Abstract
The proinflammatory and lipopolysaccharide (LPS)-inducible cytokine tumor necrosis factor α (TNF-α) has been shown to enhance primary sensory nociceptive signaling. However, the precise cellular sites of TNF-α and TNF receptors synthesis are still a matter of controversy. Therefore, we focused our study on TNF-α, TNFR1, and TNFR2 protein synthesis and expression patterns in sciatic nerve of controls and rats under systemic challenge with LPS. The enzyme-linked immunosorbent (ELISA) assay showed that the protein level of TNF-α reached peak at 6 h. Double immunofluorescence revealed that LPS-induced expression of TNF-α exclusively located in a subpopulation of Schwann cells, endothelial cells, and macrophages, which increased at late time point in the rat sciatic nerve. Positive staining of TNF receptors were also found in Schwann cells and a few endothelial cells. These observations have demonstrated the production of this proinflammatory cytokine by peripheral nerve glia especially Schwann cells. Synthesized TNF-α might directly act on peripheral nerve glia via TNF receptors, but the inherent mechanisms remain unknown. Further studies are needed to confirm the pathogenic role of tumor necrosis factor in the early stage of inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipopolysaccharide (LPS) is a major integral structural component of the outer membrane of Gram-negative bacteria and one of the most potent initiators of inflammation. LPS activates monocytes and macrophages to produce cytokines such as tumor necrosis factor-alpha (TNF-α), interleukins (IL) -1, and IL-6 that, in turn, serve as endogenous inflammatory mediators (Beutler et al. 2003; Morrison and Morrison 2005).
TNF-α, first identified 20 years ago as a cytokine with antitumor effects in vitro and in vivo (Aggarwal et al. 1985; Pennica et al. 1984 ), belongs to the TNF-α superfamily of death ligands, containing at least 19 members. TNF-α is composed of three 17-kDa polypeptides forming a compact trimer (Jones et al. 1989). It causes inflammatory, antiviral, and immunoregulatory effects, stimulates proliferation of normal cells, and exerts cytolytic and cytostatic action against tumor cells (Baker and Reddy 1998). The effects of TNF-α are mediated through two receptors, TNF receptor type 1 (TNFR1, p55) and TNF receptor type 2 (TNFR2, p75). TNFR1 contains the cytoplasmic death domain and belongs to the family of death receptors. Through the death domain, TNFR1 activates the caspase cascade, leading to cell death. However, this motif is missing in TNFR2, and the function of the latter receptor is poorly understood.
During an immune response, TNF-α production is enhanced in cells of the monocyte–macrophage lineage and in T-cells (Bette et al. 1993). After peripheral nerve injury, Schwann cells have been reported to produce TNF-α (Wagner and Myers 1996). LPS has been reported to cause hyperalgesia (Watkins et al. 1994, 1995; Reeve et al. 2000), is known to enhance not only TNF-α synthesis and release (Holst et al. 1996) but also the expression of TNFR2 and, to a lesser extent, of TNFR1 (Nadeau and Rivest 1999). Li et al. (2004) showed that LPS induced expression of TNF-α in rat DRG exclusively in non-neuronal cells, but in sciatic nerve, its expression pattern is unknown. So we carried out the experiments to study this protein molecule in rat sciatic nerve and determined the time points of TNF-α protein expression in the sciatic nerve after intraperitoneal LPS administration. TNF-α protein levels were assessed across tissues and time points using an enzyme-linked immunosorbent (ELISA) assay. Double-label immunohistochemistry showed that most of the TNF-α immunoreactivity (IR) cells are Schwann cells, suggesting that Schwann cell is most likely the main source for TNF-α in rat sciatic nerve under conditions of LPS-induced inflammatory pain. In accordance with the investigation of Li et al. (2004), TNF-α-IR was also found in macrophages. As for TNFR1 and TNFR2, the positive signals are also located in Schwann cells. These data suggested that TNF-α may have an effect on inflammatory states of rat sciatic nerve. The role of TNF-α during the process is probably via Schwann cells.
Materials and Methods
Experimental Animals and Treatments
Male Sprague–Dawley (SD) rats (Department of Animal Center, Medical College of Nantong University) were housed in plastic cages at 24 ± 1°C on a 12-h light–dark cycle and given free access to laboratory chow and water. Some of them were intraperitoneally injected with 10 mg/kg of LPS (Sigma). All animal experiments were carried out in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals.
ELISA
Rats were killed on hours 1, 3, 6, and 12 h after intraperitoneal injection of LPS (n = 3 per time point). Sciatic nerves were removed by cutting the nerve shortly. Sciatic nerves were homogenized as described (George et al. 1999). TNF-α levels were determined using ELISA kit (Biosource Europe, SA), according to the manufacturer’s instructions. The detection limits of the assays were determined to be 15 pg/ml.
Immunohistochemistry
After defined survival times, control and LPS injected rats (6 and 12 h) were terminally anesthetized and perfused through the ascending aorta with saline, followed by 4% paraformaldehyde. After perfusion, the normal and inflamed sciatic nerves were removed and postfixed in the same fixative for 3 h and then replaced with 20% sucrose for 2–3 days, after 30% sucrose for 2–3 days. Serial transverse sections (14 μm) were cut through the tissues. For single-label immunohistochemistry, all of the sections were blocked with 10% donkey or goat serum with 0.3% Triton X-100 and 1% bovine serum albumin (BSA) for 2 h at room temperature (RT) and incubated overnight at 4°C with anti-TNF-α antibody (anti-goat, 1:50; R&D Systems), anti-TNFR1 antibody (anti-mouse,1:100; Santa Cruz), or anti-TNFR2 antibody (anti-mouse,1:50; Santa Cruz). After washing in phosphate-buffered saline (PBS) three times, the second antibodies (FITC-Donkey anti Goat, 1:100, Jackson Laboratory; FITC-Goat anti Mouse, 1:100, Jackson Laboratory) were placed in a dark room and incubated for 2 h at 4°C.
For double labeling, sections were first blocked with 10% normal serum blocking solution—species the same as secondary antibody, containing 3% (w/v) bovine serum albumin (BSA) and 0.1% Triton X-100 and 0.05% Tween 20 overnight at 4°C to avoid unspecific staining. Then, the sections were incubated with both antibody specific for TNF-α and antibody for different markers as follows: S100 (Schwann cell marker, 1:100; Sigma), or NF-200 (neurofilament marker, 1:600; Sigma), ED-1 (macrophage marker, 1:50; Serotec), overnight at 4°C. After washing in PBS three times for 10 min each, the secondary antibodies (FITC-Donkey-anti-Goat, 1:50, Santa Cruz; TRITC-Donkey-anti-Mouse, 1:50/1:100, Jackson) were added in the dark room and incubated for 2–3 h at 4°C. The sections were incubated with both antibody specific for TNFR1/2 and polyclonal antibody for S100 (Schwann cell marker, 1:100; Sigma) overnight at 4°C. After washing in PBS three times for 10 min, the secondary antibodies (FITC-Goat anti Mouse, 1:100, Jackson Laboratory; TRITC-Donkey-anti-Rabbit, 1:100, Jackson) were added in the dark room and incubated for 2–3 h at 4°C. The images were captured by Leica fluorescence microscope (Germany).
Quantification and Statistics
For immunofluorescence staining in the sciatic nerve, the number of TNF-α-IR-, TNFR1-IR-, and TNFR2-IR-positive cells per section was counted. The proportions of TNF-α-, TNFR1-, and TNFR2-expressed cells were determined by counting the cell profiles that showed distinctive labeling in the sciatic nerve sections. In each rat, four to six sections of the sciatic nerve at each time point were selected randomly. The total number of positive cells was obtained for each animal across the different tissue sections. The count of positive cells and the measurement of areas of TNF-α-IR, TNFR1-IR, and TNFR2-IR were performed by a person who did not know the experiment design. All data were analyzed with Stata 7.0 statistical software. The OD of the immunoreactivity is represented as mean±SEM. Students’ t tests were used. One-way analysis of variance (ANOVA) and Dunnett t tests were also used for statistical analysis. P values less than 0.05 were considered statistically significant.
Results
LPS Induced TNF-α Secretion in Rat Sciatic Nerves
To examine the expression of TNF-α in rat sciatic nerve, ELISA assay was used. As shown in Fig. 1, TNF-α protein content of the sciatic nerve increased with time after intraperitoneal injection of LPS. In control rats, TNF-α level was low but detectable. At 1 h post-LPS administration, the expression of TNF-α elevated, the most robust increase was found at 6 h (P < 0.05). Compared with the controls, it remained elevated until 12 h after LPS injection, but was statistically not significant (P > 0.05).
Expression of TNF-α Immunoreactivity at Different Time Courses After LPS Administration
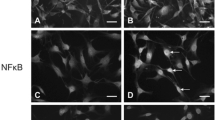
To identify the distribution of TNF-α and TNF receptors in sciatic nerve, we performed single-label immunohistochemistry with anti-TNF-α or anti-TNFR1/R2 antibodies. TNF-α-IR was induced in a time-dependent manner in the sciatic nerve; very little TNF-α-IR was detected in cells associated with the sciatic nerve of rats as control. TNF-α-IR became evident in cells especially in Schwann cells by 6 h after LPS injection (P < 0.05). After 12 h of injection, the number of positive cells is reduced (Fig. 2a, 1–3), but it was statistically significant compared with the controls (P < 0.05), as shown in Fig. 2b. Results of the single-label immunohistochemistry of TNFR1 (Fig. 2a, 4–6) and TNFR2 (Fig. 2a, 7–9) was mostly the same as TNF-α, the number of positive cells reached peak at 6 h (P < 0.05), then slightly reduced at 12 h but remained statistically significant (P < 0.05). As shown in Fig. 2a, we found that cells expressed positively the Schwann cell phenotype.
TNF-α-IR was induced in a time-dependent manner in the sciatic nerve; very little TNF-α-IR was detected in cells associated with the sciatic nerve of rats as control. Rapid increase of TNF-α-IR was found at 6 h after LPS injection. At 12 h, the number of positive cells is reduced (a, 1–3). The expression pattern of TNFR1-IR (a, 4–6) and TNFR2-IR (a, 7–9) was mostly the same as TNF-α-IR; the number of IR cells reach peak at the time course of 6 h, slight reduction was observed at 12 h but still statistically significant. Scale bar: 20 μm. As shown in b, the number of TNF-α- and TNFR1/R2-positive cells in the sciatic nerve at different time points was counted; results mean ± SEM of three independent sets of analyses (asterisk, number sign, caret sign: p < 0.05, significantly different from the control group)
Double Immunofluorescent Staining for TNF-α and S100, ED-1, and NF200
To identify the localization of TNF-α after administration of LPS, we used double immunofluorescent staining. As we had found that TNF-α-IR is mostly located in Schwann cell, we first examined the immunohistochemical colocalization of TNF-α and S100, a marker for Schwann cell. The results of the colocalization of TNF-α and S100 in the sciatic nerve at different time points were shown in Fig. 3. In the control rats, a few Schwann cells in the eyesight was TNF-α positive (Fig. 3a–c), indicating that TNF-α was expressed by part of the Schwann cells. After 6 h of LPS injection, colocalizations of TNF-α and S100 were more than that in controls, and the intensity of staining was much greater (Fig. 3d–f). A similar pattern was found at 12 h (Fig. 3g–i), the number of TNF-α-IR Schwann cells was increased compared with that of the control, but the staining intensity was less than 6 h. As a previous study showed that in response to LPS and inflammatory cytokines induced by LPS, the endothelium altered vascular tone and permeability, absorbed infectious insults, and directed leukocytes such as neutrophils and macrophages into the areas of inflammation (Cines et al. 1998), so we examined the immunohistochemical colocalization of TNF-α and ED-1 (a marker for macrophage) in rat sciatic nerve. As shown in Fig. 4a, an increase of macrophages was detected at 12 h after injection (Fig. 4a, 7–9) compared with 6 h (Fig. 4a, 4–6). In the control, sciatic nerve sections from naive rats, TNF-α-IR was occasionally found in a Schwann cell-like structure, but ED-1-IR was completely absent in the endoneurial compartment (Fig. 4a, 1–3). Furthermore, we found that TNF-α-IR was prominent in many epineurial cells with the morphology of macrophage. As shown in Fig. 4b, colocalization studies at 12 h after LPS injection confirmed that a few epineurial ED-1-positive cells, indicating macrophages, were immunoreactive for TNF-α (Fig. 4b, 4–6), and these colocalizations in epineurium were low at 6 h (Fig. 4b, 1–3). TNF-α-IR was not found in the axons identified by colocalization with NF-200 (a marker for neurofilament) at either 6 h (Fig. 5a–c) or 12 hr (Fig. 5d–f). We also found that TNF-α-IR is located in endothelial cells (Fig. 5a, arrow indicated).
Double immunofluorescent staining for TNF-α and S100 in the sciatic nerve at different time points in rats sciatic nerve. In the control rats, a few Schwann cells are in the same area, and there was some staining of TNF-α in glial cells that are positive for S100 (a–c). After 6 h of LPS injection, the number of TNF-α-IR Schwann cells was more than the control, and the intensity of staining was much greater (d–f). A similar expression pattern was found in 12 h (g–i) and still showed a marked increase, but the intensity of staining and number of positive cells were found in less than 6 h. Scale bar: 20 μm
The immunohistochemical colocalization of TNF-α and ED-1 (a marker for macrophage) in rat sciatic nerve. An increase of macrophages were detected after 12 h (a, 7–9) compared to 6 h (a, 4–6). In controls, TNF-α-IR was occasionally found in Schwann cell-like structures but was completely absent in the endoneurial compartment (a, 1–3). Furthermore, we found that TNF-α-IR was prominent in many epineurial cells with the morphology of macrophages. As shown in (b), colocalization studies at 12 h confirmed that many epineurial ED-1 positive cells, indicating macrophages were immunoreactive for TNF-α (b, 4–6), but these colocalizations in epineurium was low at 6 h (b, 1–3). Scale bar: 20 μm
Double Immunofluorescent Staining for TNF Receptors and S100
To investigate the expression of TNF receptors in rat sciatic nerve under basal conditions and after intraperitoneal injection of LPS, double immunofluorescent staining was performed on TNF receptors and S100. These colocalizations in controls were low (Fig. 6a, 1–3 and b, 1–3), after 6 and 12 h of LPS, the levels were increased in the two receptors (Fig. 6a, 4–9; b, 4–9).
Discussion
The results of the present study demonstrated that TNF-α protein level is induced in the sciatic nerve within several time points after intraperitoneal administration of LPS, as assessed by ELISA and immunohistochemistry. We first examined the TNF-α in the sciatic nerve of several time points of LPS injection and found that it could be detected in 1 h and was especially high in 6 h. The increased level of TNF-α lasted until 12 h, and the result was confirmed by immunohistochemistry, suggesting that TNF-α may function in that context of events. From these results, we know that TNF-α is expressed in rat sciatic nerve in early inflammation stage, which is similar to the study of Li et al. (2004). According to their research, the expression of TNF-α can be detected in Bouin-Hollande fixed DRG sections from rats, 3 h after LPS injection. Expressed as an integral transmembrane protein that is rapidly induced in inflammatory states, this form of TNF-α may moderate cell-to-cell communication and serve as a signal altering afferent neuronal function after inflammation.
Based on the results of double immunofluorescent staining, the TNF-α-IR mostly are locates in Schwann cells, so we conclude that Schwann cells can produce TNF-α in vivo. In the peripheral nervous system, Schwann cells form the myelin sheath and play a key role in the maintenance of the normal physiological function of the axon. The majority of Schwann cells originate from neural crest cells under the guidance of axonal cues that regulate the proliferation, survival, and differentiation of precursor cells into myelin-forming cells (Mirsky et al. 2002). Moreover, Schwann cells overexpress numerous growth factors and extracellular matrix (ECM) components and activated macrophages secrete interleukins (IL), such as IL-1 or IL-6, and growth factors, including nerve growth factor (NGF) and transforming growth factor, which stimulate, directly or indirectly, nerve fiber growth and neuronal survival. We hypothesize that Schwann cell-produced TNF-α participates critically in the pathogenesis of inflammation pain in association with the pathological process of inflammation. Our observation of TNF-α-IR in Schwann cell in the early stage of inflammation suggests that Schwann cell is most likely the main source of TNF-α in rat sciatic nerve after LPS stimulation. As glia are intimately involved in the process of systematical inflammation, our data suggests that Schwann cell may function as immunological cell.
Macrophage activation contributes to the pathology of many inflammatory diseases. In the hemophagocytic syndrome, activated macrophages in the bone marrow show excessive cytokine secretion, dub the “cytokine storm”, and devour erythrocytes (McDonald and Cassatella 1997). In the case of cancer cachexia, inflammatory cytokines such as IL-6 (Strassmann et al. 1992) and TNF-α (Costelli et al. 1992) are considered to be involved, and most likely, these cytokines are released from macrophages. Moreover, it has been reported that even tumorigenesis is caused by macrophage activation. Apart from these types of inflammation, macrophages are often observed in solid tumors, where they are called tumor-associated macrophages (Coussens and Werb 2002). Wagner and Myers (1996) detected that active macrophages were stained at late stage of Wallerian degeneration (7 days) in rat injured sciatic nerve. According to their research, the results of double immunofluorescent staining for TNF-α and ED-1 revealed that macrophages infiltrated at 6 h of LPS, then became evident at 12 h. The positive cells could be found in both endoneurium and epineurium. The infiltration of macrophages was evidently late (12 h) in rat sciatic nerve. We hypothesize that the process depends on the peritoneal injection of LPS (10 mg/kg).
Then, the results of immunohistochemistry revealed that the positive cells of TNF receptors reach peak at the time point of 6 h, then slightly reduce at 12 h but are still statistically significance. It has been reported that the two TNF receptors, TNFR1 and TNFR2, differ significantly in their intracellular signaling domains (Ksontini et al. 1998). TNFR1 contains death domains essential for the recruitment of TNFR-associated death domain (TRADD) and activation of caspases, leading to apoptosis. TNFR2 contains no such death domains. In contrast, both the two receptors contain TRAF2-binding regions that lead to involvement of NIK and proinflammatory signaling pathways. Thus, both TNF receptors should theoretically contribute to inflammatory processes, whereas TNFR1 signaling should predominate in terms of inducing apoptosis. However, in vitro and in vivo studies suggest a more complicated signaling pattern because both the TNFR1 and TNFR2 can signal apoptosis in inflammatory cells (Grell et al. 1993), but only TNFR1 agonists appear to be inflammatory in vivo (Van Zee et al. 1994; Welborn et al. 1996). TNFR1 is involved in the early establishment of the inflammatory response and that its deficiency causes a decreased inflammatory response and tissue damage following brain injury (Quintana et al. 2005). TNFR2 is involved in the proliferation of thymocytes, and the murine cytotoxic T-cell line, in the TNF-dependent proliferative response of human mononuclear cells, in the induction of GM-CSF secretion, in the inhibition of early hematopoiesis, and in downregulating activated T cells by inducing apoptosis (Grell et al. 1998; Gehr et al. 1992; Vandenabeele et al. 1992; Jacobsen et al. 1994; Zheng et al. 1995). Due to the lack of intracellular death domain, TNFR2 may use a distinct signaling pathway to induce apoptosis. Recent studies suggest that TNFR2-induced apoptosis is associated with the downregulation of the antiapoptotic proteins, Bcl-xL and/or Bcl-2 (Boise et al. 1993; Lin et al. 1997).
In our research, the positive TNF receptors can be found in Schwann cells, so it has been generally believed that TNF receptors is responsible for the majority of biological activities of TNF-α. The rapid dissociation of the TNF/TNFR2 complex may facilitate the interaction of TNF with TNFR1, suggesting a role for the TNFR2 in passing TNF to TNFR1, which has been postulated to be the main TNF signal transducer. However, recent data have demonstrated that TNFR2 is able to mediate TNF biological activity independently (Grell et al. 1998; Haridas et al. 1998). Our data also found that TNFR2-IR is evident in rat sciatic nerve at 6 h after LPS injection, but the inherent mechanisms need further detection.
Above all, we know that TNF-α and TNF receptors exist in the rat sciatic nerve during early inflammation stage, and TNF-α may play a role in this process. However, its physiological function as well as the mechanism and significance during the process remain to be further elucidated.
References
Aggarwal, B. B., Kohr, W. J., Hass, P. E., Moffat, B., Spencer, S. A., Henzel, W. J., et al. (1985). Human tumor necrosis factor. Production, purification, and characterization. Journal of Biological Chemistry, 260, 2345–2354.
Baker, S. J., & Reddy, E. P. (1998). Modulation of life and death by the TNF receptor superfamily. Oncogene, 17, 3261–3270.
Bette, M., Schafer, M. K., van Rooijen, N., Weihe, E., & Fleischer, B. (1993). Distribution and kinetics of superantigen-induced cytokine gene expression in mouse spleen. Journal of Experimental Medicine, 178, 1531–1539.
Beutler, B., Hoebe, K., Du, X., & Ulevitch, R. J. (2003). How we detect microbes and respond to them: The Toll-like receptors and their transducers. Journal of Leukocyte Biology, 74, 479–485.
Boise, L. H., Gonzalez-Garcia, M., Postema, C. E., Ding, L., Lindsten, T., Turka, L. A., et al. (1993). Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell, 74, 597–608.
Cines, D. B., Pollak, E. S., Buck, C. A., Loscalzo, J., Zimmerman, G. A., McEver, R. P., et al. (1998). Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood, 91, 3527–3561.
Costelli, P., Carbo, N., Tessitore, L., Bagby, G. J., Lopez-Soriano, F. J., Argiles, J. M., et al. (1992). Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. Journal of Clinical Investigation, 92, 2783–2789.
Coussens, L. M., & Werb, Z. (2002). Inflammation and cancer. Nature, 420, 860–867.
Gehr, G., Gentz, R., Brockhaus, M., Loetscher, H., & Lesslauer, W. (1992). Both tumor necrosis factor receptors mediate proliferative signals in human mononuclear cell activation. Journal of Immunology, 149, 911–917.
George, A., Schmidt, C., Weishaupt, A., Toyka, K. V., & Sommer, C. (1999). Serial determination of tumor necrosis factor-alpha content in rat sciatic nerve after chronic constriction injury. Experimental Neurology, 160, 124–132.
Grell, M., Scheurich, P., Meager, A., & Pfizenmaier, K. (1993). TR60 and TR80 tumor necrosis factor (TNF)-receptors can independently mediate cytolysis. Lymphokine Cytokine Research, 12, 143–148.
Grell, M., Becke, F. M., Wajant, H., Mannel, D. N., & Scheurich, P. (1998). TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. European Journal of Immunology, 28, 257–263.
Haridas, V., Darnay, B. G., Natarajan, K., Heller, R., & Aggarwal, B. B. (1998). Overexpression of the p80 TNF receptor leads to TNF-dependent apoptosis, nuclear factor-κB activation, and c-Jun kinase activation. Journal of Immunology, 160, 3152–3162.
Holst, O., Ulmer, A. J., Brade, H., Flad, H. D., & Rietschel, E. T. (1996). Biochemistry and cell biology of bacterial endotoxins. FEMS Immunology and Medical Microbiology, 16, 83–104.
Jacobsen, F. W., Rothe, M., Rusten, L., Goeddel, D. V., Smeland, E. B., Veiby, O. P., et al. (1994). Role of the 75-kDa tumor necrosis factor (TNF) receptor: Inhibition of early hematopoiesis. Proceedings of the National Academy of Sciences of the United States of America, 91, 10695–10699.
Jones, E. Y., Stuart, D. I., & Walker, N. P. C. (1989). Structure of tumor necrosis factor. Nature, 338, 225–228.
Ksontini, R., MacKay, S. L., & Moldawer, L. L. (1998). Revisiting the role of tumor necrosis factor alpha and the response to surgical injury and inflammation. Archives of Surgery, 133, 558–567.
Li, Y., Ji, A., Weihe, E., & Schafer, M. K. (2004). Cell-specific expression and lipopolysaccharide-induced regulation of tumor necrosis factor-α (TNF-α) and TNF receptors in rat dorsal root ganglion. The Journal of Neuroscience, 24, 9623–9631.
Lin, R. H., Hwang, Y. W., Yang, B. C., & Lin, C. S. (1997). TNF receptor 2-triggered apoptosis is associated with the downregulation of Bcl-xL on activated T cells and can be prevented by CD28 costimulation. Journal of Immunology, 158, 598–603.
McDonald, P. P., & Cassatella, M. A. (1997). Activation of transcription factor NF-κB by phagocytic stimuli in human neutrophils. FEBS Letters, 412, 583–586.
Mirsky, R., Jessen, K. R., Brennan, A., Parkinson, D., Dong, Z., Meier, C., et al. (2002). Schwann cells as regulators of nerve development. Journal of Physiology Paris, 96, 17–24.
Morrison, S. G., & Morrison, R. P. (2005). A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. Infection and Immunity, 175, 7536–7542.
Nadeau, S., & Rivest, S. (1999). Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (p55 and p75) in the rat brain: A view from the blood–brain barrier. Neuroscience, 93, 1449–1464.
Pennica, D., Nedwin, G. E., Hayflick, J. S., Seeburg, P. H., Derynck, R., Palladino, M. A., et al. (1984). Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature, 312, 724–729.
Quintana, A., Giralt, M., Rojas, S., Penkowa, M., Campbell, I. L., Hidalgo, J., et al. (2005). Differential role of tumor necrosis factor receptors in mouse brain inflammatory responses in cryolesion brain injury. Journal of Neuroscience Research, 82, 701–716.
Reeve, A. J., Patel, S., Fox, A., Walker, K., & Urban, L. (2000). Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. European Journal of Pain, 4, 247–257.
Strassmann, G., Fong, M., Kenny, J. S., & Jacob, C. O. (1992). Evidence for the involvement of interleukin 6 in experimental cancer cachexia. Journal of Clinical Investigation, 89, 1681–1684.
Van Zee, K. J., Stackpole, S. A., Montegut, W. J., Rogy, M. A., Calvano, S. E., Hsu, K. C., et al. (1994). A human tumor necrosis factor (TNF)—a mutant that binds exclusively to the p55 TNF receptor produces toxicity in the baboon. Journal of Experimental Medicine, 179, 1185–1191.
Vandenabeele, P., Declercq, W., Van Vercammen, D., De Craen, M., Grooten, J., Loetscher, H., et al. (1992). Functional characterization of the human tumor necrosis factor receptor p75 in a transfected rat/mouse T-cell hybridoma. Journal of Experimental Medicine, 176, 1015–1024.
Wagner, R., & Myers, R. R. (1996). Schwann cells produce tumor necrosis factor alpha: Expression in injured and non-injured nerves. Neuroscience, 73, 625–629.
Watkins, L. R., Goehler, L. E., Relton, J., Brewer, M. T., & Maier, S. F. (1995). Mechanisms of tumor necrosis factor-alpha (TNF-alpha) hyperalgesia. Brain Research, 692, 244–250.
Watkins, L. R., Wiertelak, E. P., Goehler, L. E., Smith, K. P., Martin, D., & Maier, S. F. (1994). Characterization of cytokine-induced hyperalgesia. Brain Research, 654, 15–26.
Welborn, M. B. III, Van Zee, K., Edwards, P. D., Pruitt, J. H., Kaibara, A., Vauthey, J. N., et al. (1996). A human tumor necrosis factor p75 receptor agonist stimulates in vitro T cell proliferation but does not produce inflammation or shock in the baboon. Journal of Experimental Medicine, 184, 165–171.
Zheng, L., Fisher, G., Miller, R. E., Peschon, J., Lynch, D. H., & Lenardo, M. J. (1995). Induction of apoptosis in mature T cell by tumour necrosis factor. Nature, 377, 348–351.
Acknowledgments
This work was supported by the National Natural Scientific Foundation of China Grant (No.30300099), Natural Scientific Foundation of Jiangsu Province Grant (No. BK2003035 and No. BK2006547), College and University Natural Scientific Research Programme of Jiangsu Province (No. 03KJB180109 and No. 04KJB320114), Technology Guidance Plan for Social Development of Jiangsu Province Grant (BS2004526), Health Project of Jiangsu Province (H200632), “Liu-Da-Ren-Cai-Gao-Feng” Financial Assistance of Jiangsu Province Grant (No. 2).
Youhua Wang and Dan Zhou contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Zhou, D., Shen, Q. et al. Lipopolysaccharide-Induced Upregulation of Tumor Necrosis Factor-α (TNF-α) and TNF Receptors in Rat Sciatic Nerve. J Mol Neurosci 32, 207–216 (2007). https://doi.org/10.1007/s12031-007-0036-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-007-0036-1