Abstract
Lysophosphatidic acid (LPA) is involved in a broad spectrum of biological activities, including wound healing and cancer metastasis. Autotaxin (ATX), originally isolated from a melanoma supernatant as a tumor cell motility-stimulating factor, has been shown to be molecularly identical to lysophospholipase D (lysoPLD), which is the main enzyme in the production of LPA. Although ATX/lysoPLD is known to be widely expressed in normal human tissues, the exact distribution of ATX-producing cells has not been fully investigated. In this study, we evaluated ATX/lysoPLD expression by immunohistochemical staining using a rat anti-ATX mAb in the human gastrointestinal tract and found that submucosal mast cells (MC) highly expressed this enzyme. This was confirmed by immunofluorescent double staining using mAbs to tryptase and chymase. Then, we isolated MC from human gastric tissue by an immunomagnetic method using CD117-microbeads and showed that a subpopulation of CD203c-positive MC showed positive staining for intracellular ATX/lysoPLD on flowcytometry. This was confirmed by Western blotting of the isolated cells. Moreover, a significant level of ATX/lysoPLD release could be detected in the culture supernatants of human MC by Western blot analysis. Our data suggest that submucosal MC play significant roles in various aspects of pathophysiology in the gastrointestinal tract by locally providing bioactive LPA through the production of ATX/lysoPLD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autotaxin (ATX) was originally isolated from a melanoma cell supernatant as a 125-kD glycoprotein that stimulates tumor cell motility in a pertussis toxin-sensitive manner [55, 57]. Thereafter, more evidence for a positive role of ATX in tumor metastasis has been demonstrated by both in vivo and in vitro studies [36–38, 56, 70]. ATX is strongly detected in the culture media of various cancer cells, and its mRNA is overexpressed in various human malignancies, such as lung, breast, and renal cell carcinoma [53, 70, 71]. These findings support that ATX functions as an autocrine motility factor in the step of tumor progression. ATX was initially identified as a member of the ecto-nucleotide pyrophosphatase/phosphodiesterase (NPP) family and was termed NPP2 [56]. The NPP family is composed of three ecto-enzymes that hydrolyze phosphodiester and pyrophosphate bonds in nucleotides [9, 18]; however, its molecular structure relating to stimulation of cell motility is an enigma. On the other hand, lysophospholipase D (lysoPLD), which converts lysophosphatidylcoline (LPC) to lysophosphatidic acid (LPA, 1- or 2-acyl-sn-glycero-3-phosphate), was found to be abundant in plasma and serum [3, 62]. LPA is a lipid mediator of a broad range of cellular responses, including smooth muscle contraction, platelet aggregation, neurite retraction/cell rounding, regulation of proliferation, protection from apoptosis, and modulation of chemotaxis and transcellular migration [19, 35]. LPA elicits most of its cellular responses via transduction cascades downstream of its specific G protein-coupled receptors, LPA1/Edg-2, LPA2/Edg-4, and LPA3/Ede-7, which belong to the endothelial cell differentiation gene (Edg) family [2, 4, 11, 24]. Some of these cellular responses implicate LPA as a mediator of tumor progression [31, 34, 50–52]. Recently, molecular cloning of lysoPLD revealed that this enzyme was identical to ATX [63, 66]. As LPC is abundantly present in plasma and tissues (exceeding 100 μM), ATX/LysoPLD is considered to be a key enzyme in the production of LPA in vivo [3, 12, 61]. Therefore, it is generally accepted that the effects of ATX/LysoPLD in cancer progression (including proliferation, migration, and angiogenesis) are mostly attributable to the production of LPA from LPC or more complex lysophospholipids [66, 67]. Although the mechanism of catalysis of ATX/lysoPLD is well characterized, its posttranslational processing, regulation of expression, and mechanism of release from cells are not well understood. ATX is widely expressed, with highest mRNA levels detected in brain, placenta, intestine, and ovary [36]. However, the cellular expression of ATX/lysoPLD in each tissue remains unclear. Herein, by immunohistochemical study using a specific monoclonal antibody, we assessed the cellular distribution of ATX/LysoPLD in the human gastrointestinal tract, and found that submucosal mast cells (MC) constitutively express ATX/lysoPLD.
Materials and methods
Reagents and human samples
Rat anti-ATX/lysoPLD monoclonal antibodies (2A12, 4F1) were generated by the immunization of rat with a polypeptide (amino acids 58–182 of human autotaxin) at the Graduate School of Pharmaceutical Sciences, The University of Tokyo, Tokyo, Japan. The specificity [60] and immunoreactivity for tissue section of these mAbs were evaluated previously [5, 46]. Antihuman tryptase mAb (AA1) and antihuman chymase mAb (CC1) were purchased from Dako (Carpinteria, CA) and Serotic (Oxford, UK), respectively, and both of their subclasses were IgG1.
Surgical specimens were obtained from seven patients with primary gastric carcinoma treated by total gastrectomy and seven patients with primary colon cancer treated by right hemicolectomy in the University of Tokyo, with written informed consent. Normal parts of the stomach or colon in those specimens, more than 5 cm from the tumor edge, were used for staining experiments. The ileum of the oral margin of right hemicolectomy specimens and the jejunal margin used for reconstruction after gastrectomy was used for evaluation of the small intestine.
Immunohistochemical study
Paraffin-embedded sections, 5 μm thick, were deparaffinized in xylene, hydrated through a graded series of ethanol, then immersed in 3% hydrogen peroxide in 100% methanol for 30 min to inhibit endogenous peroxidase activity. To activate the antigens, the sections were boiled in 10 mM citrate buffer pH 6.0 for 15 min. After being rinsed in phosphate-buffered saline (PBS), the sections were incubated with normal rabbit serum for 30 min and incubated overnight at 4°C in humid chambers with primary antibodies to tryptase (AA1, dilution 1:100), chymase (CC1, dilution 1:100), or ATX/lysoPLD (2A12, dilution 1:50). After washing with PBS, the sections were incubated with biotinylated rabbit anti-mouse immunoglobulin (Nichirei, Tokyo, Japan) for 20 min. After washing again with PBS, the slides were treated with peroxidase-conjugated streptavidin for 10 min and developed by immersion in 0.01% H2O2 and 0.05% diaminobenzidine tetrahydrochloride. Light counterstaining with Mayer’s hematoxylin was performed. All cases had a negative control that was run simultaneously with the test slide, in which control rat IgG was used as the primary antibody. Tryptase-, chymase-, and ATX/LysoPLD-positive cells were counted in five different fields selected at random, using a microscope (and expressed as number per ×200 field).
Immunofluorescent double staining
To identify the two molecules in the same specimen, immunofluorescent double staining was performed on frozen sections. The surgically removed specimen was immediately fixed with acetone for 10 min and immersed in 3% hydrogen peroxide in 100% methanol for 30 min. Then, sections were incubated with normal rabbit serum for 30 min and incubated overnight at 4°C in shaded humid chambers with primary antibodies to tryptase or chymase. After washing with PBS, sections were incubated for 2 h with rhodamine-conjugated rabbit anti-mouse IgG (Chemicon International, Temecula, CA). Then, sections were incubated overnight with rat anti-ATX antibody chemically conjugated with fluorescein isothiocyanate (FITC) (FITC-conjugated 4F1, dilution 1:10). The sections were observed with a confocal laser microscope (Fluoview, Olympus, Tokyo, Japan).
Buffer for cell preparation
Tris–ethylenediaminetetraacetic acid (EDTA) (TE) buffer is Eagle’s minimum essential medium (MEM, Gibco, Berlin, Germany) containing 2 mM EDTA. TGMD buffer is TE buffer supplemented with 1 mg/ml gelatin, 1.23 mM MgCl2, and 15 μg/ml DNAse. Hepes buffer contains 20 mM Hepes, 125 mM NaCl, 5 mM KCl, and 0.5 mM glucose. HA buffer is Hepes buffer plus 0.25 mg/ml bovine serum albumin (BSA).
Cell preparation
The normal parts of the gastric wall were resected, and tissues containing the mucosal and submucosal layers were separated from the muscularis propria layer using scissors and used for cell separation. MC were isolated by a four-step enzymatic tissue dispersion method as described previously with some modifications [7, 8, 49]. The tissues were cut into fragments with scissors and incubated in TE buffer containing 1 mg/ml acetylcysteine for 10 min at room temperature to remove mucus and then in TE buffer containing 5 mM EDTA for 15 min at 37°C to detach epithelial cells. After washing in TE buffer, the tissue was incubated in TE buffer containing 3 mg/ml pronase and 0.75 mg/ml chymopapain at room temperature. Pronase and chymopapain were purchased from Roche (Mannheim, Germany). During this first digestion step, the tissue was cut into smaller pieces using scalpels. After 30 min, the free cells (fraction 1) were separated from tissue fragments by filtration through a polyamide Nybolt filter (pore size 300 μm). The remaining tissue fragments were washed in TE buffer, and the first digestion step was repeated at 37°C (fraction 2). The tissue fragments were then washed in TGMD buffer and incubated twice for 30 min at 37°C in TGMD buffer containing 1.5 mg/ml collagenase D (Roche, Mannheim, Germany) and 0.15 mg/ml elastase (Roche, Mannheim, Germany). The freed cells were separated from the digested tissue by filtration (fraction 3 and 4). Fraction 3 and 4 were filtered through a Nyblot filter (pore size 100 μm) again and resuspended in HA buffer. Cell viability was measured using trypan blue staining, and MC counts were performed using Alcian blue at each step.
Mast cell purification
Dispersed cells containing approximately 1% MC were purified as described previously with some modifications [7, 39, 49]. In brief, the dispersed cells were incubated with anti-CD117 mAb-conjugated microbeads (Miltenyi Biotec,Bergisch Gladbach, Germany) for 15 min at 4°C. Then, CD117-positive cells were enriched by passing through a magnetic column twice at 1 ml/min using the “POSSEL DS” program of the AutoMACS system which enables the fast and efficient positive selection of rare population with low immunogenic epitope (Miltenyi Biotec, Bergisch Gladbach, Germany). “POSSEL DS” means “double sensitive positive selection”.
The purified MC were dispersed on slides by centrifugation for 10 min (×500 rpm) and fixed with acetone. Then, the slide samples were incubated with biotinylated anti-tryptase (AA1) or anti-chymase (CC1) mAbs followed by incubation with peroxidase-conjugated streptavidin and development by diaminobenzidine tetrahydrochloride as described above. Under the microscope, 100 cells were randomly selected in five to seven different fields, and cells positive for tryptase or chymase in those cell populations were calculated (per ×200 field).
Detection of ATX/lysoPLD by flow cytometry
Dispersed cells obtained after the four enzymatic steps were washed twice with PBS and fixed with acetone for 10 min at 4°C. Then they were permeabilized with 4% paraformaldehyde (PFA) for 10 min at 4°C. For two-color staining, cells were incubated with FITC-conjugated anti-ATX mAb and PE-conjugated anti-CD203c antibody (Immunotech, Marseille, France). Data were collected in a fluorescent activated cell sorting (FACS) Calibur (Becton Dickinson, Mountain View, CA) and analyzed using CellQuest software (Becton Dickinson).
Detection of ATX/lysoPLD by Western blot analysis
Immunoselected MC (2 × 106), which contained more than 70% Alcian blue-stained cells, were cultured in 8 ml Roswell Park Memorial Institute (RPMI) supplemented with 0.1% BSA, 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies, Inc., Grand Island, NY). As a control, the same numbers of negatively selected cells were also cultured in the same medium. After culture for 48 h, the proteins of the culture supernatants were extracted as described previously [66]. Then, 8 ml culture supernatant was concentrated to 100 μl by a Centricon Plus-20 (Millipore, Bedford, MA), and electrophoresed in sodium dodecyl sulfate (sodium dodecyl sulfate (SDS)) 7.5% polyacrylamide gel for 45 min at 200 V. Then, the protein was transferred onto an Immobilon transfer membrane (Millipore, Bedford, MA) for sequential incubation with 5% reconstituted nonfat milk powder to block nonspecific sites, dilutions of anti-ATX antibody, and horseradish peroxidase-labeled rabbit polyclonal anti-rat IgG, before development with a standard enhanced chemiluminenscence kit (Amersham, Inc., Buckinghamshire, United Kingdom).
Results
Immunostaining of MC in the gastrointestinal tract
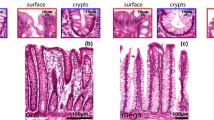
MC were clearly identified in the stomach, small intestine, and colon by immunostaining with anti-tryptase antibody (AA1), as reported previously [27, 69]. Granules in the MC cytoplasm were stained brown. In the stomach, tryptase-positive MC were diffusely present from the mucosal to the muscular layer, but the number of MC in the submucosal layer was significantly greater than that in the mucosal or muscular layer (Fig. 1b). MC were also diffusely present in the small intestinal and colonic tissues (Fig. 1d).
Immunohistochemical staining of the stomach (a, b, e, f) or terminal ileum (c, d) with anti-ATX/lysoPLD mAb (2A12) (a, c, e) or anti-tryptase mAb (AA1) (b, d, f). Serial sections of submucosal area of the stomach with high magnification (e, f). Bar indicates 1,000 μm (a, b), 500 μm (c, d), and 50 μm (e, f). Immunoreactivity for ATX/lysoPLD was clearly detected in a subpopulation of tryptase positive cells
Immunodetection of ATX/lysoPLD in the gastrointestinal tract
As shown in Fig. 1, there were many large oval cells whose cytoplasm was clearly stained by anti-ATX/lysoPLD antibody (2A12). The positive cells were detected in the mesenchymal area in the submucosal layer of the gastrointestinal tract, while they were rarely observed in the mesenchymal area of the mucosal or muscular layers (Table 1). Interestingly, in serial sections, most of the ATX-positive cells were morphologically similar to MC detected by anti-tryptase antibody (Fig. 1e,f).
ATX/lysoPLD-positive cells were identified as a subtype of MC
To confirm that ATX/lysoPLD-positive cells are identical to MC, we performed immunofluorescent double staining in surgically removed gastric tissue. As shown in Fig. 2, when tryptase-positive MC were stained red and ATX/LysoPLD-positive MC green, ATX/LysoPLD-positive cells were detected with yellow fluorescence in merged views (Fig. 2c). On the other hand, some MC were detected as red in merged views, suggesting that some MC did not express ATX/LysoPLD (Fig. 2f). No cells were stained green in these frozen sections. This clearly indicates that the cells expressing ATX/LysoPLD in merged views are confined to a subpopulation of MC in the gastrointestinal tract.
Immunofluorescent double staining with anti-tryptase (AA1), anti-chymase (CC1), and anti-ATX/lysoPLD (4F1) mAbs. A frozen section of normal gastric tissue was stained as described in the Materials and Methods section. The section was observed with a confocal laser microscope (Fluoview, Olympus, Tokyo, Japan). The bar indicates 10 μm (a–f). Double staining for tryptase in red (a, d) and ATX/lysoPLD (b, e) in green. In the merged view, a double-positive cell is stained yellow, indicating that the cell expresses ATX/lysoPLD and tryptase (white arrows in c, f). In contrast, a cell stained red in the merged view expresses tryptase but not ATX/lysoPLD (white arrow head in f). No cells are stained green in the merged view, indicating that ATX/lysoPLD-producing cells are restricted to a subtype of MC. (g–i) Double staining for chymase in red (g) and ATX/lysoPLD (h) in green. In the merged view, most of the cells are stained yellow [open arrow in (i)], indicating that most chymase-positive MC coexpress ATX/lysoPLD
Then, we performed double immunofluorescent staining with anti-chymase antibody (CC1). Most of the ATX/lysoPLD-positive MC were stained yellow in the merged view (Fig. 2i). When the number of ATX/lysoPLD-positive MC in tryptase- or chymase-positive MC was counted in 20 fields selected at random, 92% (82–96%) of chymase-positive cells expressed ATX/lysoPLD, whereas 68% (61–86%) of tryptase-positive cells expressed ATX/LysoPLD (Table 2). A previous study on human intestine showed that tryptase-positive MC exist in both the mucosal and submucosal layers, while most chymase-positive MC exist in the submucosal layer [27]. This appears to be consistent with our result that most of the MC in submucosal, but not mucosal layers, highly expressed ATX/lysoPLD.
Proportion of ATX/lysoPLD-positive MC in the gastrointestinal tracts
The proportion of ATX/lysoPLD-positive cells in tryptase-positive MC was comparatively evaluated in the stomach, intestine, and colon. As shown in Table 1, more than 50% of MC in the submucosal layer expressed ATX/lysoPLD in all organs examined (stomach: 52%, small intestine: 63%, colon: 56%). In contrast, only a small proportion of MC in the mucosal or muscular layer expressed ATX/lysoPLD (stomach: 7.1%, 8%, small intestine: 6.7%, 8%, colon: 14%, 4.1%).
Detection of intracellular ATX/lysoPLD in MC isolated from gut tissue
Enzymatic isolation provided at least 2 × 108 single cells containing about 1% MC, and their viability was at least 90%. After positive selection using magnetic affinity purification with anti-CD117, the majority of the cells were stained blue by Alcian blue (Fig. 3a) and anti-tryptase mAb (Fig. 3b). In contrast, cells stained with anti-chymase mAb were fewer than tryptase-positive cells (Fig. 3b). Then, to detect intracellular expression of ATX/lysoPLD, we performed double staining of the dispersed cells with anti-ATX/lysoPLD and anti-CD203c, which was determined as a specific cell surface antigen of human MC and basophils [17]. FACS analysis showed that more than 70% of the cell population positively expressed CD203c (Fig. 3c). When the CD203c-positive population was gated, 22% (13–27%) of CD-203c positive cells were clearly positive for intracellular ATX/lysoPLD, whereas the CD203c-negative population lacked expression of ATX/lysoPLD (Fig. 3d).
Characterization of MC isolated from gastrointestinal tissue. The cells recovered after enzymatic digestion of gastrointestinal mucosal tissue were positively selected using anti-CD117 mAb. a A representative MC stained by Alcian blue (b); Immunochemical staining with anti-tryptase (upper) and anti-chymase (lower). The majority of cells are positive for tryptase, but not for chymase. b Flowcytometric profile of double staining by CD203c and ATX/lysoPLD. The MC-enriched fraction was fixed, permeabilized, and double stained with PE-conjugated anti-CD203c mAb and FITC-conjugated anti-ATX/lysoPLD mAb (c). The CD203c-negative (left) and -positive (right) areas were gated, and the FITC level was examined in each gated fraction. The red line represents negative control. The figure shows the representative data from four different experiments (Table 4)
Expression of tryptase or chymase in cell population isolated from gut tissue
As shown in Fig. 3b, 82% of the cells were positive for tryptase, while only 17% MC were positive for chymase (Table 3). This result is consistent with Gebhardt’s report, showing that about 20% of purified MC expressed both tryptase and chymase [16]. From these results, it is supposed that the cell isolates from gut tissue by this method contain relatively more mucosal MC than submucosal MC.
Detection of the release of ATX/lysoPLD from MC by Western blot analysis
Finally, we examined the protein expression of ATX/lysoPLD and tryptase and chymase in MC-rich population. Positively selected cells expressed ATX/lysoPLD, whereas negatively selected cells did not (Fig. 4a). As shown in Fig. 4a, tryptase and chymase were also detectable in cell lysates of MC-rich population.
a Western blotting of lysates of MC-enriched cell population from gastrointestinal tract for ATX/lysoPLD, tryptase, and chymase. b Detection of release of ATX/lysoPLD from MC-enriched population. MC-enriched cells (2 × 106) were cultured. Then, the supernatant was concentrated and the protein expression of ATX/lysoPLD was evaluated by Western blotting. For negative control, negatively selected cells with anti-CD117 mAb were cultured in the same conditions, and the culture supernatant was assessed for the presence of ATX/lysoPLD. The figure shows representative data from three different experiments
Then, we evaluated whether MC extracellularly secrete ATX/lysoPLD. As shown in Fig. 4b, ATX/lysoPLD protein was detected in the concentrated culture supernatant of MC-enriched population cells isolated from gastric tissue, but not in the supernatant of negatively selected cell populations. This finding indicates that a subpopulation of the human MC in gastric tissue release soluble ATX/lysoPLD into the culture medium.
Discussion
LPA is involved in the etiology of a variety of diseases such as atherosclerosis, obesity, and cancer and in physiological responses such as wound healing, vascular tone, vascular integrity, and reproduction [34, 48]. However, the molecular mechanism of the production of this bioactive phospholipid is poorly understood, although a large amount of LPA is known to be produced by activated platelets or stimulated fibroblasts in pathological conditions. Recently, it has been postulated that at least two pathways exist for LPA production, intracellular synthesis from phosphatidic acid and extracellular conversion from other lysophospholipids. In the latter pathway, ATX/lysoPLD is shown to be a key enzyme in the production of LPA by the catalysis of LPC, which is known to be abundantly present in plasma and tissue fluids [3, 64, 66]. In this study, we found a subtype of MC in the human gastrointestinal tract that showed strong staining for ATX/lysoPLD. This reports characterized the cellular producer of ATX/lysoPLD in normal tissue, while a recent study has demonstrated that ATX/LysoPLD is overexpressed in Epstein–Barr virus-infected Hodgkin’s lymphoma cells [5]. That study used the same antibody as us and showed similar cytosolic staining of ATX/LysoPLD in lymphoma cells, suggesting that ATX/lysoPLD is stored in the cytosol in contrast to the other NPP family proteins, which are predominantly distributed in the plasma membrane [54]. By Western blotting of culture supernatants, we confirmed the secretion of ATX/lysoPLD from the MC-enriched fraction. These data clearly indicate that those MC undergo membrane-proximal cleavage to yield soluble ATX/lysoPLD, and thus, have important physiological roles through the local supply of a high concentration of LPA in gastrointestinal mucosal tissue.
Tissue MC are known to derive from specific bone marrow progenitor cells and migrate into tissues, where they mature depending on the microenvironment and acquire the capability to produce a wide range of mediators including histamine, heparin, prostaglandins, leukotrienes, and many inflammatory cytokines such as interleukin-8, vascular endothelial cell growth factor (VEGF), and platelet-derived growth factor (PDGF), and exert their biological effects by releasing such mediators [6, 15, 21, 26, 30]. In rodents, MC are phenotypically divided into two subtypes, mucosal-type MC (MMC) and connective tissue-type MC (CTMC), that produce distinct types of mediators. MC in the human gastrointestinal tract are also subdivided by immunohistochemical detection of two proteases; CTMC that contain tryptase and chymase, and MMC containing tryptase but not chymase [1, 27–29]. Those studies have shown that MMC are preferentially located in the mucosal surface, while CTMC are detected mainly in submucosal tissue in the gut. Our immunostaining results revealed that MC in the submucosal layer highly expressed ATX/lysoPLD, but MC located in the mucosal and muscular layers did not. Moreover, double staining experiments showed that most of the chymase-positive MC also expressed ATX/lysoPLD, while about half of the tryptase-positive cells lacked the expression of ATX/lysoPLD. This is consistent with previous findings and indicates that ATX/lysoPLD is constitutively produced mainly by submucosal CTMC in the gastrointestinal tract.
Using flowcytometry, we confirmed that intracellular staining of ATX/lysoPLD was detected by CD203c-positive MC isolated from gut tissue. In FACS analysis, however, only 10–20% CD203c-positive MC are positive for ATX /lysoPLD, which does not agree with the immunostaining data indicating that most of the submucosal MC are positive for ATX /lysoPLD (Table 4). In fact, cells separated by this immunomagnetic method contained many tryptase-positive MC but fewer chymase-positive MC. This suggests that submucosal MC are collected less efficiently than mucosal MC by this isolation process, which may contribute to the discrepancy between FACS analysis and immunohistochemistry.
The expression of ATX/lysoPLD in submucosal MC is considered to have physiological relevance. MC are essential for allergic and late phase reactions and T-cell-mediated immunity [14, 33, 42]. LPA has been reported to lead to overexpression of adhesion molecules and chemokines in endothelial cells, and thus, induces the recruitment of mononuclear phagocytic cells [40, 45]. For helper T cells, LPA has been shown to induce chemotactic migration of naïve CD4(+) T cells expressing the specific receptor Edg4 (LPA2), while enhancing proliferation and IL-2 production in activated CD4(+) T cells expressing Edg2 (LPA1) predominantly, but rather, inhibit the migration of these activated CD4(+) T cells [20, 73]. This strongly suggests that the production of ATX/lysoPLD in submucosal MC has an important role in preparation of CD4(+) helper T cells’ repertoire to respond to a variety of antigens in gastrointestinal mucosal tissue. Once T cells are stimulated with certain antigens, ATX/lysoPLD can augment T cell-mediated responses in mucosal tissue through the production of LPA.
On the other hand, LPA is considered to have suppressive effects on neutrophil-induced inflammation in vivo. LPA reduces IL-8-induced migration of human neutrophils in patients with pneumonia and inhibits the production of oxygen species in human neutrophils in response to stimulation with PMA [10, 43]. In the gastrointestinal tract, LPA reduces the degree of colonic inflammation induced by ethanol and trinitrobenzene sulfonic acid in a rat colitis model in vivo [59]. These findings suggest the possibility that ATX/lysoPLD released from submucosal MC plays a protective role against acute phase inflammatory bowel disease (IBD) [23]. Because the number of MC is markedly increased in IBD, it would be intriguing to examine the production of ATX/lysoPLD in MC in the intestinal mucosa in patients with IBD. In addition, recent studies have demonstrated that LPA also activates enteric glia cells, which are considered to play critical immunoregulatory roles in gut tissue [47, 68]. Taken together, these findings suggest that MC-derived ATX/lysoPLD has pivotal effects on mucosal immunity in the gastrointestinal tract.
LPA is also essential in wound healing. When superficial mucosal injury occurs, the mucosal defect is rapidly closed through a process termed epithelial restitution in physiological conditions [25, 58]. LPA is considered to play major roles in this step, as it strongly promotes epithelial cell migration and proliferation in vitro [41]. In fact, rectally applied LPA stimulates wound healing of the intestinal epithelium and reduces the size of ulcers in the rat [58]. Therefore, the production of ATX/lysoPLD in gastrointestinal CTMC is considered to be necessary for the maintenance of mucosal barrier function, as they can constitutively provide a considerable amount of LPA in the submucosal area, and thus, epithelial cells can rapidly respond to repair the mucosal injury.
Finally, ATX/lysoPLD has an essential role in the progression of malignant diseases in the gastrointestinal tract. Many studies have provided evidence for positive roles of LPA in the initiation or progression of malignancy, including melanoma, ovary, prostate, breast, head and neck, colon, and stomach cancer [13, 22, 72]. This is reasonable, because LPA can function as a growth factor for cancer cells and also stimulates angiogenesis [34]. More than 40 years ago, it has been reported that a large number of MC accumulate around solid tumors and a large number of tumor-associated MC were significantly correlated with high vascular density and poor outcome [32, 44, 65]. In those studies, MC-derived secretalogues, such as histamine, heparin, or VEGF are considered to be responsible for the new vessel formation. However, our results strongly support additional mechanisms of MC to promote the malignant potential of cancer through the production of ATX/lysoPLD.
In summary, we discovered that a subpopulation of submucosal CTMC constitutively secrete ATX/lysoPLD. MC are considered to play important roles in the pathophysiology of gastrointestinal diseases, such as allergic enteritis, inflammatory bowel disease and malignancy. The effects of MC may be partly attributable to ATX/lysoPLD by providing LPA in gastrointestinal mucosal tissue, although circulating ATX/lysoPLD synthesized in different locales may also modulate LPA levels. As ATX/lysoPLD is a relatively stable protein compared to the bioactive phospholipid product, further research on this enzyme may be useful for the evaluation of functional role.
Abbreviations
- ATX:
-
autotaxin
- lysoPLD:
-
lysophospholipase D
- LPA:
-
lysophosphatidic acid
- MC:
-
mast cell
- NPP:
-
ecto-nucleotide pyrophosphatase / phosphodiesterase
References
Aldenborg F, Enerback L (1994) The immunohistochemical demonstration of chymase and tryptase in human intestinal mast cells. Histochem J 267:587–596
An S, Bleu T, Hallmark OG, Goetzl EJ (1998) Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem 27314:7906–7910
Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, Mizuno K, Saku K, Taguchi R, Arai H (2002) Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem 27750:48737–48744
Bandoh K, Aoki J, Hosono H, Kobayashi S, Kobayashi T, Murakami-Murofushi K, Tsujimoto M, Arai H, Inoue K (1999) Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem 27439:27776–27785
Baumforth KR, Flavell JR, Reynolds GM, Davies G, Pettitt TR, Wei W, Morgan S, Stankovic T, Kishi Y, Arai H, Nowakova M, Pratt G, Aoki J, Wakelam MJ, Young LS, Murray PG (2005) Induction of autotaxin by the Epstein–Barr virus promotes the growth and survival of Hodgkin’s lymphoma cells. Blood 106(6):2138–2146
Bischoff SC (1996) Mucosal allergy: role of mast cells and eosinophil granulocytes in the gut. Baillieres Clin Gastroenterol 103:443–459
Bischoff SC, Lorentz A, Schwengberg S, Weier G, Raab R, Manns MP (1999) Mast cells are an important cellular source of tumour necrosis factor alpha in human intestinal tissue. Gut 445:643–652
Bischoff SC, Schwengberg S, Wordelmann K, Weimann A, Raab R, Manns MP (1996) Effect of c-kit ligand, stem cell factor, on mediator release by human intestinal mast cells isolated from patients with inflammatory bowel disease and controls. Gut 381:104–114
Bollen M, Gijsbers R, Ceulemans H, Stalmans W, Stefan C (2000) Nucleotide pyrophosphatases/phosphodiesterases on the move. Crit Rev Biochem Mol Biol 356:393–432
Chettibi S, Lawrence AJ, Stevenson RD, Young JD (1994) Effect of lysophosphatidic acid on motility, polarisation and metabolic burst of human neutrophils. FEMS Immunol Med Microbiol 83:271–281
Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J (2000) Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci USA 9724:13384–13389
Croset M, Brossard N, Polette A, Lagarde M (2000) Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem J 345(Pt 1):61–67
Fang X, Yu S, Bast RC, Liu S, Xu HJ, Hu SX, LaPushin R, Claret FX, Aggarwal BB, Lu Y, Mills GB (2004) Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J Biol Chem 27910:9653–9661
Galli SJ (2000) Mast cells and basophils. Curr Opin Hematol 71:32–39
Galli SJ (1993) New concepts about the mast cell. N Engl J Med 3284:257–265
Gebhardt T, Lorentz A, Detmer F, Trautwein C, Bektas H, Manns MP, Bischoff SC (2005) Growth, phenotype, and function of human intestinal mast cells are tightly regulated by transforming growth factor beta1. Gut 547:928–934
Ghannadan M, Hauswirth AW, Schernthaner GH, Muller MR, Klepetko W, Schatzl G, Sperr WR, Buhring HJ, Valent P (2002) Detection of novel CD antigens on the surface of human mast cells and basophils. Int Arch Allergy Immunol 1274:299–307
Goding JW, Grobben B, Slegers H (2003) Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim Biophys Acta 16381:1–19
Goetzl EJ, An S (1998) Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J 1215:1589–1598
Goetzl EJ, Kong Y, Voice JK (2000) Cutting edge: differential constitutive expression of functional receptors for lysophosphatidic acid by human blood lymphocytes. J Immunol 16410:4996–4999
Goldstein SM, Kaempfer CE, Kealey JT, Wintroub BU (1989) Human mast cell carboxypeptidase. Purification and characterization. J Clin Invest 835:1630–1636
Gschwind A, Hart S, Fischer OM, Ullrich A (2003) TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. EMBO J 2210:2411–2421
He SH (2004) Key role of mast cells and their major secretory products in inflammatory bowel disease. World J Gastroenterol 103:309–318
Hecht JH, Weiner JA, Post SR, Chun J (1996) Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol 1354:1071–1083
Hines OJ, Ryder N, Chu J, McFadden D (2000) Lysophosphatidic acid stimulates intestinal restitution via cytoskeletal activation and remodeling. J Surg Res 921:23–28
Holgate ST (2000) The role of mast cells and basophils in inflammation. Clin Exp Allergy 30(Suppl 1):28–32
Irani AM, Bradford TR, Kepley CL, Schechter NM, Schwartz LB (1989) Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem 3710:1509–1515
Irani AM, Craig SS, DeBlois G, Elson CO, Schechter NM, Schwartz LB (1987) Deficiency of the tryptase-positive, chymase-negative mast cell type in gastrointestinal mucosa of patients with defective T lymphocyte function. J Immunol 13812:4381–4386
Irani AM, Goldstein SM, Wintroub BU, Bradford T, Schwartz LB (1991) Human mast cell carboxypeptidase. Selective localization to MCTC cells. J Immunol 1471:247–253
Johnson RG, Carty SE, Fingerhood BJ, Scarpa A (1980) The internal pH of mast cell granules. FEBS Lett 1201:75–79
Kitayama J, Shida D, Sako A, Ishikawa M, Hama K, Aoki J, Arai H, Nagawa H (2004) Over-expression of lysophosphatidic acid receptor-2 in human invasive ductal carcinoma. Breast Cancer Res 66:R640–R646
Kondo K, Muramatsu M, Okamoto Y, Jin D, Takai S, Tanigawa N, Miyazaki M (2006) Expression of chymase-positive cells in gastric cancer and its correlation with the angiogenesis. J Surg Oncol 931:36–42; discussion 42–33
Mekori YA, Metcalfe DD (2000) Mast cells in innate immunity. Immunol Rev 173:131–140
Mills GB, Moolenaar WH (2003) The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer 38:582–591
Moolenaar WH (1995) Lysophosphatidic acid, a multifunctional phospholipid messenger. J Biol Chem 27022:12949–12952
Murata J, Lee HY, Clair T, Krutzsch HC, Arestad AA, Sobel ME, Liotta LA, Stracke ML (1994) cDNA cloning of the human tumor motility-stimulating protein, autotaxin, reveals a homology with phosphodiesterases. J Biol Chem 26948:30479–30484
Nam SW, Clair T, Campo CK, Lee HY, Liotta LA, Stracke ML (2000) Autotaxin (ATX), a potent tumor motogen, augments invasive and metastatic potential of ras-transformed cells. Oncogene 192:241–247
Nam SW, Clair T, Kim YS, McMarlin A, Schiffmann E, Liotta LA, Stracke ML (2001) Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Res 6118:6938–6944
Okayama Y, Hunt TC, Kassel O, Ashman LK, Church MK (1994) Assessment of the anti-c-kit monoclonal antibody YB5.B8 in affinity magnetic enrichment of human lung mast cells. J Immunol Methods 1692:153–161
Palmetshofer A, Robson SC, Nehls V (1999) Lysophosphatidic acid activates nuclear factor kappa B and induces proinflammatory gene expression in endothelial cells. Thromb Haemost 825:1532–1537
Panetti TS, Nowlen J, Mosher DF (2000) Sphingosine-1-phosphate and lysophosphatidic acid stimulate endothelial cell migration. Arterioscler Thromb Vasc Biol 204:1013–1019
Pedotti R, De Voss JJ, Steinman L, Galli SJ (2003) Involvement of both ‘allergic’ and ‘autoimmune’ mechanisms in EAE, MS and other autoimmune diseases. Trends Immunol 249:479–484
Rahaman M, Costello RW, Belmonte KE, Gendy SS, Walsh MT (2006) Neutrophil sphingosine 1-phosphate and lysophosphatidic acid receptors in pneumonia. Am J Respir Cell Mol Biol 342:233–241
Ribatti D, Ennas MG, Vacca A, Ferreli F, Nico B, Orru S, Sirigu P (2003) Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur J Clin Invest 335:420–425
Rizza C, Leitinger N, Yue J, Fischer DJ, Wang DA, Shih PT, Lee H, Tigyi G, Berliner JA (1999) Lysophosphatidic acid as a regulator of endothelial/leukocyte interaction. Lab Invest 7910:1227–1235
Savaskan NE, Rocha L, Kotter MR, Baer A, Lubec G, van Meeteren LA, Kishi Y, Aoki J, Moolenaar WH, Nitsch R, Brauer AU (2007) Autotaxin (NPP-2) in the brain: cell type-specific expression and regulation during development and after neurotrauma. Cell Mol Life Sci 642:230–243
Segura BJ, Zhang W, Cowles RA, Xiao L, Lin TR, Logsdon C, Mulholland MW (2004) Lysophosphatidic acid stimulates calcium transients in enteric glia. Neuroscience 1233:687–693
Sengupta S, Wang Z, Tipps R, Xu Y (2004) Biology of LPA in health and disease. Semin Cell Dev Biol 155:503–512
Shah PM, Husby S, Damsgaard TE, Nielsen HV, Schiotz PO (1998) Purification of human colonic and gastric mast cells. J Immunol Methods 214:141–148
Shida D, Kitayama J, Yamaguchi H, Hama K, Aoki J, Arai H, Yamashita H, Mori K, Sako A, Konishi T, Watanabe T, Sakai T, Suzuki R, Ohta H, Takuwa Y, Nagawa H (2004) Dual mode regulation of migration by lysophosphatidic acid in human gastric cancer cells. Exp Cell Res 3012:168–178
Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, Watanabe T, Takuwa Y, Nagawa H (2003) Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res 637:1706–1711
Shida D, Watanabe T, Aoki J, Hama K, Kitayama J, Sonoda H, Kishi Y, Yamaguchi H, Sasaki S, Sako A, Konishi T, Arai H, Nagawa H (2004) Aberrant expression of lysophosphatidic acid (LPA) receptors in human colorectal cancer. Lab Invest 8410:1352–1362
Stassar MJ, Devitt G, Brosius M, Rinnab L, Prang J, Schradin T, Simon J, Petersen S, Kopp-Schneider A, Zoller M (2001) Identification of human renal cell carcinoma associated genes by suppression subtractive hybridization. Br J Cancer 859:1372–1382
Stefan C, Jansen S, Bollen M (2005) NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci 3010:542–550
Stracke M, Liotta LA, Schiffmann E (1993) The role of autotaxin and other motility stimulating factors in the regulation of tumor cell motility. Symp Soc Exp Biol 47:197–214
Stracke ML, Clair T, Liotta LA (1997) Autotaxin, tumor motility-stimulating exophosphodiesterase. Adv Enzyme Regul 37:135–144
Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, Liotta LA (1992) Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem 2674:2524–2529
Sturm A, Sudermann T, Schulte KM, Goebell H, Dignass AU (1999) Modulation of intestinal epithelial wound healing in vitro and in vivo by lysophosphatidic acid. Gastroenterology 1172:368–377
Sturm A, Zeeh J, Sudermann T, Rath H, Gerken G, Dignass AU (2002) Lisofylline and lysophospholipids ameliorate experimental colitis in rats. Digestion 661:23–29
Tanaka M, Kishi Y, Takanezawa Y, Kakehi Y, Aoki J, Arai H (2004) Prostatic acid phosphatase degrades lysophosphatidic acid in seminal plasma. FEBS Lett 571:197–204
Thies F, Delachambre MC, Bentejac M, Lagarde M, Lecerf J (1992) Unsaturated fatty acids esterified in 2-acyl-l-lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. J Neurochem 593:1110–1116
Tokumura A, Fujimoto H, Yoshimoto O, Nishioka Y, Miyake M, Fukuzawa K (1999) Production of lysophosphatidic acid by lysophospholipase D in incubated plasma of spontaneously hypertensive rats and Wistar Kyoto rats. Life Sci 653:245–253
Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K (2002) Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem 27742:39436–39442
Tokumura A, Tominaga K, Yasuda K, Kanzaki H, Kogure K, Fukuzawa K (2002) Lack of significant differences in the corrected activity of lysophospholipase D, producer of phospholipid mediator lysophosphatidic acid, in incubated serum from women with and without ovarian tumors. Cancer 941:141–151
Tomita M, Matsuzaki Y, Edagawa M, Shimizu T, Hara M, Sekiya R, Onitsuka T (2001) Association of mast cells with tumor angiogenesis in esophageal squamous cell carcinoma. Dis Esophagus 142:135–138
Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H (2002) Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol 1582:227–233
Umezu-Goto M, Tanyi J, Lahad J, Liu S, Yu S, Lapushin R, Hasegawa Y, Lu Y, Trost R, Bevers T, Jonasch E, Aldape K, Liu J, James RD, Ferguson CG, Xu Y, Prestwich GD, Mills GB (2004) Lysophosphatidic acid production and action: validated targets in cancer? J Cell Biochem 926:1115–1140
von Boyen GB, Steinkamp M, Reinshagen M, Schafer KH, Adler G, Kirsch J (2004) Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 532:222–228
Walls AF, Jones DB, Williams JH, Church MK, Holgate ST (1990) Immunohistochemical identification of mast cells in formaldehyde-fixed tissue using monoclonal antibodies specific for tryptase. J Pathol 1622:119–126
Yang SY, Lee J, Park CG, Kim S, Hong S, Chung HC, Min SK, Han JW, Lee HW, Lee HY (2002) Expression of autotaxin (NPP-2) is closely linked to invasiveness of breast cancer cells. Clin Exp Metastasis 197:603–608
Yang Y, Mou L, Liu N, Tsao MS (1999) Autotaxin expression in non-small-cell lung cancer. Am J Respir Cell Mol Biol 212:216–222
Zhang G, Zhao Z, Xu S, Ni L, Wang X (1999) Expression of autotaxin mRNA in human hepatocellular carcinoma. Chin Med J (Engl) 1124:330–332
Zheng Y, Voice JK, Kong Y, Goetzl EJ (2000) Altered expression and functional profile of lysophosphatidic acid receptors in mitogen-activated human blood T lymphocytes. FASEB J 1415:2387–2389
Acknowledgements
This work was supported partly by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and partly by a grant from the Ministry of Health, Labor and Welfare of Japan. We thank Dr. M. Uchikawa for his kind help to conjugate FITC to anti-ATX mAb, and Ms. C. Uchikawa and Ms. K. Amitani for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mori, K., Kitayama, J., Aoki, J. et al. Submucosal connective tissue-type mast cells contribute to the production of lysophosphatidic acid (LPA) in the gastrointestinal tract through the secretion of autotaxin (ATX)/lysophospholipase D (lysoPLD). Virchows Arch 451, 47–56 (2007). https://doi.org/10.1007/s00428-007-0425-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-007-0425-4