Abstract
Purpose
Autotaxin (ATX) is molecularly identical to lysophospholipase D (lysoPLD) and is a main enzyme producing lysophosphatidic acid (LPA), which mediates a broad range of cellular responses including stimulation of cell motility.
Patients and Methods
Using immunohistochemical staining, we examined the expression of ATX/lysoPLD in 98 cases of early colorectal cancer with submucosal invasion. ATX/lysoPLD was highly expressed in infiltrating cells in tumor tissue in the submucosal layer, which were characterized as mast cells.
Results
The number of ATX/lysoPLD-positive cells was significantly greater in tumors with a macroscopically depressed lesion than in tumors without depression. The density of ATX/lysoPLD-positive cells tended to have a positive correlation with microvessel vascular density (MVD), while it was not correlated with vessel invasion and nodal metastases as well as lymphovascular vessel density (LVD).
Conclusion
Our results suggest that local production of LPA through ATX/lysoPLD may weakly correlate with formation of a depressive lesion and tumor angiogenesis in the early stage of colorectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The autocrine motility theory, that tumor cells with high malignant potential secrete a factor that promotes their own motility, and acquire high permeation and metastatic potential, was advocated by Liotta [1]. Such autocrine motility function has thus far been detected for insulin-like growth factor-II (IGF-II) [2], hepatocyte growth factor (HGF) [3], and autotaxin (ATX) [4]. ATX was originally isolated from a melanoma cell (A-2058) supernatant as a 125 kD glycoprotein that stimulates tumor cell motility in a pertussis toxin-sensitive manner by Stracke [5]. ATX is synthesized as a transmembrane propeptide with a short intracellular tail, and the extracellular peptide includes four potential N-linked glycosylation sites, a proteolytic cleavage site, two adjacent somatomedin B-like domains of vitronectin with a putative plasminogen activator inhibitor (PAI) binding site, a phosphodiesterase catalytic domain, arginine-glycine-aspartic acid (RGD) tripeptide motifs and a loop region of a calcium-binding EF-hand domain. The phosphodiesterase catalytic activity of ATX and dephosphorylation of Thr210 appear essential for the motility function of ATX [6]. Moreover, ATX is homologous to a family of exo/ecto nucleotide-pyrophosphatase phosphodiesterases (NPPs) that includes the B cell activation marker, PC-1, the neural differentiation antigen, B10 [7], the brain-type PDE I-nucleotide pyrophosphatase gene 2, PD-Iα [8], and the rat neural differentiation antigen, bp130RB13-6 [9]. Recombinant ATX (r-ATX) shows potent motility-stimulating activity in several tumor cell lines derived from human breast cancer (MBA-NB231) [10], prostate carcinoma (PC-3) [11] and neuroblastoma (SF295) [12]. Moreover, Nam et al. demonstrated that ATX modulates angiogenesis both directly and indirectly, suggesting that ATX could contribute to the metastatic cascade through multiple mechanisms [13]. These facts suggest that ATX may have critical roles in tumor invasion and metastasis.
Recently, molecular cloning of lysophospholipase D (lysoPLD) revealed that this enzyme was identical to ATX [14, 15]. LysoPLD is the main enzyme producing lysophosphatidic acid (LPA, 1- or 2-acyl-sn-glycero-3-phosphate) from lysophosphatidylcoline (LPC), which is abundant in plasma and serum [16, 17]. LPA, the simplest glycerophospholipid, mediates a broad range of cellular responses of smooth muscle contraction, platelet aggregation, neurite retraction/cell rounding, regulation of proliferation, protection from apoptosis, and modulation of chemotaxis and transcellular migration [18, 19]. These facts raise the possibility that the effect of ATX to stimulate tumor motility may be, at least in part, dependent on LPA [20].
ATX/lysoPLD is widely expressed in brain, placenta, intestine, and ovary [21, 22]. We have recently found that submucosal mast cells in the gastrointestinal tract highly express ATX/lysoPLD, which might play various roles in the pathophysiology of gastrointestinal diseases [23]. In the present study, we hypothesized that mast cells expressing ATX/lysoPLD may play positive roles in cancer invasion and metastases, and assessed the distribution of ATX/lysoPLD immunohistochemically using a specific monoclonal antibody. In this study, we selected early colon cancer tissues with submucosal invasion, since those tissues are considered to reflect the initial phase of cancer invasion.
Patients and Methods
Patients and Specimens
Between March 1990 and October 2006, a total of 98 patients with submucosal invasive colorectal cancer underwent surgical resection and systematic lymph node dissection at the Department of Surgical Oncology, The University of Tokyo, Tokyo, Japan. Patients who underwent local excision without lymph node dissection, and those with synchronous advanced colorectal cancer, ulcerative colitis or familial polyposis were excluded from this study. All patients had curative resection and none of them received adjuvant chemotherapy.
The resected specimens were immediately fixed in 10% buffered formalin and the entire cancerous lesion was cut into step-wise sections and embedded in paraffin. In order to confirm the existence of risk factors, the median number of serial sections cut from each cancerous lesion was 6 (range, 1-24). Conventional pathological diagnosis of the primary lesion and dissected lymph nodes was performed on hematoxylin and eosin (H.E.)-stained sections. ATX/lysoPLD expression in the submucosal layer was examined by immunohistochemical staining. Moreover, the risk factors for lymph node and distant metastasis were assessed in greater detail by the following methods.
Histopathological Characterization (Table 1)
Histological type and macroscopic appearance were determined according to World Health Organization classifications. Macroscopically, we defined the depressed lesion as a tumor showing either a purely depressed lesion or depression within elevated lesion. Moreover, the tumor with depression was classified into pure depressed type, which is defined that the depression is formed by a reduction in the thickness of the neoplastic mucosa, and ulcerated type by examining the specimens of the tumor microscopically. Depth of submucosal invasion was defined as the distance determined by microscopic observation of specimens using an optical micrometer (Olympus, Tokyo, Japan). The method for measurement of submucosal invasion depth was described by Kitajima et al. [24]. To examine risk factors such as lymphovascular invasion, microvascular density and tumor budding, five 3-μm-thick sections from each paraffin block were used in the present study. The first section was stained with H.E., and the next section was used for Victoria blue elastic fiber staining. This staining visualized elastic fibers in the venous wall, and was used to examine venous invasion. The third section was used for immunostaining for D2-40 to examine lymphatic invasion and lymphatic vessel density (LVD). The fourth section was used for immunostaining for CD34 to examine microvessel density (MVD). The last section was stained with anti-cytokeratin antibody CAM5.2 for histological confirmation of the presence of tumor budding.
Lymph Nodes
A total of 1,599 lymph nodes were dissected from the 98 patients. The mean number of lymph nodes dissected per patient was 16.3 (range, 1-60). Lymph nodes were routinely examined in one H.E section through the center of each lymph node. Based on a previous study that demonstrated that staining of a total thickness of 27 μm staining was sufficient to detect lymph node micrometastasis in colorectal cancer [25], we examined three 10-μm-thick sections from each paraffin block of lymph nodes by immunohistochemical technique in the present study. The immunohistochemical method using anticytokeratin antibody CAM5.2 is described below.
ATX/lysoPLD Immunohistochemical Staining and Assessment
Rat anti-ATX/lysoPLD monoclonal antibodies (2A12, 4F1) were generated by immunization of a rat with a polypeptide (amino acid 58-182 of human autotaxin) at the Graduate School of Pharmaceutical Sciences, The University of Tokyo, Tokyo, Japan [26]. The specificity and immunoreactivity of these mAbs in tissue sections were evaluated previously [27, 28]. The antibodies against ATX/lysoPLD were used at a dilution of 1:50. The method of immunohistochemical staining was described previously [23]. Because Mori et al. reported that a large number of positive cells were detected in the mesenchymal area in the submucosal layer, we counted ATX/lysoPLD-positive cells in five different fields of the submucosal layer selected at random, under a microscope (expressed as number per x200 field). The mean value of the five fields for each case was used as an index of ATX/lysoPLD-positive cells.
Assessment of Microvascular Vessel Density (MVD) and Lymph Vascular Vessel Density (LVD)
The anti-human lymphatic endothelial cell antibody D2-40 (Signet, Detham, MA, USA) was used at a dilution of 1:100, and the anti-human small-vessel endothelial cell antibody CD34 (BI-3C5, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used at a dilution of 1:200. MVD and LVD were assessed by counting microvessels immunostained for CD34 or D2-40 antigen under a light microscope based on the standard method for MVD assessment proposed by the First International Consensus [29, 30]. Initially, the CD34- or D2-40- stained sections were scanned at low power (x40), and the areas of specimens with the highest number of capillaries and small venules stained for CD34 or D2-40 (hot spots) were selected. Subsequently, microvessel counting was performed in three fields in the hot spots at ×200 (×20 objective lens and ×10 ocular lens) magnification. The mean value of the three fields for each case was used for further analysis.
Definition of Tumor Budding and Lymph Node Metastasis
To detect tumor budding and lymph node micrometastasis, the anti-cytokeratin antibody CAM5.2 (Becton Dickinson, San Jose, CA, USA) was used at a dilution of 1:3. The streptavidin-biotin immunoperoxidase method was performed as described previously [31]. Tumor budding was defined as microscopic microtubular cancer nests and undifferentiated cancer cells at the invasive margin of the cancerous lesion, as previously reported by Morodomi et al. [32]. Tumor budding is a cancer cell cluster that is immunopositive for CAM5.2 and is composed of not more than five cancer cells or undifferentiated cells. Sections that had 5 or more budding formations at the invasive front under light microscopy at ×200 magnification were defined as budding-positive sections.
Tumor deposits within lymph nodes were classified according to the revised guidelines set by the American Committee on Cancer (AJCC) [33]. Lymph nodes were classified as pN0 (pN0i-, pN0i+), pN1mi, pN1, pN2mi, or pN2 according to the current AJCC criteria for colorectal cancer. Tumor cells that measured no more than 0.2 mm detected by H.E. and/or immunohistochemical staining were defined as isolated tumor cells (ITCs) (pN0i+). Moreover, micrometastasis (pN1mi, pN2mi) was diagnosed when a tumor nodule in the lymph node was smaller than 2 mm in diameter [33]. Cases with lymph node metastasis or micrometastasis were classified as the node-positive group; cases with ITCs or without lymph node metastasis were classified as the node-negative group.
Statistical Analysis
All statistical calculations were carried out with JMP7 statistical software (JMP for Macintosh, version 7; SAS Institute, Tokyo, Japan). Chi-squared test and Student's t-test were used to analyze data. Simple regression analysis was used to analyze the correlation between the index of ATX/lysoPLD-positive cells and MVD and LVD. A P value <0.05 was considered to indicate statistical significance.
Results
Patient Data
As shown in Table 1, there were 67 men and 31 women with a median age of 63.6 (range, 40-83) years. All tumors were treated by primary resection and had a mean size of 23.3 (range, 6-95) mm. Three tumors were located in the cecum (3.1%), 13 in the ascending colon (13.3%), 14 in the transverse colon (14.3%), 2 in the descending colon (2.0%), 39 in the sigmoid colon (40.0%), and 27 in the rectum (27.6%). Macroscopic type was classed as pedunculated in 51 cases (52.0%), and sessile in 47 cases (48.0%). The number of cases with depression was 34 (34.7%). In 34 cases with depressed lesions, 32 cases were sessile group, while two cases were pedunculated group. The size of depressed lesions is 10.5 ± 4.8 (5-22) mm. Among 34 cases with depression, 7 (20.6%) cases were diagnosed pure depressed type, while 27 (79.4%) cases were ulcerated type, respectively. The number of cases with lymph node metastasis, micrometastasis, and ITCs was 13 (13.2%), 2 (2.0%), and 16 (16.3%), respectively. The histological type was classed as well differentiated adenocarcinoma in 84 cases (85.7%), moderately differentiated adenocarcinoma in 13 (13.3%) and poorly differentiated adenocarcinoma in one (1.0%). Lymphatic invasion and venous invasion were identified in 17 (17.3%) and 42 cases (42.9%), respectively. Among 17 cases with lymphatic involvement, eight were diagnosed by H.E. staining, while an additional nine that were not detected by conventional H.E. staining were detected by D2-40 immunostaining. Among 42 cases with venous involvement, 32 were diagnosed by H.E. staining and 10 by Victoria blue elastic fiber staining; the latter cases were not detected by H.E. Tumor budding was found in 55 cases (56.1%) using CAM5.2 staining. The mean objective submucosal invasive depth was 3,200 μm (range, 500-8,100).
Immunodetection of ATX/lysoPLD in Submucosal Layer
ATX/lysoPLD-positve cells could be clearly detected in the mesenchymal area of the tumor tissue with submucosal invasion, as reported by Mori et al. (Fig. 1). The mean index of ATX/lysoPLD-positive cells in each field was 4.9 (range, 1.2-19.6). We then examined the relationship between the density of ATX/lysoPLD-positive cells and clinicopathological features. As shown in Table 2, it did not show a significant relation with most features. Interestingly, however, tumors with macroscopic depression on colonoscopy showed significantly more ATX/lysoPLD-positve cells than those without depression (6.458 ± 4.087 vs 4.059 ± 2.219, P = 0.003). Tumors with ulceration (5.919 ± 3.508) showed significantly more ATX/lysoPLD-positve cells than those without depression (P = 0.015). Tumors with pure depression (8.543 ± 5.671) also tended to show more ATX/lysoPLD-positve cells than those without depression (P = 0.083), but not significantly.
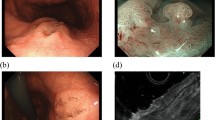
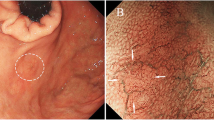
Submucosal sigmoid colon cancer, depressed type. a Colonoscopy showing the tumor with depression and redness. b Macroscopic appearance of depressed-type cancer resected surgically. c Immunohistochemical staining using a monoclonal antibody, ATX/lysoPLD, identified mast cells in the submucosal layer (original magnification, ×25). d Immunohistochemical staining using a monoclonal antibody, ATX/lysoPLD, showed 8.8 mast cells per field in the submucosal layer (original magnification, ×50)
Correlation Between ATX/lysoPLD Expression and MVD and LVD
Next, we assessed MVD and LVD in the submucosal layer and assessed their relation with the density of ATX/lysoPLD-positve cells. Mean MVD and LVD were 47.7 (range, 10.7-179.0) and 15.6 (range, 4.3-35.3), respectively. As shown in Fig. 2, the density of ATX/lysoPLD-positive cells tended to have a positive correlation with MVD, although the p-value did not reach statistical significance (MVD = 40.3 + 1.5xATX, p = 0.0655, respectively), while it showed no correlation with LVD.
Discussion
LPA is involved in the etiology of a variety of diseases such as atherosclerosis, obesity, and cancer as well as in physiological responses such as wound healing, vascular tone, vascular integrity, and reproduction [34]. Since LPA is usually rapidly degraded in the body, the production of LPA in local areas is considered to be physiologically important. ATX/lysoPLD is a key enzyme in the production of LPA through the catalysis of LPC, which is abundant in plasma and tissue fluids [14, 15]. Therefore, the detection of ATX/lysoPLD may be extremely important to realize the role of LPA in physiological and pathological conditions.
In our previous study, we found that submucosal mast cells in the gastrointestinal tract highly expressed ATX/lysoPLD, suggesting those mast cells may play important roles in the pathogenesis of gastrointestinal diseases. It has been shown that mast cells regulate immune responses in local areas through the production of various inflammatory cytokines and mediators such as IL-1, IL-4, IL-5, IL-6, TNF-α, prostaglandins, leukotrienes, and platelet activating factor [35]. Moreover, mast cells have been shown to produce various angiogenic and vascular permeability factors as well as many proteases, which are known to be critically involved in tumor invasion and metastasis [36, 37].
These findings raise the hypothesis that the density of ATX/lysoPLD-positive mast cells may be closely related to the characteristics of colorectal cancer, especially tumor budding, vessel invasion and nodal metastasis, and we thus examined the correlation with clinicopathological features in submucosal colorectal cancer. In our results, however, the density did not show any significant correlation with budding, venous or lymphatic invasion as well as nodal metastasis. These results might be caused by the fact that the number of patients was too low to find significant associations. Instead, it did correlate with macroscopic findings. In fact, tumors with macroscopic depression, especially tumors of ulcerated type, contained significantly more ATX/lysoPLD-positive mast cells than those without depression. This finding raises the possibility that local production of LPA may be important for the formation of a depressed lesion in a tumor. Another possibility is that the presence of a depressed lesion may result in the recruitment of many ATX/lysoPLD-positive mast cells in tumor tissue. In fact, an increase in intestinal mast cells was observed in ulcerative lesions associated with inflammatory conditions in both in rats [38] and humans [39].
Another finding of our study was that the density of ATX/lysoPLD-positive mast cells showed a possible correlation with MVD in tumor tissue. This is consistent with the recent finding that LPA can promote angiogenesis [40–42]. In fact, ATX has been shown to be essential for the development of blood vessels in mouse embryogenesis [43]. These findings suggest the possibility that pharmacological inhibition of ATX/lysoPLD may be potentially effective to suppress tumor development or metastasis through interference with LPA signaling.
A recent study by Lin et al. has shown that LPA enhanced VEGF-C production and expression of a lymphatic vessel marker in human endothelial cells both in vitro and in vivo, and suggested that LPA may also have a functional property to promote lymphoangiogenesis [44]. In our immunostaining results, however, LVD, in contrast to MVD, showed no correlation with the density of ATX/lysoPLD-positive mast cells, suggesting that LPA does not play a major role in lymphatic vessel formation. Since LPA elicits most of its cellular responses via a redundant receptor system [45, 46], the discrepancy may be explained by different receptor usage, or that the presence of other major lymphangiogenic factors in human cancer tissue may mask the effect of LPA.
In summary, ATX/lysoPLD-positive cells can be detected in early colorectal cancer tissue, and their density is correlated with the formation of a macroscopically depressed lesion in tumors. Although the density of ATX/lysoPLD-positive cells is not an indicator of tumor aggressiveness, it might show a positive association with microvascular density, supporting a functional correlation between LPA-derived signaling and tumor angiogenesis.
References
Liotta LA, Guirguis RA, Schiffmann E. Tumor autocrine motility factor. Prog Clin Biol Res. 1986;212:17–24.
Stracke ML, Engel JD, Wilson LW, Rechler MM, Liotta LA, Schiffmann E. The type I insulin-like growth factor receptor is a motility receptor in human melanoma cells. J Biol Chem. 1989;264(36):21544–9.
Matsumoto K, Nakamura T, Kramer RH. Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. J Biol Chem. 1994;269(50):31807–13.
Stracke ML, Clair T, Liotta LA. Autotaxin, tumor motility-stimulating exophosphodiesterase. Adv Enzyme Regul. 1997;37:135–44.
Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, et al. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267(4):2524–9.
Lee HY, Clair T, Mulvaney PT, Woodhouse EC, Aznavoorian S, Liotta LA, et al. Stimulation of tumor cell motility linked to phosphodiesterase catalytic site of autotaxin. J Biol Chem. 1996;271(40):24408–12.
Stefan C, Gijsbers R, Stalmans W, Bollen M. Differential regulation of the expression of nucleotide pyrophosphatases/phosphodiesterases in rat liver. Biochim Biophys Acta. 1999;1450(1):45–52.
Kawagoe H, Soma O, Goji J, Nishimura N, Narita M, Inazawa J, et al. Molecular cloning and chromosomal assignment of the human brain-type phosphodiesterase I/nucleotide pyrophosphatase gene (PDNP2). Genomics. 1995;30(2):380–4.
Deissler H, Lottspeich F, Rajewsky MF. Affinity purification and cDNA cloning of rat neural differentiation and tumor cell surface antigen gp130RB13-6 reveals relationship to human and murine PC-1. J Biol Chem. 1995;270(17):9849–55.
MacDonald NJ, Freije JM, Stracke ML, Manrow RE, Steeg PS. Site-directed mutagenesis of nm23-H1. Mutation of proline 96 or serine 120 abrogates its motility inhibitory activity upon transfection into human breast carcinoma cells. J Biol Chem. 1996;271(41):25107–16.
Mulvaney PT, Stracke ML, Nam SW, Woodhouse E, O'Keefe M, Clair T, et al. Cyclocreatine inhibits stimulated motility in tumor cells possessing creatine kinase. Int J Cancer. 1998;78(1):46–52.
Kawagoe H, Stracke ML, Nakamura H, Sano K. Expression and transcriptional regulation of the PD-Ialpha/autotaxin gene in neuroblastoma. Cancer Res. 1997;57(12):2516–21.
Nam SW, Clair T, Kim YS, McMarlin A, Schiffmann E, Liotta LA, et al. Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Res. 2001;61(18):6938–44.
Tokumura A, Tominaga K, Yasuda K, Kanzaki H, Kogure K, Fukuzawa K. Lack of significant differences in the corrected activity of lysophospholipase D, producer of phospholipid mediator lysophosphatidic acid, in incubated serum from women with and without ovarian tumors. Cancer. 2002;94(1):141–51.
Umezu-Goto M, Tanyi J, Lahad J, Liu S, Yu S, Lapushin R, et al. Lysophosphatidic acid production and action: validated targets in cancer? J Cell Biochem. 2004;92(6):1115–40.
Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, et al. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem. 2002;277(50):48737–44.
Tokumura A, Fujimoto H, Yoshimoto O, Nishioka Y, Miyake M, Fukuzawa K. Production of lysophosphatidic acid by lysophospholipase D in incubated plasma of spontaneously hypertensive rats and Wistar Kyoto rats. Life Sci. 1999;65(3):245–53.
Goetzl EJ, An S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J. 1998;12(15):1589–98.
Yuelling LM, Fuss B. Autotaxin (ATX): a multi-functional and multi-modular protein possessing enzymatic lysoPLD activity and matricellular properties. Biochim Biophys Acta. 2008;1781(9):525–30.
Gaetano CG, Samadi N, Tomsig JL, Macdonald TL, Lynch KR, Brindley DN. Inhibition of autotaxin production or activity blocks lysophosphatidylcholine-induced migration of human breast cancer and melanoma cells. Mol Carcinog. 2009;48(9):801–9.
Narita M, Goji J, Nakamura H, Sano K. Molecular cloning, expression, and localization of a brain-specific phosphodiesterase I/nucleotide pyrophosphatase (PD-I alpha) from rat brain. J Biol Chem. 1994;269(45):28235–42.
Lee HY, Murata J, Clair T, Polymeropoulos MH, Torres R, Manrow RE, et al. Cloning, chromosomal localization, and tissue expression of autotaxin from human teratocarcinoma cells. Biochem Biophys Res Commun. 1996;218(3):714–9.
Mori K, Kitayama J, Aoki J, Kishi Y, Shida D, Yamashita H, et al. Submucosal connective tissue-type mast cells contribute to the production of lysophosphatidic acid (LPA) in the gastrointestinal tract through the secretion of autotaxin (ATX)/lysophospholipase D (lysoPLD). Virchows Arch. 2007;451(1):47–56.
Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39(6):534–43.
Sasaki M, Watanabe H, Jass JR, Ajioka Y, Kobayashi M, Hatakeyama K. Immunoperoxidase staining for cytokeratins 8 and 18 is very sensitive for detection of occult node metastasis of colorectal cancer: a comparison with genetic analysis of K-ras. Histopathology. 1998;32(3):199–208.
Tanaka M, Kishi Y, Takanezawa Y, Kakehi Y, Aoki J, Arai H. Prostatic acid phosphatase degrades lysophosphatidic acid in seminal plasma. FEBS Lett. 2004;571(1–3):197–204.
Baumforth KR, Flavell JR, Reynolds GM, Davies G, Pettit TR, Wei W, et al. Induction of autotaxin by the Epstein-Barr virus promotes the growth and survival of Hodgkin lymphoma cells. Blood. 2005;106(6):2138–46.
Savaskan NE, Rocha L, Kotter MR, Baer A, Lubec G, van Meeteren LA, et al. Autotaxin (NPP-2) in the brain: cell type-specific expression and regulation during development and after neurotrauma. Cell Mol Life Sci. 2007;64(2):230–43.
Gao J, Knutsen A, Arbman G, Carstensen J, Franlund B, Sun XF. Clinical and biological significance of angiogenesis and lymphangiogenesis in colorectal cancer. Dig Liver Dis. 2009;41(2):116–22.
Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, et al. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94(12):1823–32.
Kazama S, Watanabe T, Ajioka Y, Kanazawa T, Nagawa H. Tumour budding at the deepest invasive margin correlates with lymph node metastasis in submucosal colorectal cancer detected by anticytokeratin antibody CAM5.2. Br J Cancer. 2006;94(2):293–8.
Morodomi T, Isomoto H, Shirouzu K, Kakegawa K, Irie K, Morimatsu M. An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer. 1989;63(3):539–43.
Hermanek P, Hutter RV, Sobin LH, Wittekind C, International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. Cancer. 1999;86(12):2668–73.
Sengupta S, Wang Z, Tipps R, Xu Y. Biology of LPA in health and disease. Semin Cell Dev Biol. 2004;15(5):503–12.
Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6(2):135–42.
Hiromatsu Y, Toda S. Mast cells and angiogenesis. Microsc Res Tech. 2003;60(1):64–9.
Noël A, Jost M, Maquoi E. Matrix metalloproteinases at cancer tumor-host interface. Semin Cell Dev Biol. 2008;19(1):52–60.
Morris GP, Beck P, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96(3):795–803.
Fox CC, Lichtenstein L, Roche JK. Intestinal mast cell responses in idiopathic inflammatory bowel disease. Histamine release from human intestinal mast cells in response to gut epithelial proteins. Dig Dis Sci. 1993;38(6):1105–12.
Sun B, Nishihira J, Suzuki M, Fukushima N, Ishibashi T, Kondo M, et al. Induction of macrophage migration inhibitory factor by lysophosphatidic acid: relevance to tumor growth and angiogenesis. Int J Mol Med. 2003;12(4):633–41.
Lee J, Park S, Lee EK, Park CG, Chung HC, Rha SY, et al. Activation of hypoxia-inducible factor-1alpha is necessary for lysophosphatidic acid-induced vascular endothelial growth factor expression. Clin Cancer Res. 2006;12(21):6351–8.
Rivera-Lopez CM, Tucker A, Lynch KR. Lysophosphatidic acid (LPA) and angiogenesis. Angiogenesis. 2008;11(3):301–10.
van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradère JP, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26(13):5015–22.
Lin CI, Chen C, Huang MT, Lee SJ, Lin CH, Chang CC, et al. Lysophosphatidic acid up-regulates vascular endothelial growth factor-C and lymphatic marker expressions in human endothelial cells. Cell Mol Life Sci. 2008;65(17):2740–51.
An S, Bleu T, Hallmark OG, Goetzl EJ. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem. 1998;273(14):7906–10.
Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci USA. 2000;97(24):13384–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kazama, S., Kitayama, J., Aoki, J. et al. Immunohistochemical Detection of Autotaxin (ATX)/Lysophospholipase D (lysoPLD) in Submucosal Invasive Colorectal Cancer. J Gastrointest Canc 42, 204–211 (2011). https://doi.org/10.1007/s12029-010-9186-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-010-9186-4