97602013000300004
Boggs JM, Rangaraj G (2000) Interaction of lipid-bound myelin basic protein with actin filaments and calmodulin. Biochemistry. 39(26):7799–7806
Richter-Landsberg C (2008) The cytoskeleton in oligodendrocytes. Microtubule dynamics in health and disease. J Mol Neurosci 35(1):55–63
Owlanj H, Jie Yang H, Wei Feng Z (2012) Nucleoside diphosphate kinase Nm23-M1 involves in oligodendroglial versus neuronal cell fate decision in vitro. Differentiation. 84(4):281–293. https://doi.org/10.1016/j.diff.2012.08.007
Choi YN, Lee SK, Seo TW, Lee JS, Yoo SJ (2014) C-terminus of Hsc70-interacting protein regulates profilin1 and breast cancer cell migration. Biochem Biophys Res Commun 446(4):1060–1066. https://doi.org/10.1016/j.bbrc.2014.03.061
Ni XG, Zhou L, Wang GQ, Liu SM, Bai XF, Liu F, Peppelenbosch MP, Zhao P (2008) The ubiquitin-proteasome pathway mediates gelsolin protein downregulation in pancreatic cancer. Mol Med 14(9–10):582–589. https://doi.org/10.2119/2008-00020.Ni
Belogurov A Jr, Kudriaeva A, Kuzina E, Smirnov I, Bobik T, Ponomarenko N, Kravtsova-Ivantsiv Y, Ciechanover A et al (2014) Multiple sclerosis autoantigen myelin basic protein escapes control by ubiquitination during proteasomal degradation. J Biol Chem 289(25):17758–17766. https://doi.org/10.1074/jbc.M113.544247
Millet V, Moiola CP, Pasquini JM, Soto EF, Pasquini LA (2009) Partial inhibition of proteasome activity enhances remyelination after cuprizone-induced demyelination. Exp Neurol 217(2):282–296. https://doi.org/10.1016/j.expneurol.2009.03.005
Pasquini LA, Paez PM, Moreno MA, Pasquini JM, Soto EF (2003) Inhibition of the proteasome by lactacystin enhances oligodendroglial cell differentiation. J Neurosci 23(11):4635–4644
Doukhanine E, Gavino C, Haines JD, Almazan G, Richard S (2010) The QKI-6 RNA binding protein regulates actin-interacting protein-1 mRNA stability during oligodendrocyte differentiation. Mol Biol Cell 21(17):3029–3040. https://doi.org/10.1091/mbc.E10-04-0305
Acknowledgements
We thank Cytoscape organization for providing us Cytoscape software for ontogenetic analysis
Funding
This study was supported by grants from the Argentine Agency for Promotion of Science and Technology (PICT 2014-3116; PICT 2016-0319) and the University of Buenos Aires (UBACYT- 20020170100285BA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Thomas, L., Pasquini, L.A. Galectin-3 Exerts a Pro-differentiating and Pro-myelinating Effect Within a Temporal Window Spanning Precursors and Pre-oligodendrocytes: Insights into the Mechanisms of Action. Mol Neurobiol 57, 976–987 (2020). https://doi.org/10.1007/s12035-019-01787-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01787-3