Abstract
Background and Aims
In vitro studies have shown that clusterin modulates treatment sensitivity in a number of human cancers; however, the interaction between clusterin expression and hypoxia in controlling treatment response in CRC has not previously been examined. The aim of this study was to assess the effect of clusterin overexpression in CRC cells on sensitivity to 5-fluorouracil (5-FU), oxaliplatin and FOLFOX treatment under normoxic and graded hypoxic conditions.
Methods
SW480 colon cancer cells were transfected with full length Clusterin cDNA to generate a clusterin overexpressing cell line. Overexpression was confirmed by western blot analysis. The response of parental and clusterin overexpressing cells to 5-FU, oxaliplatin and FOLFOX was examined using a crystal violet-based proliferation assay under normoxic conditions, 3% and 1% hypoxic conditions. The levels of apoptosis and G2/M arrest in FOLFOX-treated cells were assessed by flow cytometry.
Results
Under normoxic conditions, clusterin overexpressing cells were more sensitive to FOLFOX treatment (p = 0.01); under 3% and 1% hypoxic conditions, overexpressing clusterin cells were more sensitive to 5-FU, oxaliplatin and FOLFOX, p values <0.05 for all conditions. Under normoxic conditions, overexpressing clusterin cells showed significantly higher levels of apoptosis when treated with FOLFOX compared to untransfected cells; levels of G2M cells were not significantly different. Under both 3% and 1% hypoxia, the percentage of cells undergoing apoptosis following FOLFOX treatment was significantly higher in overexpressing clusterin cells.
Conclusion
These in vitro findings suggest that tumours expressing high levels of clusterin, particularly if hypoxic in nature, may benefit from treatments such as FOLFOX.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clusterin is a disulfide-linked heterodimeric-secreted glycoprotein which is ubiquitously expressed in most mammalian tissues and found in plasma, urine and cerebrospinal fluid across a broad range of species [1, 2]. We and others have previously shown that Clusterin is a heavily glycosylated protein [3]; however, non-glycosylated cytoplasmic and nuclear isoforms have also been identified [3–6].
Clusterin binds to and forms complexes with lipids, immunoglobulins, heparin, complement components, beta amyloid, bacteria, paraoxonase and leptin [1, 7–13]. It is also hypothesised to be involved in tissue remodelling, cellular debris clearance, complement inhibition, cell aggregation and adhesion and matrix metalloproteinase inhibition [1, 3, 14]. Despite advances in the understanding of the multifactorial nature of clusterin’s functions and its recognition as an important player in tumour growth and disease progression, its precise role remains unclear with studies supporting both pro- [15] and anti-apoptotic effects [16, 17].
In vitro studies have shown that clusterin can modulate treatment sensitivity in human prostate cancer cell lines (LNCaP and PC-3) [16, 18], bladder cancer cell lines (UM-UC-3P and KoTCC-1) [19, 20], and breast cancer cell lines (MCF-7 and 734B) [21]. These in vitro models showed that increased clusterin expression conveyed resistance to chemotherapy and radiotherapy [1, 3, 16, 19, 21]. The downregulation of clusterin using anti-sense oligonucleotides has been shown to reverse this resistance, therefore enhancing the cytotoxicity of chemotherapeutic and radiation treatments [1, 19, 21, 22]. While tumour hypoxia is also classically associated with resistance to radiotherapy and chemotherapy [23, 24], the interaction between clusterin expression and hypoxia in controlling treatment response has not previously been examined. The aim of this study was to assess the effect of clusterin overexpression in colorectal cancer cells on sensitivity to 5-fluorouracil (5-FU), oxaliplatin and FOLFOX treatment under normoxic and graded hypoxic conditions.
Methods
Cell Culture and Transfection
To assess the effect of clusterin overexpression on chemotherapy treatment sensitivity in vivo, SW480 colon cancer cells were transfected with full-length Clusterin cDNA which was kindly provided by Prof. Martin Tenniswood (GEN*NY*Sis Center for Excellence in Cancer Genomics, NY). Clusterin cDNA was inserted into the pcDNA DEST47 mammalian expression vector. This vector contained a neomycin resistance gene used to select for clusterin overexpressing transfected cells. SW480 cells were obtained from the American Type Culture Collection (www.atcc.org). Cells were grown in RPMI medium, supplemented with 10% fetal calf serum plus penicillin (100 U/ml), streptomycin (100 μg/ml) and Fungizone amphotericin B (4 μg/ml; Invitrogen, Paisley, UK). Cells were seeded at 500,000 cells in a 6-well plate 24 h prior to transfection with clusterin cDNA. Transfection was carried out following the manufacturer’s protocol (Lipofectamine™ 2000 reagent, Invitrogen). Briefly, 500 ng of DNA was diluted in serum-free media containing 20 μl of lipofectamine™ 2000 reagent and incubated for 20 min at room temperature. This DNA/lipid mixture was added to cells in serum-free media and incubated for 16 h. Transfection medium was replaced with fresh medium supplemented with 500 μg/ml G418 (Geneticin, Invitrogen) to select for clusterin overexpressing stably transfected cells for a period of 7 days.
Western Blot Analysis
The expression levels of clusterin in parental SW480 cells and cells transfected with empty vector or clusterin cDNA were determined by western blotting analysis. The cells were harvested, and the pellets were washed in cold PBS. Whole-cell lysates were prepared in ice-cold RIPA buffer containing 50 mM Tris/HCl (pH 7.4), 425 mM NaCl, 0.1% Triton X100, 0.25% sodium deoxycholate and protease inhibitors (Halt™ Protease Inhibitor Cocktail Kit, Pierce Technology, Rockford, IL), 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (Pierce Biotechnology, Rockford, IL) and 1 mM sodium orthovanadate (Sigma, Dublin, Ireland). Lysates were gently shaken at 4°C for 20 min and centrifuged at 13,000 rpm at 4°C for 5 min. Protein concentrations were determined using the BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL). Fifty microgrammes of protein was run on a 10% SDS-PAGE gel and transferred to a PVDF Transfer Membrane (Pierce Biotechnology Rockford, IL). Antibodies to human clusterin (1:100; Fitzgerald, USA), and β-actin (1:8,000; Sigma) were used. A secondary antibody, antimouse-IgG HRP-conjugated (Pierce Biotechnology, Rockford, IL), was used at a final dilution of 1:20,000. Signals were detected using a SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL). The blots were scanned using an AutoChemi System, and the bands were densitometrically analysed using LabWorks II image acquisition and analysis software (UVP Bioimaging Systems, Cambridge, UK).
Immunofluorescence
Immunofluorescence was performed on parental and clusterin overexpressing SW480 cells. Cells were prepared for immunofluorescence as follows. Cells were seeded at a density of 50,000 cells/well in 8-well cell culture-treated glass chamber slides. The cells were fixed using 3.7% paraformaldehyde in PBS for 25 min at 4°C. Two 5-min PBS washes were then performed. The cells were permeabilised using 0.2% Triton X100 in PBS. Two further 5-min PBS washes were then performed. The chamber slides were then incubated with clusterin 2D9 antibody [5] for 1.5 h at room temperature. Three 5-min PBS washes were performed. Biotinylated antimouse IgG (Vector Laboratories, Burlingame, CA, USA) 1:200 was incubated on slides for 30 min, followed by three 5-min PBS washes. Slides were then incubated with monoclonal anti-biotin–Cy3 antibody (clone BN-34, Sigma-Aldrich, St Louis, MO, USA) 1:100 for 30 min. Three further 5-min PBS washes were performed, and slides were then incubated with 10 ng/ml DAPI for 5 min at room temperature. Following three final 5-min PBS washes, slides were incubated with ProLong Gold antifade reagent (Invitrogen, Oregon, USA) and stored at −20°C until visualised using a confocal microscope (Zeiss).
Cell Growth Assay
The response of parental, empty vector and clusterin overexpressing cells to chemotherapeutic treatments, 10 μM 5-fluorouracil, 5 μM oxaliplatin and FOLFOX (5-FU, oxaliplatin and 2.5 μg/ml folinic acid), was examined using a crystal violet-based proliferation assay. Fifteen thousand cells were seeded in 96-well plates and incubated overnight under normoxic (21% oxygen levels), 3% hypoxic (partial pressure of oxygen, 22.8 mmHg) and 1% hypoxic (partial pressure of oxygen, 7.6 mmHg) conditions, and cell proliferation was evaluated at after 24, 48, 72 and 96 h. At each time point, the medium was removed; the cells were washed with PBS, fixed with 1% glutaraldehyde for 15 min at room temperature and stained with a 0.1% crystal violet solution (Pro-Lab Diagnostics, Cheshire, UK) for 30 min. The cells were washed extensively in tap water and air dried. Dye was extracted from cells using 1% solution of Triton X100 and absorbance read at 550 nm using a plate reader (Multiskan Ascent, Labsystems, Helsinki, Finland). The level of growth inhibition induced by each treatment was calculated as a percentage of the control.
Flow Cytometry
To assess the levels of apoptosis and G2/M arrest by flow cytometry, cells were seeded in 6-well plates and incubated in the absence and presence of FOLFOX (and under normoxic and graded hypoxic levels). At 48 and 96 h, cells and media were harvested, pooled and centrifuged. Cell pellets were fixed in 4% paraformaldehyde for 20 min at room temperature. Cells were permeabilised in PBA + 0.25% Triton at 4 C for 5 min and stained with 10ug/mL propidium iodide for 30 min in the dark. The samples were analysed, and the cells were post-fixed in 1% paraformaldehyde and stored at 4°C until run on a Becton Dickinson FACScan flow cytometer using CellQuest software (BD Biosciences, San Jose, CA).
Clusterin, VEGF and IL-8 Secretions
Cell culture media collected at 48 and 96 h time points were analysed for secretion levels of clusterin, VEGF and IL-8 concentrations using ELISA technique. Human clusterin competitive ELISA kit (AdipoGen, Incheon, Korea) was used to assess levels of clusterin secretion as per manufacturer’s instruction. The absorbances were read at 450 nm, and the concentration of clusterin was determined by linear regression from a standard curve of the known clusterin standards. VEGF and IL-8 levels were quantified using ELISA’s from R&D as per manufacturer’s instructions.
Statistical Analysis
Data was analysed using the Statistical Package for the Social sciences (SPSS, Chicago, IL, USA), version 11.0. Continuous data were presented as mean and standard deviation. Time-dependent variables such as cell growth rates were assessed using repeated measures analysis of variance. All p values were two-sided, and p values less than 0.05 were considered statistically significant in all analyses.
Results
Clusterin Overexpression in SW480 Cells
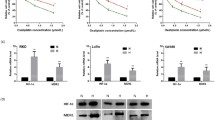
Figure 1a shows levels of clusterin (three different isoforms, 35, 50 and 75 kDa) in parental (untransfected) and transfected clusterin cells. Densitometry analysis demonstrated approximately fivefold overexpression of the 30–35 and 50 kDa isoforms (Fig. 1b). Figure 1c shows representative immunofluorescence images demonstrating the overexpression of cytoplasmic clusterin in the transfected (CLU) versus untransfected cells (PAR). No morphological cellular changes were detected in the clusterin overexpressing cells.
Clusterin is overexpressed in SW480 cells lines. a A representative example of a western blot for clusterin with increased signal seen at 35, 50, and 75 kDa fractions comparing transfected (CLU) to parental (PAR) cell lines. b Densitometry analysis confirming approximately fivefold overexpression of >50 and 30–35 kDa clusterin isoforms. c Representative immunofluorescence images demonstrating increased amounts of cytoplasmic clusterin in CLU compared to PAR cell lines
Treatment Sensitivity of Clusterin Overexpressing Cells
Cell growth rates and growth inhibition were assessed using the crystal violet assay over a 4-day time course. Overexpressing clusterin cells did not show significant differences in cell growth rates compared to parental and empty vector lines in untreated conditions. Under normoxic conditions, clusterin overexpression was not associated with altered sensitivity to 5-FU and oxaliplatin (Fig. 2a, b); however, for FOLFOX treatment, the overexpressing clusterin cells were more sensitive (p = 0.01), Fig. 2c. Under 1% hypoxic conditions (Fig. 2d–f), overexpressing clusterin cells were more sensitive to 5-FU, oxaliplatin and FOLFOX, p values 0.003, <0.001 and <0.001, respectively. Under 3% hypoxic conditions (Fig. 2, g–i), overexpressing clusterin cells were again more sensitive to 5-FU, oxaliplatin and FOLFOX, p values 0.03, <0.001 and <0.001, respectively.
The growth inhibitory effects of 5-fluorouracil, oxaliplatin, and FOLFOX in parental and clusterin overexpressing SW480 cell lines cultured under normoxic, 1% hypoxic, and 3% hypoxic conditions. Under normoxic conditions, clusterin overexpression was not associated with altered sensitivity to 5-FU and oxaliplatin (a, b); however, for FOLFOX treatment, the overexpressing clusterin cells were more sensitive (p = 0.01; c). Under 1% hypoxic conditions (d–f), overexpressing clusterin cells were more sensitive to 5-FU, oxaliplatin, and FOLFOX; p values of 0.003, <0.001, and <0.001, respectively. Under 3% hypoxic conditions (g–i), overexpressing clusterin cells were again more sensitive to 5-FU, oxaliplatin, and FOLFOX; p values of 0.03, <0.001, and <0.001, respectively
Levels of Apoptosis and G2M Arrest in Clusterin Overexpressing Cells
The increased sensitivity of clusterin overexpressing cells under graded hypoxic levels was further evaluated to determine if this effect was due to the elevated rates of apoptosis or the altered G2M arrest cells. This was performed for FOLFOX treatment at 48 and 96 h. Under normoxic conditions, overexpressing clusterin cells showed significantly higher levels of apoptosis when treated with FOLFOX compared to untransfected cells (Fig. 3a). The levels of G2M cells were not significantly different (Fig. 3b). Under both 3% and 1% hypoxia, the percentage of cells undergoing apoptosis following FOLFOX treatment was significantly higher in overexpressing clusterin cells (Fig. 3c, e).
Levels of apoptosis and G2M arrest in clusterin overexpressing cells. Under normoxic conditions, overexpressing clusterin cells showed significantly higher levels of apoptosis when treated with FOLFOX compared to untransfected cells (a) and levels of G2M cells were not significantly different (b). Under both 3% and 1% hypoxia, the percentage of cells undergoing apoptosis following FOLFOX treatment was significantly higher in overexpressing clusterin cells (c, e)
VEGF and IL-8 Secretions
The levels of secreted VEGF and IL-8 were assessed in conditioned media from untransfected and transfected cells under normoxic and 3% and 1% hypoxic conditions. Table 1 shows that the levels of these factors at 48 and 96 h. Under normoxic untreated conditions, clusterin overexpressing cells had lower levels of VEGF secretions but higher IL-8 secretions compared to untransfected cells (p < 0.001). Treatment under normoxic conditions with FOLFOX reversed this trend (all p values ≤0.002). Under 3% hypoxia conditions, there was no significant difference in the levels of VEGF secretions between untransfected and transfected cells; however, for this grade of hypoxia, there was a significant higher level of IL-8 secretion in clusterin overexpressing (p = 0.003). In 3% hypoxia FOLFOX condition, VEGF was lower and IL-8 levels were higher in overexpressing clusterin cells compared to untransfected cells (p values 0.006 and 0.02, respectively). In 1% hypoxia condition with and without FOLFOX treatment, overexpressing clusterin cells show lower VEGF and higher IL-8 secretions (all p values ≤0.03). There was no significant difference in secreted clusterin levels comparing untransfected to transfected cells for any condition with or without FOLFOX treatment.
Discussion
This study has shown that clusterin overexpression enhances the sensitivity of colon cancer cells to chemotherapy, an effect which is enhanced under graded hypoxic conditions. Flow cytometry analysis demonstrated that the enhanced treatment sensitivity of clusterin overexpressing cells is mediated via increased apoptosis.
Clusterin has been implicated in tumourigenesis in many different in vitro and in vivo tumour models; however, the results of these studies are conflicting with some promoting clusterin as a cell survival signal [22, 25] and others as a pro-apoptotic protein [15]. These apparently ambiguous functions may be attributable to the existence of two different but related clusterin isoforms, secreted clusterin (sCLU) and nuclear clusterin (nCLU), with may have distinct biological activities [3] and can be distinguished immunologically [5, 6]. sCLU has pro-survival activity and is known to convey treatment resistance in some cancers while nCLU has pro-apoptotic effects [2]. We demonstrate, for the first time, that clusterin overexpression in colorectal cancer cells paradoxically conveys increased treatment sensitivity to 5-fluorouracil, oxaliplatin and FOLFOX, an effect which is even further enhanced under graded hypoxic conditions. While hypoxia alone caused resistance to the treatments, overexpression of clusterin reversed these effects. This has not been previously reported.
The mechanism by which clusterin overexpression contributes to this increased chemotherapy-induced growth inhibition in the SW480 cell line was assessed using flow cytometry and the levels of apoptosis and G2M arrest evaluated. We demonstrated that the higher rates of growth inhibition in overexpressing clusterin cells compared to untransfected cells were due to higher levels of apoptosis, a finding which was significantly more pronounced under both hypoxic conditions. Clusterin has been reported to play a role in cell cycle regulation. Transient clusterin overexpression in human prostate epithelial cells resulted in increased accumulation of cells in the G(0)/G(1) phases of the cell cycle, accompanied by the slowdown of cell cycle progression and decrease of DNA synthesis [26]; while overexpression of nuclear clusterin in human breast cancer cells dramatically reduced cell growth and colony-forming ability concomitant with increased G(1) cell cycle checkpoint arrest and increased cell death [27]. Despite these data, the overall function of clusterin in cell cycle regulation remains unclear. In our study, we found increased apoptosis in clusterin overexpressing cells treated with chemotherapy suggesting that increased expression of this protein may promote changes in the cell cycle which favour apoptotic cell death following exposure to chemotherapy. In contrast to our findings, other in vitro studies have shown that treatment resistance is mediated by clusterin overexpression [1, 3, 16, 19, 21] and clusterin anti-sense oligonucleotides reversed this effect [1, 19, 21, 22]. However, these studies did not examine clusterin’s treatment sensitivity/resistance effect in colorectal cells nor were these effects evaluated under graded hypoxia levels.
Tumour hypoxia occurs frequently in solid tumours including colorectal carcinoma [28] and is one of the key factors in inducing the development of cell clones with an aggressive and treatment-resistant phenotype. A number of mechanisms contribute to its development including: unrestrained growth and accelerated oxygen consumption by tumour cells, poor lymphatic drainage of tumours resulting in high interstitial pressures and the development of immature, disordered tumour vasculature [29, 30]. Hypoxia may result in a variety of changes within a given tumour. It may have anti-proliferative effects, restricting cell proliferation, promoting differentiation and inducing apoptosis and necrosis. Conversely, clones within the tumour may react to hypoxic stress with adaptive processes through the modification of gene expression, which confer an aggressive phenotype, promoting local and distant spread [28, 30, 31]. Hypoxia is known to promote treatment resistance in a number of cancers [23, 32] and can convey a growth advantage to tumour cells by inducing the production of VEGF [33] and promoting the switch from oxidative to glycolytic metabolism [34]. Hypoxia is known to increase VEGF secretion via the HIF pathway which can promote and drive tumour progression [33]. We demonstrated VEGF secretion to be generally decreased in clusterin overexpressing colorectal cancer cells, while VEGF levels were generally increased in untransfected cells comparing normoxic and hypoxic conditions. Consequently, increased clusterin expression may enhance the growth inhibitory effects of chemotherapeutic agents via attenuation of VEGF production. This would be in concordance with previous data which demonstrated that inhibition of VEGF expression using epidermal growth factor receptor inhibitors increased sensitivity to chemotherapy and radiotherapy [35].
IL-8 is a pro-inflammatory cytokine which is induced under hypoxic conditions whose predominant function is to mediate the activation and migration of neutrophils [36]. IL-8 has also been implicated in tumourigenesis through its potential functions as a mitogenic and angiogenic factor [37]. Increased serum and tissue IL-8 levels have been demonstrated in CRC patients, and recombinant human IL-8 has been reported to enhance the in vitro proliferation of CRC cells [38]. The effect of IL-8 on treatment sensitivity has been investigated in a number of cancers. IL-8 has been shown to promote treatment resistance in ovarian cancer [39], while IL-8 signalling has been demonstrated to promote treatment resistance in prostate carcinoma by reducing levels of apoptosis [40]. The influencer of IL-8 on treatment sensitivity in colorectal cancer has not been previously evaluated. We demonstrated IL-8 production to be generally increased in clusterin overexpressing cells under both normoxic and hypoxic conditions, suggesting that IL-8 may have some role in enhancing treatment sensitivity in colorectal cancer; however, these findings clearly require further study and validation.
The form of clusterin that is overexpressed in our system is the intracellular form; we do not see expression of the nuclear clusterin isoform. From Fig. 1, the pro form (50 kDa), active form (35 kDa) and glycosylated form (75 kDa) are all overexpressed. Furthermore, there were no differences observed in secreted clusterin levels comparing transfected and untransfected cells for any condition, with or without FOLFOX treatment, suggesting that the increased treatment sensitivity observed in clusterin overexpressing cells was mediated by intracellular clusterin isoforms. We hypothesise that it is the overexpression of this active intracellular isoform that is contributing to the effects that we see under hypoxic conditions.
Several groups including ours have examined the prognostic significance of clusterin in vivo. Cytoplasmic clusterin expression correlates with poor prognosis in prostate adenocarcinoma [41], renal cell carcinoma [42], ovarian carcinoma [43], cervical cancer [44] and hepatocellular carcinoma [45]. In contrast, cytoplamic clusterin expression correlates with a good prognosis in pancreatic adenocarcinoma [46] and non-small cell lung cancer [47]. Our group have recently reported that increased cytoplamic clusterin correlates with poor outcome in stage II colorectal cancer [48]. These in vitro findings that we report suggest that in vivo tumours expressing high levels of clusterin, particularly if these tumours are hypoxic in nature, may benefit from treatments such as FOLFOX. This may be of particular importance in early-stage cancer where patients with clusterin overexpressing tumours have poorer prognosis yet may be more sensitive to combined chemotherapy treatments.
References
Trougakos IP, Gonos ES. Clusterin/apolipoprotein J in human aging and cancer. Int J Biochem Cell Biol. 2002;34(11):1430–48.
Pucci S, Bonanno E, Pichiorri F, Angeloni C, Spagnoli LG. Modulation of different clusterin isoforms in human colon tumorigenesis. Oncogene. 2004;23(13):2298–304. doi:10.1038/sj.onc.1207404.
Shannan B, Seifert M, Leskov K, Willis J, Boothman D, Tilgen W, et al. Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death Differ. 2006;13(1):12–9. doi:10.1038/sj.cdd.4401779.
Andersen CL, Schepeler T, Thorsen K, Birkenkamp-Demtroder K, Mansilla F, Aaltonen LA, et al. Clusterin expression in normal mucosa and colorectal cancer. Mol Cell Proteomics. 2007;6(6):1039–48. doi:10.1074/mcp.M600261-MCP200.
Lakins J, Bennett SA, Chen JH, Arnold JM, Morrissey C, Wong P, et al. Clusterin biogenesis is altered during apoptosis in the regressing rat ventral prostate. J Biol Chem. 1998;273(43):27887–95.
O’Sullivan J, Whyte L, Drake J, Tenniswood M. Alterations in the post-translational modification and intracellular trafficking of clusterin in MCF-7 cells during apoptosis. Cell Death Differ. 2003;10(8):914–27. doi:10.1038/sj.cdd.4401254.
Wilson MR, Easterbrook-Smith SB. Clusterin binds by a multivalent mechanism to the Fc and Fab regions of IgG. Biochim Biophys Acta. 1992;1159(3):319–26.
de Silva HV, Harmony JA, Stuart WD, Gil CM, Robbins J. Apolipoprotein J: structure and tissue distribution. Biochemistry. 1990;29(22):5380–9.
Jenne DE, Lowin B, Peitsch MC, Bottcher A, Schmitz G, Tschopp J. Clusterin (complement lysis inhibitor) forms a high density lipoprotein complex with apolipoprotein A-I in human plasma. J Biol Chem. 1991;266(17):11030–6.
Li DQ, Ljungh A. Binding of human clusterin by Staphylococcus epidermidis. FEMS Immunol Med Microbiol. 2001;31(3):197–202.
Matsubara E, Soto C, Governale S, Frangione B, Ghiso J. Apolipoprotein J and Alzheimer’s amyloid beta solubility. Biochem J. 1996;316(Pt 2):671–9.
Kujiraoka T, Hattori H, Miwa Y, Ishihara M, Ueno T, Ishii J, et al. Serum apolipoprotein j in health, coronary heart disease and type 2 diabetes mellitus. J Atheroscler Thromb. 2006;13(6):314–22.
Bajari TM, Strasser V, Nimpf J, Schneider WJ. A model for modulation of leptin activity by association with clusterin. FASEB J. 2003;17(11):1505–7. doi:10.1096/fj.02-1106fje.
Matsuda A, Itoh Y, Koshikawa N, Akizawa T, Yana I, Seiki M. Clusterin, an abundant serum factor, is a possible negative regulator of MT6-MMP/MMP-25 produced by neutrophils. J Biol Chem. 2003;278(38):36350–7. doi:10.1074/jbc.M301509200.
Leskov KS, Klokov DY, Li J, Kinsella TJ, Boothman DA. Synthesis and functional analyses of nuclear clusterin, a cell death protein. J Biol Chem. 2003;278(13):11590–600. doi:10.1074/jbc.M209233200.
Miyake H, Nelson C, Rennie PS, Gleave ME. Acquisition of chemoresistant phenotype by overexpression of the antiapoptotic gene testosterone-repressed prostate message-2 in prostate cancer xenograft models. Cancer Res. 2000;60(9):2547–54.
Cervellera M, Raschella G, Santilli G, Tanno B, Ventura A, Mancini C, et al. Direct transactivation of the anti-apoptotic gene apolipoprotein J (clusterin) by B-MYB. J Biol Chem. 2000;275(28):21055–60. doi:10.1074/jbc.M002055200.
Zellweger T, Chi K, Miyake H, Adomat H, Kiyama S, Skov K, et al. Enhanced radiation sensitivity in prostate cancer by inhibition of the cell survival protein clusterin. Clin Cancer Res. 2002;8(10):3276–84.
Muramaki M, So A, Hayashi N, Sowery R, Miyake H, Fujisawa M, et al. Chemosensitization of gemcitabine-resistant human bladder cancer cell line both in vitro and in vivo using antisense oligonucleotide targeting the anti-apoptotic gene, clusterin. BJU Int. 2009;103(3):384–90. doi:10.1111/j.1464-410X.2008.08098.x.
Yamanaka K, Gleave M, Muramaki M, Hara I, Miyake H. Enhanced radiosensitivity by inhibition of the anti-apoptotic gene clusterin using antisense oligodeoxynucleotide in a human bladder cancer model. Oncol Rep. 2005;13(5):885–90.
Toffanin S, Daidone MG, Miodini P, De Cecco L, Gandellini P, Cappelletti V. Clusterin: a potential target for improving response to antiestrogens. Int J Oncol. 2008;33(4):791–8.
Gleave M, Miyake H. Use of antisense oligonucleotides targeting the cytoprotective gene, clusterin, to enhance androgen- and chemo-sensitivity in prostate cancer. World J Urol. 2005;23(1):38–46. doi:10.1007/s00345-004-0474-0.
Vaupel P, Thews O, Hoeckel M. Treatment resistance of solid tumors: role of hypoxia and anemia. Med Oncol. 2001;18(4):243–59. doi:10.1385/MO:18:4:243.
Vaupel P, Mayer A, Hockel M. Tumor hypoxia and malignant progression. Methods Enzymol. 2004;381:335–54. doi:10.1016/S0076-6879(04)81023-1.
Trougakos IP, So A, Jansen B, Gleave ME, Gonos ES. Silencing expression of the clusterin/apolipoprotein j gene in human cancer cells using small interfering RNA induces spontaneous apoptosis, reduced growth ability, and cell sensitization to genotoxic and oxidative stress. Cancer Res. 2004;64(5):1834–42.
Bettuzzi S, Scorcioni F, Astancolle S, Davalli P, Scaltriti M, Corti A. Clusterin (SGP-2) transient overexpression decreases proliferation rate of SV40-immortalized human prostate epithelial cells by slowing down cell cycle progression. Oncogene. 2002;21(27):4328–34. doi:10.1038/sj.onc.1205594.
Yang CR, Leskov K, Hosley-Eberlein K, Criswell T, Pink JJ, Kinsella TJ, et al. Nuclear clusterin/XIP8, an X-ray-induced Ku70-binding protein that signals cell death. Proc Natl Acad Sci USA. 2000;97(11):5907–12.
Yotnda P, Wu D, Swanson AM. Hypoxic tumors and their effect on immune cells and cancer therapy. Methods Mol Biol. 2010;651:1–29. doi:10.1007/978-1-60761-786-0_1.
Giatromanolaki A, Harris AL. Tumour hypoxia, hypoxia signaling pathways and hypoxia inducible factor expression in human cancer. Anticancer Res. 2001;21(6B):4317–24.
Voss MJ, Niggemann B, Zanker KS, Entschladen F. Tumour reactions to hypoxia. Curr Mol Med. 2010;10(4):381–6.
Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia and cancer. J Mol Med. 2007;85(12):1301–7. doi:10.1007/s00109-007-0281-3.
Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9 Suppl 5:10–7. doi:10.1634/theoncologist.9-90005-10.
Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26(2):281–90. doi:10.1007/s10555-007-9066-y.
Kim JW, Gao P, Dang CV. Effects of hypoxia on tumor metabolism. Cancer Metastasis Rev. 2007;26(2):291–8. doi:10.1007/s10555-007-9060-4.
Cerniglia GJ, Pore N, Tsai JH, Schultz S, Mick R, Choe R, et al. Epidermal growth factor receptor inhibition modulates the microenvironment by vascular normalization to improve chemotherapy and radiotherapy efficacy. PLoS ONE. 2009;4(8):e6539. doi:10.1371/journal.pone.0006539.
Tamm M, Bihl M, Eickelberg O, Stulz P, Perruchoud AP, Roth M. Hypoxia-induced interleukin-6 and interleukin-8 production is mediated by platelet-activating factor and platelet-derived growth factor in primary human lung cells. Am J Respir Cell Mol Biol. 1998;19(4):653–61.
Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307(1):97–101.
Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G, et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128(9):2038–49. doi:10.1002/ijc.25562.
Wang Y, Guo XQ, Niu XL, Wu J, Zhu YQ, Mao LQ. Relationship of IL-6 and IL-8 secretion in epithelial ovarian cancer cell lines with their sensitivity to tamoxifen as well as MAPK, Akt and estrogen receptor phosphorylation. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2010;26(1):21–4.
Wilson C, Wilson T, Johnston PG, Longley DB, Waugh DJ. Interleukin-8 signaling attenuates TRAIL- and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells. Mol Cancer Ther. 2008;7(9):2649–61. doi:10.1158/1535-7163.MCT-08-0148.
Miyake H, Yamanaka K, Muramaki M, Kurahashi T, Gleave M, Hara I. Enhanced expression of the secreted form of clusterin following neoadjuvant hormonal therapy as a prognostic predictor in patients undergoing radical prostatectomy for prostate cancer. Oncol Rep. 2005;14(5):1371–5.
Kurahashi T, Muramaki M, Yamanaka K, Hara I, Miyake H. Expression of the secreted form of clusterin protein in renal cell carcinoma as a predictor of disease extension. BJU Int. 2005;96(6):895–9. doi:10.1111/j.1464-410X.2005.05733.x.
Xie D, Lau SH, Sham JS, Wu QL, Fang Y, Liang LZ, et al. Up-regulated expression of cytoplasmic clusterin in human ovarian carcinoma. Cancer. 2005;103(2):277–83. doi:10.1002/cncr.20765.
Watari H, Ohta Y, Hassan MK, Xiong Y, Tanaka S, Sakuragi N. Clusterin expression predicts survival of invasive cervical cancer patients treated with radical hysterectomy and systematic lymphadenectomy. Gynecol Oncol. 2008;108(3):527–32. doi:10.1016/j.ygyno.2007.11.026.
Kang YK, Hong SW, Lee H, Kim WH. Overexpression of clusterin in human hepatocellular carcinoma. Hum Pathol. 2004;35(11):1340–6. doi:10.1016/j.humpath.2004.07.021.
Xie MJ, Motoo Y, Su SB, Mouri H, Ohtsubo K, Matsubara F, et al. Expression of clusterin in human pancreatic cancer. Pancreas. 2002;25(3):234–8.
Albert JM, Gonzalez A, Massion PP, Chen H, Olson SJ, Shyr Y, et al. Cytoplasmic clusterin expression is associated with longer survival in patients with resected non small cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1845–51. doi:10.1158/1055-9965.EPI-07-0146.
Kevans D, Foley J, Tenniswood M, Sheahan K, Hyland J, O’Donoghue D, et al. High clusterin expression correlates with a poor outcome in stage II colorectal cancers. Cancer Epidemiol Biomarkers Prev. 2009;18(2):393–9. doi:10.1158/1055-9965.EPI-08-0302.
Disclosures
The authors declare that they have no conflict of interest or financial support to disclose in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kevans, D., Gorman, S., Tosetto, M. et al. Clusterin and Chemotherapy Sensitivity Under Normoxic and Graded Hypoxic Conditions in Colorectal Cancer. J Gastrointest Canc 43, 305–313 (2012). https://doi.org/10.1007/s12029-011-9277-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-011-9277-x