Abstract
Background
Both seizures and spreading depolarizations (SDs) are commonly detected using electrocorticography (ECoG) after severe traumatic brain injury (TBI). A close relationship between seizures and SDs has been described, but the implications of detecting either or both remain unclear. We sought to characterize the relationship between these two phenomena and their clinical significance.
Methods
We performed a post hoc analysis of a prospective observational clinical study of patients with severe TBI requiring neurosurgery at five academic neurotrauma centers. A subdural electrode array was placed intraoperatively and ECoG was recorded during intensive care. SDs, seizures, and high-frequency background characteristics were quantified offline using published standards and terminology. The primary outcome was the Glasgow Outcome Scale-Extended score at 6 months post injury.
Results
There were 138 patients with valid ECoG recordings; the mean age was 47 ± 19 years, and 104 (75%) were men. Overall, 2,219 ECoG-detected seizures occurred in 38 of 138 (28%) patients in a bimodal pattern, with peak incidences at 1.7–1.8 days and 3.8–4.0 days post injury. Seizures detected on scalp electroencephalography (EEG) were diagnosed by standard clinical care in only 18 of 138 (13%). Of 15 patients with ECoG-detected seizures and contemporaneous scalp EEG, seven (47%) had no definite scalp EEG correlate. ECoG-detected seizures were significantly associated with the severity and number of SDs, which occurred in 83 of 138 (60%) of patients. Temporal interactions were observed in 17 of 24 (70.8%) patients with both ECoG-detected seizures and SDs. After controlling for known prognostic covariates and the presence of SDs, seizures detected on either ECoG or scalp EEG did not have an independent association with 6-month functional outcome but portended worse outcome among those with clustered or isoelectric SDs.
Conclusions
In patients with severe TBI requiring neurosurgery, seizures were half as common as SDs. Seizures would have gone undetected without ECoG monitoring in 20% of patients. Although seizures alone did not influence 6-month functional outcomes in this cohort, they were independently associated with electrographic worsening and a lack of motor improvement following surgery. Temporal interactions between ECoG-detected seizures and SDs were common and held prognostic implications. Together, seizures and SDs may occur along a dynamic continuum of factors critical to the development of secondary brain injury. ECoG provides information integral to the clinical management of patients with TBI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe traumatic brain injury (TBI) results in 56,000 deaths in the United States each year [1], and an estimated 30,000 survivors require intensive care [2]. A major focus of neurocritical care is the prevention, detection, and treatment of secondary brain injuries [3], which are defined as pathological changes that evolve after a primary injury. Widespread adoption of continuous scalp electroencephalography (EEG) in the intensive care unit setting has led to the recognition that seizures occur in up to 33% of patients with TBI and are often nonconvulsive [4]. More recently, recordings from electrodes placed on or within the brain tissue (electrocorticography [ECoG]) have documented an even higher seizure incidence of up to 61% of patients with severe TBI, suggesting that many seizures are quite focal and evade detection by continuous EEG [5, 6]. Simultaneously, ECoG studies have shown that spreading depolarizations (SDs) also occur frequently within the human cortex after injury. SDs are defined by a massive wave of near-complete depolarization and near-complete breakdown of the transmembrane ion gradients of neural tissue associated with neuronal influx of water and swelling [7]. In electrically active tissue, SDs typically induce spreading depression of high-frequency activity [7, 8]. These slow propagating waves are difficult to detect using scalp EEG [9, 10], but ECoG studies have documented SDs in as many as 60% of patients with severe TBI requiring surgery [11].
Seizures and SDs are distinct pathophysiologic events. Yet from their first description, SDs were recognized as related, alternate, or interacting manifestations of hyperexcitable and injured tissue [12]. Translational studies and small cohort studies have subsequently demonstrated the occurrence of seizures that precede the appearance of SDs, “spreading convulsions,” which emerge from the end of SDs when high-frequency activity remains depressed, and the development of the paroxysmal depolarization shifts in association with recovery from SDs [13,14,15]. Others have highlighted that seizures cause SDs in some cases [16,17,18,19,20], and a short-term intrinsic antiseizure function of SDs has been postulated [21]. However, the longer-term effect may be the opposite, as suggested by prior work in which acute and subacute SDs after cortical injury were associated with a higher risk of developing chronic epilepsy, possibly mediated by SD-induced blood–brain barrier dysfunction [14].
Seizures and SDs have been implicated in the development of secondary brain injuries. Both may create or worsen critical metabolic disturbances within the cortex and play a role in mediating excitotoxicity [6, 22, 23]. Seizures have been linked to hippocampal atrophy [24] and posttraumatic epilepsy [25], whereas SDs exhibit a causal link with infarct development [26] and lesion expansion and have been associated with worse functional outcome following TBI [11, 27]. Clinically, the implications of detecting either pattern or both remain somewhat less clear, and optimal methods and potential benefits of treating SDs have not been investigated [28]. To better understand the interaction between seizures and SDs, we performed a post hoc analysis of a prospective observational clinical study [11] of patients who required surgical management of severe TBI and were monitored with ECoG strips during intensive care. We used standardized definitions and terminology to characterize both the DC signal and the high-frequency activity of the ECoG.
Methods
Patients
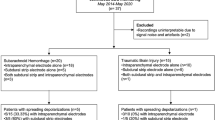
We performed a post hoc analysis of a prospective observational study (ClinicalTrials.gov identifier NCT00803036) performed at five academic medical centers as reported previously [11]. Adult patients with severe TBI who required a neurosurgical procedure for clinical purposes within 1 week of injury were eligible. Patients with nonsurvivable injuries were excluded; polytrauma, penetrating head injuries, and other preexisting disorders were not excluded. Surrogate consent was obtained in all cases. Approval was provided by the ethics boards at each participating institution, and research was conducted in accordance with the Declaration of Helsinki.
Procedures
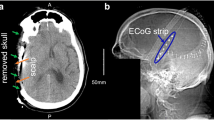
As previously described [11], a single six-contact linear electrode array was placed on the cerebral cortex under direct visualization during surgery. All patients underwent postoperative care in an intensive care unit, and treatment teams were blinded to ECoG recordings. Clinical management, including the use of additional neuromonitoring devices, was performed according to local practice. Electrode strips were removed at the bedside at the conclusion of clinical neuromonitoring or after a maximum of 1 week.
Data Collection
Clinical data were collected, including demographics, markers of injury severity, and radiographic studies. Preoperative head imaging was evaluated by a central neuroradiologist blinded to ECoG data. Neurologic outcome was obtained by telephone or in person, as available, using the Glasgow Outcome Scale-Extended (GOS-E) by an assessor blinded to clinical and ECoG data. Reports from routine and continuous scalp EEG performed during the study period were collected from the medical record. Scalp EEG was recorded with clinically standard equipment, and electrodes were applied according to the International 10–20 System. All interpretations were provided by local board-certified electroencephalographers, as part of clinical care, who were blinded to ECoG data.
ECoG
All ECoG recordings were scored by study investigators (JAH, HL, BF) offline using LabChart (ADInstruments, Oxford, UK). For each patient, low-frequency activity (< 0.1 Hz) to identify SDs was reviewed alongside the conventional high-frequency activity (0.5–50 Hz) displayed in five sequential bipolar channels derived from the six-electrode ECoG strip. SDs were identified and measured as described previously [11] per consensus recommendations [29]. Depolarizations were classified as occurring in clusters if three or more occurred within a 120-min period and as isoelectric if at least one channel depolarized while already electrically silent. The number of depolarizations and the duration for each associated spreading depression of high-frequency activity were measured.
Each recording was further divided in 4-h epochs for further analysis of high-frequency ECoG patterns by a board-certified clinical neurophysiologist (BF) and neurologist (HL), both certified in the use of the American Clinical Neurophysiology Society standardized terminology [30]. The background activity of each epoch was classified on the basis of visual inspection into one of four categories: (1) continuous activity ≥ 10 µV in amplitude; (2) discontinuous activity, characterized by periods of attenuation or suppression making up 10–49% of the 4-h epoch; (3) burst suppression, consisting of periods of suppression for > 50% of the epoch; or (4) suppressed activity, in which the entire background exhibited only low-amplitude (< 10 µV) activity. Worsening background activity was defined as increasing discontinuity relative to the initial background activity recorded on ECoG.
Ictal–interictal patterns were classified on the basis of their prevalence and average duration within each epoch. The frequency of periodic or rhythmic patterns was stratified as < 1 Hz, 1–2 Hz, or 2–3 Hz. For ictal–interictal patterns, the burden (in hours) of each pattern was quantified by multiplying the prevalence and the duration of each pattern during each epoch. The pattern index was measured by multiplying the burden by the frequency of the pattern (Hz-hour) as previously defined [30, 31].
Seizures were defined in ECoG recordings as any pattern consisting of either (1) spike- or sharp-wave discharges > 2.5 Hz lasting for more than 10 s or (2) patterns with evolution in frequency, spatial involvement, or morphology. Status epilepticus was defined as a seizure burden of > 60 min over the course of the recording, continuous status epilepticus was defined as any seizure lasting > 20 min, and cyclic status epilepticus was defined as 20 or more seizures (or 12 or more on average per day for shorter recordings) with a regular pattern. Arterial pulse artifact has been described during SD [14] and is characterized by a spindle-shaped, pseudo-evolving pattern that is often superimposed on the peak of SDs. This pattern was excluded using the arterial blood pressure waveform or electrocardiogram as needed. The seizure burden was quantified directly as the duration (in minutes) of seizure activity. Quantitative parameters for SDs and seizures were normalized by the duration of the ECoG recording.
Statistical Methods
Univariate analysis was performed using χ2 and Wilcoxon rank-sum tests as appropriate and expressed as proportions or median and interquartile range (IQR). Data visualization was conducted using ggplot [32] and ggjoy in R version 4.0.3 [33]. Subsequently, an ordinal regression model was developed using the MASS package [34] to characterize associations between seizures, SDs, and relevant clinical outcomes. Covariates were identified a priori on the basis of the core International Mission for Prognosis and Analysis of Clinical Trials in TBI model [35] as age, motor Glasgow Coma Scale (GCS) score, and pupillary reactivity after resuscitation from initial injury. Model summaries were expressed as proportional odds alongside 95th percentile confidence intervals (CIs). For all comparisons, the threshold for significance was a p value of < 0.05.
Results
Seizures During ECoG After Surgical TBI
Of the 157 patients enrolled, we included 138 for analysis, as reported previously [11]. Table 1 contains patient demographics and injury characteristics. ECoG began a median of 12.8 h (8.5–23.8 h) from injury and continued for a median of 75.5 h (42.2–117.1 h). In total, 2,219 ECoG-detected seizures occurred in 38 of 138 (27.5%) patients (Fig. 1a). Of these, 23 of 38 (61%) patients had ECoG-detected seizures that met criteria for electrographic status epilepticus. Nearly half of patients had ECoG-detected seizures arising within only a restricted region of the ECoG strip (47.4%); other patients had more than one independent seizure focus or experienced seizures broadly across the ECoG strip. Most ECoG-detected seizures occurred within the first 72 h of injury (31 of 38 [82%]). The overall temporal profile of ECoG-detected seizures in this cohort showed a clear bimodal pattern with peak seizure occurrence at 1.7–1.8 days (40–44 h) and 3.8–4.0 days (92–96 h) following injury (Fig. 1b). This pattern was similar to, but more pronounced than, the bimodal pattern seen across 2,837 SDs that occurred in 83 of 138 (60.1%) patients. Notably, the first peak occurrence of SDs occurred earlier and was more sustained between 0.5 and 1.5 days (12 and 36 h) post injury compared with the emergence of ECoG-detected seizures beginning at a median of 39.5 h (34.1–64.4 h).
a Raster plot of electrocorticography data from each individual patient who had seizures during the study recording (n = 38) is shown across the post-injury period, shown in days (x-axis). Thin gray horizontal bars show periods of valid recording. Vertical hash marks indicate the timing of event: spreading depolarizations are in blue, isoelectric depolarizations in red, and seizures in green. The right margin of the Figure shows the 6-month Glasgow Outcome Scale Score-Extended for each patient. b The total number of depolarizations (gray bars) and seizures (pink bars) across each 4-h was divided by the number of patients monitored with ECoG (gray outline) during the corresponding time period in order to calculate adjusted rates of each event over time. All patients (n = 138) were included
Seizures During ECoG vs. Scalp EEG
Continuous scalp EEG was recorded for clinical purposes in 52 of 138 (37.7%) patients over a median of 3 days (IQR 1–7 days); routine scalp EEG was recorded in a further 8 of 138 (5.8%). Scalp EEG-detected seizures were diagnosed as part of standard clinical care in 18 of 138 (13.0%); in most cases [14 of 18 (77.8%)], there was no reported clinical correlate. Of 15 patients with ECoG-detected seizures and contemporaneous scalp EEG, seven (46.7%) had no definite scalp correlate (Fig. 2). Scalp EEG by contrast detected seizures in one patient contralateral to ECoG strip recording and in one patient with three brief focal scalp EEG-detected seizures arising from the occipital region remote from the location of the ECoG strip. In five patients, scalp EEG-detected seizures consisted of rhythmic or periodic patterns with spatial evolution; ECoG demonstrated concordant periodic patterns without definite evolution (n = 3) and/or brief potentially ictal rhythmic discharges (n = 2) in these cases. Overall, ECoG diagnosed seizures in 28 of 138 (20.3%) patients in whom seizures would have gone undetected with standard clinical practice alone.
Comparison of seizure detection using ECoG versus scalp EEG. Flow chart demonstrating the detection of seizures by ECoG, scalp EEG, or both. N values refer to individual patients whereas counts in parentheses reflect the occurrence of seizures detected by each modality at different points during the study period; a single patient could be represented within more than one box. Highlighted in the green box are seizures that were concordant on both ECoG and scalp EEG. In contrast, events in the yellow box were interpreted as seizures on scalp EEG but not by ECoG and events in the red boxes reflect seizures detected by ECoG but missed by scalp EEG
Clinical Predictors of Seizures
Both ECoG- and scalp EEG-detected seizures were associated with older age but not with clinical markers of injury severity. Patients with either ECoG- or scalp EEG-detected seizures (n = 46) were more likely to have experienced intracerebral hemorrhage (12 of 44 [27.3%] vs. 10 of 89 [11.2%] patients without seizures; p = 0.04), although this association did not retain significance when we considered only those with ECoG-detected seizures (10 of 37 [27.0%] vs. 12 of 96 [12.5%] without seizures; p = 0.08). ECoG- and scalp EEG-detected seizures were not associated with a past medical history of epilepsy (2 of 46 [4.4%] vs. 11 of 92 [12.0%] without seizures; p = 0.23) or with the presence of prehospital clinical seizures (7 of 46 [15.2%] vs. 11 of 92 [12.0%] without seizures; p = 0.85). All 46 patients with ECoG- or scalp EEG-detected seizures and 89 of 92 (96.7%) of those without seizures received seizure prophylaxis with antiepileptic medications.
Association Between ECoG-Detected Seizures and SDs
Multiple lines of evidence demonstrated significant co-occurrence and interaction between ECoG-detected seizures and SDs. As shown in Table 2, ECoG-detected seizures were more likely to occur in patients with clustered or isoelectric SDs (22 of 51 [43.1] vs. 16 of 87 [18.4%] of those with sporadic or no SDs; p = 0.002). Similarly, patients with ECoG-detected seizures had a higher number of depolarizations per day (median 8.3 [0–20.5] vs. 0.7 [0–4.3] in those without seizures; p = 0.01; Fig. 3a) and had an increased percentage of recordings that were depressed by SDs (median 5.1% [0.0–17.2%] vs. 0.3% [0.0–2.8%] in those without seizures; p = 0.01; Fig. 3b).
Relationships between seizures, ictal-interictal patterns, spreading depolarizations, and overall outcome. a A box plot showing the number of daily depolarizations (y-axis) in those with (red box) vs without (gray box) seizures. b A box plot showing the daily seizure burden (in minutes; y-axis) for patients based on categories of spreading depolarization (x-axis). The highest seizure burden is shown in those with clustered (orange box) or isoelectric (red box) spreading depolarizations. Scale is restricted to up to 60 min to highlight differences between groups. c A box plot showing the daily burden of ictal-interictal continuum patterns (in Hz-hour; y-axis) across categories of spreading depolarization (x-axis). d A stacked bar plot showing the relative proportions of patients (x-axis) based on 6-month Glasgow Outcome Scale-Extended scores across different temporal interactions between seizures and spreading depolarizations (y-axis)
Examination of the 24 of 38 (63.2%) patients in whom both ECoG-detected seizures and SDs occurred revealed several patterns of temporal relationship between these phenomena (Fig. 1a). In three cases, ECoG-detected seizures occurred only at the beginning of repetitive SDs, which then continued for several days. In eight cases, ECoG-detected seizures were interspersed between SDs, and in four cases, status epilepticus developed on ECoG after SDs ceased. Individual ECoG-detected seizures were linked temporally with SDs for at least a portion of the recording in most cases (17 of 24 [70.8%]). Figure 4 highlights the occurrence of ECoG-detected seizures within 30 min of SD occurrence. For 10 of 24 (41.7%) patients, there was a clear modulating effect in which ECoG-detected seizures or SDs blocked each other, as illustrated in Figs. 5a–d and 6c. Still, most patients (16 of 24 [66.7%]) also exhibited ECoG-detected seizures that appeared independent of SDs at some point during the monitoring. Only 6 of 24 (25%) patients exhibited ECoG-detected seizures that did not appear temporally related to any SDs, four of whom had only sporadic seizures.
Density raster of spreading depolarizations relative to individual ECoG-detected seizures. Each row represents patients in whom seizures and spreading depolarizations occurred contemporaneously. The start of each seizure (black vertical hash mark) is shown relative to the closest spreading depolarization (represented by time 0; x-axis). Seizures are plotted only once. Superimposed upon the raster plot is a density plot (pink) highlighting the close temporal relationship between seizures and depolarizations for most patients. The median time interval between seizures and spreading depolarizations that coexisted within the 60-min window across all 17 patients was 0.94 min (interquartile range -6.0 to 7.7 min). The x-axis extends from 30 min before to 30 min after the depolarization; 266/985 (27%) of seizures occurred with nearest SD > 30 min (median 76.8 min) and these were not included
Case example of seizures modulating spreading depolarizations and associated electrographic worsening. A 64-year-old man presented following non-penetrating traumatic brain injury with subdural hemorrhage and associated midline shift requiring operative evacuation. The legend at the top shows the bipolar ECoG channels displayed (ECoG 4–5 [pink], ECoG 5–6 [purple]) and the time course of the patient recording. For all figures, near-DC traces (top) are displayed using low-pass filter of 0.1 Hz and a high-pass filter of 0.01 Hz to eliminate drift. Standard AC traces (bottom) are shown after bandpass filtering at 1-30 Hz. During the first two days following injury, sporadic spreading depolarizations (SDs) were observed on ECoG monitoring. a Starting after the third day, clusters of SDs began to occur as regularly as 3–4/hour (two top traces). During a period of regularly spaced SDs, a seizure occurred (bottom two traces highlighted in dashed boxes); one example is shown in more detail in panel b. Seizures began in ECoG 4–5 and spread to ECoG 5–6. During the recording, a low-amplitude SD was observed, modulated by a seizure which continues to evolve prior to an abrupt offset at the time the SD would be expected to resolve. Subsequently, clustered SDs recurred until another seizure (shown in more detail in panel c) during which there was no apparent slow potential change, although the appearance of the seizures itself appeared to be modulated, suggesting a co-relationship between the two phenomenon. d As the recording continued, seizures within ECoG 5–6 became frequent and repetitive, occurring every 3–5 min (bottom traces; highlighted in dashed boxes). SDs were seen less frequently relative to their previous pattern, yet the occurrence of SDs appeared to break up the repetitive pattern of seizure activity. e Finally, four days following injury, the SDs resolved while seizures continued to occur independently on ECoG 5–6 (bottom two traces, purple arrowheads). f High-frequency ECoG activity is shown from the beginning of the recording period on the day of trauma, which exhibits a normal amplitude admixture of delta and superimposed faster alpha/beta frequencies. By the end of the recording, g high-frequency ECoG activity reflects electrographic worsening with a discontinuous pattern of low-amplitude delta frequencies
Case example of the variability in temporal relationship between seizures and spreading depolarizations. A 61-year-old woman presented following non-penetrating traumatic brain injury with subdural hemorrhage and associated midline shift requiring operative evacuation. The legend at the top shows the bipolar ECoG channels displayed (ECoG 1–2 [red], ECoG 2–3 [blue]) and the time course of the patient recording. During the first day post-trauma, regular clusters of spreading depolarizations (SDs) began to occur, at times on an electrically silent background (isoelectric SDs). During the following 24-h period, clusters of SDs became regular and tightly coupled with seizures which occurred within the same bipolar channels. a Over the course of two hours, variable relationships can be seen between SDs (top two traces; near-DC low-pass 0.1 Hz and high-pass 0.01 Hz; black arrows) and seizures (bottom two traces; AC bandpass 1-30 Hz). Some seizures occur during recovery of high-frequency activity (red arrowheads), some appear linked with the slow potential change (dashed box, B inset), and some begin during the depression period following SD (blue arrowhead). b Evolving ictal activity was observed on several occasions in conjunction with the slow potential change associated with spreading depolarization (top two traces; full-band low-pass 30 Hz and high-pass 0.01 Hz). As the depolarization is completed in this example, the seizure resolved with a superimposed, spindle-shaped pulse artifact which can be seen in the high-frequency traces (bottom two traces; AC bandpass 1-30 Hz). c One seizure began prior to the depolarizing wave, which appeared to modulate the overall amplitude of the underlying ictal activity. Subsequently, the amplitude of the seizure increased gradually throughout the depression period. The seizure resolved prior to the gradual emergence of interictal high-frequency activity. d, In some cases, seizures occurred during a period of relative electrical inactivity. In this example, an isoelectric SD is seen followed by evolving ictal activity
Channel-Specific Impact of ECoG-Detected Seizures
To determine the impact of ECoG-detected seizures on background ECoG activity, we separately analyzed each of five bipolar channels per patient for a total of 181 valid channels across n = 38 patients with ECoG-detected seizures. ECoG-detected seizures occurred in 138 of 181 channels (76.2%) and arose most commonly from a background activity that was continuous (35 of 138 [25.4%]) or discontinuous (40 of 138 [29.0%]). However, the ECoG background activity at the end of recordings worsened more frequently in channels with ECoG-detected seizures (73 of 138 [52.9%]) than in channels without seizures (11 of 43 [25.6%]; p = 0.003) (Figs. 5f, g and 7). Worsening ECoG background activity occurred in 26 of 38 (68.4%) patients, of whom most (19 of 26 [73.1%]) exhibited worsening in only some, but not all, ECoG channels.
Case example of status epilepticus in association with electrographic worsening. A 65-year-old man presented following non-penetrating traumatic brain injury with subdural hemorrhage and associated midline shift requiring operative evacuation. In the legend at the top is a representation of the bipolar ECoG channels displayed (ECoG 1–2 [red], ECoG 2–3 [blue], ECoG 3–4 [green], ECoG 4–5 [pink] and ECoG 5–6 [purple]) and the time course of the patient recording. a Starting shortly after the monitoring period began on post-trauma day 1, there were recurrent seizures (Seizures) every 3–4 min across ECoG 1–2, 2–3, and 3–4 with variable spread every third Seizures into ECoG 4–5 and ECoG 5–6. b High-frequency ECoG activity is shown from the beginning of the recording period and exhibits an admixture of delta frequencies with frequent sharply-configured waveforms and intermittent superimposed faster frequency activity. c, By the end of the recording, the ECoG high-frequency activity is largely suppressed in ECoG 1–2 and ECoG 2–3, whereas channels in which there was lesser Seizures burden (ECoG 4–5 and ECoG 5–6), an attenuated delta pattern remains. An ictal-interictal periodic pattern is seen in ECoG 3–4 between these two channel groups. For all figures, standard AC traces are shown using a low-pass filter at 1 Hz and a high-pass filter at 30 Hz
Ictal–Interictal Patterns
Periodic patterns with a frequency between 1 and 2.5 Hz occurred in 96 of 138 (69.6%) of patients during ECoG monitoring. The daily pattern burden index was a median of 0.56 Hz-hours (0.0–4.7 Hz-hours). Periodic patterns composed a median of 1.6% (0.0–12.9%) of the recording time. In contrast to ECoG-detected seizures, the incidence and burden of these patterns did not have an association with categories of SD occurrence (Fig. 3c) or with quantitative measures of ECoG-detected seizures or SDs. However, the occurrence of slow (≤ 0.5 Hz) periodic delta patterns was more common in those with clustered or isoelectric SDs (48 of 51 [94.1%] vs. 60 of 87 [69.0%] of those with sporadic or no SDs).
Associations Between ECoG-Detected Seizures and Clinical Outcome
Patients with and without ECoG-detected seizures did not differ in the proportion who were nonlocalizing (motor GCS score < 5) prior to surgery (17 of 38 [44.7%] vs. 63 of 100 [63.0%], respectively; p = 0.08). However, at the end of ECoG recording, 31 of 100 (31.0%) patients without ECoG-detected seizures had improved to at least localization to pain (motor GCS score ≥ 5) compared with 3 of 38 (7.9%) patients with ECoG-detected seizures (p = 0.01).
At 6 months following injury, the median GOS-E score was 3 (IQR 1–5) and 99 of 138 (71.7%) had poor outcomes of death, unresponsive wakefulness, and severe disability. The proportion of poor outcomes did not differ between those with and without ECoG-detected seizures (30 of 38 [79.0%] vs. 69 of 100 [69.0%]; p = 0.34). However, patients with frequent repetitive ECoG-detected seizures in close temporal relation with SD during the first 48–72 h following trauma had disproportionately better outcome relative to those with status epilepticus or in whom ECoG-detected seizures led to or were interspersed between predominantly clustered or isoelectric SDs (Fig. 3d; p < 0.001).
Seizures on either ECoG or scalp EEG had an independent impact on the odds of improvement in the neurological examination result by the end of the ECoG monitoring period (odds ratio 0.26, 95% CI 0.07–0.80, p = 0.03) in multivariable regression models controlling for the core International Mission for Prognosis and Analysis of Clinical Trials in TBI variables age, motor GCS score, and pupillary examination result, in addition to SD category (Table 3). The presence and burden of ECoG- or scalp EEG-detected seizures overall did not show an independent impact on 6-month GOS-E scores when controlling for the same factors. However, the presence of ECoG-detected seizures in patients with no or sporadic SDs was associated with better outcome (odds ratio 0.16, 95% CI 0.03–0.72, p = 0.02). The presence of temporally linked frequent repetitive ECoG-detected seizures in patients with clustered and/or isoelectric SDs decreased the odds for worse outcome relative to the odds calculated for patients with clustered or isoelectric SDs overall (Table 4).
Discussion
In this post hoc analysis of a prospective observational study of patients with severe TBI requiring neurosurgery and undergoing ECoG monitoring, we found that seizures were half as common as SDs (28% vs. 60%). Yet these two phenomena tended to co-occur, with a higher proportion of seizures in patients with clustered or isoelectric SDs (43%) than in those with no or sporadic SDs only (18%). Seizures and SDs showed a variety of temporal interactions within and across given patients, evidencing the heterogeneous and dynamic nature of secondary brain injury after TBI. Seizures were associated with electrographic worsening and less improvement in the GCS motor score over the period of monitoring independent of SDs. Although seizures were not independently associated with 6-month functional outcome, patterns of temporal interaction between seizures and SDs held prognostic significance.
The incidence of seizures during ECoG (27.5%) and available scalp EEG (30.0%) in this study was comparable with that in a recent prospective cohort of patients with moderate-to-severe TBI, in which 26.7% had clinical or electrographic seizures on continuous scalp EEG within 1 week of injury [25], but was far higher than the 2.6% incidence reported in a post hoc analysis of a randomized controlled trial [31]. This discrepancy is likely related to TBI severity in these cohorts because surgery was required more commonly in cohorts with a higher incidence of seizures (55.6% vs. 6.5%) and injury severity has been linked with seizures after moderate-to-severe TBI [31]. We did not find that injury severity was associated with either ECoG- or scalp EEG-detected seizures, but this is likely attributable to similarities in injury severity due to selective enrollment of only those requiring surgery. We also did not find an association between a history of epilepsy and clinical seizure activity, a validated risk factor for EEG-confirmed seizures [36] in the general hospital population undergoing continuous EEG.
This study is the largest clinical series documenting the association between SDs and ECoG seizures and confirms results from prior series. In a previous report of 63 patients undergoing ECoG monitoring, ECoG-detected seizures occurred almost exclusively (n = 10) in the subset of 32 patients who had SDs; only one patient had ECoG-detected seizures without SDs [13]. Several patient-specific patterns were described, including seizures that occurred reliably prior to SDs, seizures that appeared to occur at temporally distinct periods during the recording, recurrent seizures interrupted by SDs, and seizures that appeared to trigger SDs that propagated away from an ictal focus [13]. Interestingly, one reported patient exhibited seizures that preceded frequent SDs by 5–7 min. Here we similarly found that ECoG-detected seizures were more common in those with frequent SDs and that these were often temporally associated with SDs. We found that ECoG-detected seizures could either follow or precede SDs such that the central tendency of the time delay between ECoG-detected seizures and the nearest SD was a median of 1 min (IQR − 6 to 8 min). Within patients, the relationship between ECoG-detected seizures and SDs was often consistent for a period but then frequently evolved or fluctuated between different patterns through the course of monitoring. Similarly, in a study of ECoG after aneurysmal subarachnoid hemorrhage in 25 patients, ECoG-detected seizures could occur before, alternating with, or after SDs even within the same patient recording [14].
Secondary brain injury after TBI is common and contributes to clinical outcome. Recent evidence has pointed to the key role of SDs as a direct pathomechanism for secondary brain injury [37]. The presence of recurrent clusters of SDs and the development of isoelectric SDs have been associated with a lack of improvement in GCS motor scores and poor functional outcome [11]. The links between seizures and secondary brain injuries are more indirect, although ECoG-detected seizures, in particular, have been shown to produce detrimental physiologic changes and scalp EEG-detected seizures have been implicated in longer-term development of hippocampal atrophy and eventually posttraumatic epilepsy [24, 25, 38, 39]. Here, we found that ECoG-detected seizures were clearly linked with electrographic worsening characterized by increasing discontinuity in ECoG background activity from the beginning of the monitoring period relative to the end of the recording. We also found that both ECoG- and scalp EEG-detected seizures were independently associated with a lack of postoperative motor improvement on clinical examination even after controlling for the presence of SDs. However, seizures were not independently associated with functional outcome in this cohort of surgically managed patients with TBI.
Whereas SDs propagate, impacting tissue distal to the site of monitoring, seizures may remain focal, even to the point of being undetectable by scalp EEG. We found this to be the case in 7 of 15 (47%) of those with ECoG-detected seizures during simultaneous scalp EEG monitoring, and others have reported similar proportions (42.9%) of seizures missed by scalp EEG alone [6]. Employing ECoG monitoring, we found that standard clinical care missed a diagnosis of seizures in 20% of patients enrolled in this study. Given these findings and the prognostic value of detecting their co-occurrence, we suggest that more direct methods for monitoring the brain after injury (such as ECoG) provide information integral to multimodal brain monitoring after TBI relative to scalp EEG alone.
The relationship between seizures and SDs has been a source of debate. The cellular mechanism of ictal activity is the paroxysmal depolarizing shift in resting membrane potential, which nonetheless remains sufficiently charged to propagate bursts of action potentials that correlate with epileptiform spikes on EEG. In contrast, SDs reflect a near-complete and sustained loss of resting membrane potential within the tissue such that the cortex is unable to generate the postsynaptic potentials associated with high-frequency EEG. This distinction rests on the important finding that SDs are associated with substantially larger changes in transmembrane ion and neurotransmitter concentrations. During seizures, for instance, extracellular sodium concentrations fall from ~ 150 to ~ 140 mM and extracellular calcium concentrations fall from 1,200 to 1,000 µM. In contrast, during SDs the extracellular sodium concentration reaches as low as ~ 60 mM and the extracellular calcium concentration reaches a nadir of 80 µM [40]. The extracellular glutamate concentration during seizures increases from ~ 2 to ~ 4 µM [41], but during SDs, glutamate concentrations increase to 100 µM [42]. These changes result in an estimated free energy release of ~ 3 J/L during seizures compared with ~ 22 J/L during SD [20]. The neurophysiologic manifestation of these changes, the negative DC potential shift, reflects this with a shift in voltage of 0.6 mV (IQR 0.2-–0.9 mV) during seizures compared with 8.4 mV (IQR 6.2–10.9 mV) during SDs [43]. This distinction is critical insofar as levels of intraneuronal calcium function as a central second messenger in myriad subcellular systems, including programmed cell death: intraneuronal calcium concentrations increase from ~ 60 to ~ 130 nM during seizures but to at least 25 µM during SDs [40, 44,45,46].
Yet both seizures and SDs are precipitated by direct cortical injury, hypoxia, or ischemia. SDs have been shown at times to be triggered by seizures [16,17,18,19,20], and some have described a temporary interruption of the epileptic activity that may then result [19]. This was recently interpreted as a fundamental antiseizure function of SDs, which block or modulate the spread of focal seizures [21]. Our data suggest that SDs occur frequently before ECoG-detected seizures and that SDs may be followed by or even replaced by ECoG-detected seizures, as shown in Figs. 1a and 4.
GABA inhibitory tone is involved in both seizures and SDs [13,14,15, 47] but does not necessarily affect both similarly. SDs may selectively delay the recovery of GABAergic inhibitory interneurons after injury [14], and while GABA is released during SDs, the influx of chloride ions and water [40, 48, 49] could also reduce overall GABA currents [15]. The sodium channel NaV1.1 is primarily expressed along inhibitory interneurons. Gain-of-function mutations in this channel lead to familial hemiplegic migraine (type III) and provide a Mendelian model of SDs. Recently, these mutations were found to impair inactivation of the channel [50], which should lead to an increase in the inhibition of the network. In contrast, loss-of-function mutations have been associated with epilepsy syndromes, such as Dravet syndrome (a pharmacoresistant developmental and epileptic encephalopathy) and genetic epilepsy with febrile seizures plus [51, 52]. Interactions between SD and GABA-related inhibitory tone may be involved in the experimental observation that paroxysmal depolarizing shifts, the sine qua non of ictal activity, begin to occur approximately 10–20 min after an SD [15].
Another link reflects the association between seizures, SDs, and the blood–brain barrier. SDs have been associated with dysfunction of the blood–brain barrier [53, 54], development of cerebral edema [55,56,57], and inflammation [58], all of which have also been implicated in the development of seizures [59]. The spectrum of hemodynamic responses to both seizures and SDs from hyperemia to ischemia may mirror the integrity of the blood–brain barrier after injury [60] and further contribute to lesion development. In one clinical study, acute and subacute SDs after cortical injury were associated with the development of blood–brain barrier dysfunction and a higher risk of developing postinjury epilepsy [14]. Interestingly, during epileptogenesis, the long-term plastic process that leads to epilepsy, the propensity for SDs appears to decrease despite an increase in the propensity for spontaneous seizures [19, 61, 62].
Computational models suggest that seizures and SDs occur as part of a “dynamical continuum of the neuronal membrane potential, ion concentrations, metabolic energy, and volume” [57]. In the setting of hypoxia, there is a fusion of the dynamics that mediate the occurrence of SDs and seizures [63]. In addition, cellular swelling, which occurs commonly after brain trauma, and changes in glial [K+] buffering capacity or impaired [K+] diffusion give rise to transition points in which the tissue may exhibit seizures or SDs [57]. Given the spatiotemporal heterogeneity of hypoxic and ischemic injury following TBI [64], our findings of variably temporally associated ECoG-detected seizures and SDs may reflect the aggregate balance of oxygen delivery and use, cerebral edema, and extracellular [K+] clearance in heterogeneously injured tissue.
Limitations to this study include its generalizability to the broader TBI population because we focused on patients with severe TBI requiring neurosurgery, for whom consent to undergo ECoG monitoring was obtained. Additionally, there were challenges in recording quality, including noise and artifacts encountered with ECoG in the intensive care unit environment, which impacted the total available data per patient and caused exclusion of some patients entirely. ECoG data were acquired and analyzed using a near-DC bipolar configuration, which might have precluded a more nuanced analysis of the channel-by-channel relationship between SDs and ECoG-detected seizures that could be facilitated with monopolar full-band DC recordings that are now available [65], and inherent variability occurred in the placement of subdural electrode strips across noncontiguous gyri or sulci, further limiting more direct study of the causal relationship between the two phenomena. Although we used available standards in terminology both for SDs and seizure scoring and characterization, interrater disagreement remains possible within ECoG data, and scalp EEG findings were also subject to the interpretation of clinical reviewers. Finally, decisions regarding removal of ECoG strip electrodes were made by clinical teams which may have biased our findings regarding motor GCS at the end of the recording period.
In summary, we found that seizures are half as common as SDs in patients with severe TBI requiring neurosurgery undergoing ECoG monitoring but that their detection has important implications. We noted that scalp EEG missed nearly half of seizures seen on simultaneous ECoG recording and that, furthermore, seizures would have been missed in up to 20% of patients on the basis of standard clinical care alone. In our study, evidence linked ECoG-detected seizures with worsening electrographic activity and decreased odds for clinical motor recovery, independent of the occurrence of SDs. We confirmed the long-held observation that seizures and SDs are related and, in fact, found that they commonly co-occur, often with a close temporal relationship. Although neither ECoG- nor scalp EEG-detected seizures had an independent association with 6-month functional outcome, patterns of temporal interactions between ECoG-detected seizures and SDs modified prognostic estimates. We conclude that seizures and SDs are interrelated and may operate in synergy along a dynamic continuum of factors critical to the development of secondary brain injury in metabolically vulnerable injured tissue and that ECoG provides information integral to the clinical management of patients with TBI.
References
Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1.
Reddy GD, Gopinath S, Robertson C. Critical Care Management of the Patient with Traumatic Brain Injury. Semin Neurol. 2016;36(6):570–6.
Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care : a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(9):1189–209.
Herman ST, Abend NS, Bleck TP, Chapman KE, Drislane FW, Emerson RG, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87–95.
Waziri A, Claassen J, Stuart RM, Arif H, Schmidt JM, Mayer SA, et al. Intracortical electroencephalography in acute brain injury. Ann Neurol. 2009;66(3):366–77.
Vespa P, Tubi M, Claassen J, Buitrago-Blanco M, McArthur D, Velazquez AG, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016;79(4):579–90.
Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17(4):439–47.
Hartings JA. Spreading depolarization monitoring in neurocritical care of acute brain injury. Curr Opin Crit Care. 2017;23(2):94–102.
Drenckhahn C, Winkler MK, Major S, Scheel M, Kang EJ, Pinczolits A, et al. Correlates of spreading depolarization in human scalp electroencephalography. Brain. 2012;135(Pt 3):853–68.
Hartings JA, Wilson JA, Hinzman JM, Pollandt S, Dreier JP, DiNapoli V, et al. Spreading depression in continuous electroencephalography of brain trauma. Ann Neurol. 2014;76(5):681–94.
Hartings JA, Andaluz N, Bullock MR, Hinzman JM, Mathern B, Pahl C, et al. Prognostic Value of Spreading Depolarizations in Patients With Severe Traumatic Brain Injury. JAMA Neurol. 2020;77(4):489–99.
Leao AAP. SPREADING DEPRESSION OF ACTIVITY IN THE CEREBRAL CORTEX. J Neurophysiol. 1944;7(6):359–90.
Fabricius M, Fuhr S, Willumsen L, Dreier JP, Bhatia R, Boutelle MG, et al. Association of seizures with cortical spreading depression and peri-infarct depolarisations in the acutely injured human brain. Clin Neurophysiol. 2008;119(9):1973–84.
Dreier JP, Major S, Pannek HW, Woitzik J, Scheel M, Wiesenthal D, et al. Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain. 2012;135(Pt 1):259–75.
Eickhoff M, Kovac S, Shahabi P, Ghadiri MK, Dreier JP, Stummer W, et al. Spreading depression triggers ictaform activity in partially disinhibited neuronal tissues. Exp Neurol. 2014;253:1–15.
Hablitz JJ, Heinemann U. Alterations in the microenvironment during spreading depression associated with epileptiform activity in the immature neocortex. Dev Brain Res. 1989;46(2):243–52.
Mody I, Lambert J, Heinemann U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J Neurophysiol. 1987;57(3):869–88.
Avoli M, Drapeau C, Louvel J, Pumain R, Olivier A, Villemure JG. Epileptiform activity induced by low extracellular magnesium in the human cortex maintained in vitro. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1991;30(4):589–96.
Koroleva V, Bureš J. Cortical penicillin focus as a generator of repetitive spike-triggered waves of spreading depression in rats. Exp Brain Res. 1983;51(2):291–7.
Dreier JP, Isele T, Reiffurth C, Offenhauser N, Kirov SA, Dahlem MA, et al. Is spreading depolarization characterized by an abrupt, massive release of gibbs free energy from the human brain cortex? Neuroscientist. 2013;19(1):25–42.
Tamim I, Chung DY, de Morais AL, Loonen IC, Qin T, Misra A, et al. Spreading depression as an innate antiseizure mechanism. Nat Commun. 2021;12(1):1–15.
Hinzman JM, DiNapoli VA, Mahoney EJ, Gerhardt GA, Hartings JA. Spreading depolarizations mediate excitotoxicity in the development of acute cortical lesions. Exp Neurol. 2015;267:243–53.
Hinzman JM, Wilson JA, Mazzeo AT, Bullock MR, Hartings JA. Excitotoxicity and metabolic crisis are associated with spreading depolarizations in severe traumatic brain injury patients. J Neurotrauma. 2016;33(19):1775–83.
Vespa P, McArthur D, Xu Y, Eliseo M, Etchepare M, Dinov I, et al. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75(9):792–8.
Tubi MA, Lutkenhoff E, Blanco MB, McArthur D, Villablanca P, Ellingson B, et al. Early seizures and temporal lobe trauma predict post-traumatic epilepsy: a longitudinal study. Neurobiol Dis. 2019;123:115–21.
Lückl J, Lemale CL, Kola V, Horst V, Khojasteh U, Oliveira-Ferreira AI, et al. The negative ultraslow potential, electrophysiological correlate of infarction in the human cortex. Brain. 2018;141(6):1734–52.
Hartings JA, Watanabe T, Bullock MR, Okonkwo DO, Fabricius M, Woitzik J, et al. Spreading depolarizations have prolonged direct current shifts and are associated with poor outcome in brain trauma. Brain. 2011;134(5):1529–40.
Helbok R, Hartings JA, Schiefecker A, Balança B, Jewel S, Foreman B, et al., editors. What should a clinician do when spreading depolarizations are observed in a patient? Neurocritical care; 2020: Springer.
Dreier JP, Fabricius M, Ayata C, Sakowitz OW, William Shuttleworth C, Dohmen C, et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: review and recommendations of the COSBID research group. J Cereb Blood Flow Metab. 2017;37(5):1595–625.
Hirsch L, LaRoche S, Gaspard N, Gerard E, Svoronos A, Herman S, et al. American clinical neurophysiology society’s standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30(1):1–27.
Lee H, Mizrahi MA, Hartings JA, Sharma S, Pahren L, Ngwenya LB, et al. Continuous Electroencephalography After Moderate to Severe Traumatic Brain Injury. Crit Care Med. 2019;47(4):574–82.
Wickham H, Chang W, Wickham MH. Package ‘ggplot2.’ Create Elegant Data Visualisations Using the Grammar of Graphics Version. 2016;2(1):1–189.
Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020.
Venables W, Ripley B, Hornik K, Gebhardt A. Package ‘Mass’. https://cran.r-project.org/web/packages/MASS/.
Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5(8):e165; discussion e.
Struck AF, Tabaeizadeh M, Schmitt SE, Ruiz AR, Swisher CB, Subramaniam T, et al. Assessment of the validity of the 2HELPS2B score for inpatient seizure risk prediction. JAMA Neurol. 2020;77(4):500–7.
Hartings JA, Shuttleworth CW, Kirov SA, Ayata C, Hinzman JM, Foreman B, et al. The continuum of spreading depolarizations in acute cortical lesion development: Examining Leao’s legacy. J Cereb Blood Flow Metab. 2017;37(5):1571–94.
Claassen J, Perotte A, Albers D, Kleinberg S, Schmidt JM, Tu B, et al. Nonconvulsive seizures after subarachnoid hemorrhage: Multimodal detection and outcomes. Ann Neurol. 2013;74(1):53–64.
Vespa PM, Miller C, McArthur D, Eliseo M, Etchepare M, Hirt D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35(12):2830.
Dreier JP, Reiffurth C. The stroke-migraine depolarization continuum. Neuron. 2015;86(4):902–22.
Li Z, Song Y, Xiao G, Gao F, Xu S, Wang M, et al. Bio-electrochemical microelectrode arrays for glutamate and electrophysiology detection in hippocampus of temporal lobe epileptic rats. Anal Biochem. 2018;550:123–31.
Zhou N, Rungta RL, Malik A, Han H, Wu DC, MacVicar BA. Regenerative glutamate release by presynaptic NMDA receptors contributes to spreading depression. J Cereb Blood Flow Metab. 2013;33(10):1582–94.
Revankar GS, Winkler MK, Major S, Schoknecht K, Heinemann U, Woitzik J, et al. Spreading depolarizations and seizures in clinical subdural electrocorticographic recordings. Seizures in Critical Care: Springer; 2017. p. 77–90.
Revah O, Lasser-Katz E, Fleidervish IA, Gutnick MJ. The earliest neuronal responses to hypoxia in the neocortical circuit are glutamate-dependent. Neurobiol Dis. 2016;95:158–67.
Dietz RM, Weiss JH, Shuttleworth CW. Zn2+ influx is critical for some forms of spreading depression in brain slices. J Neurosci. 2008;28(32):8014–24.
Aiba I, Shuttleworth CW. Sustained NMDA receptor activation by spreading depolarizations can initiate excitotoxic injury in metabolically compromised neurons. J Physiol. 2012;590(22):5877–93.
Köhling R, Koch U, Hagemann G, Redecker C, Straub H, Speckmann E-J. Differential sensitivity to induction of spreading depression by partial disinhibition in chronically epileptic human and rat as compared to native rat neocortical tissue. Brain Res. 2003;975(1–2):129–34.
Allen NJ, Rossi DJ, Attwell D. Sequential release of GABA by exocytosis and reversed uptake leads to neuronal swelling in simulated ischemia of hippocampal slices. J Neurosci. 2004;24(15):3837–49.
Fabricius M, Jensen LH, Lauritzen M. Microdialysis of interstitial amino acids during spreading depression and anoxic depolarization in rat neocortex. Brain Res. 1993;612(1–2):61–9.
Jansen NA, Dehghani A, Linssen MM, Breukel C, Tolner EA, van den Maagdenberg AM. First FHM3 mouse model shows spontaneous cortical spreading depolarizations. Annals of clinical and translational neurology. 2020;7(1):132–8.
Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. The American Journal of Human Genetics. 2001;68(6):1327–32.
Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, An-Gourfinkel I, et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+ 2. Nat Genet. 2000;24(4):343–5.
Gursoy-Ozdemir Y, Qiu J, Matsuoka N, Bolay H, Bermpohl D, Jin H, et al. Cortical spreading depression activates and upregulates MMP-9. J Clin Investig. 2004;113(10):1447–55.
Sadeghian H, Lacoste B, Qin T, Toussay X, Rosa R, Oka F, et al. Spreading depolarizations trigger caveolin-1–dependent endothelial transcytosis. Ann Neurol. 2018;84(3):409–23.
Dreier JP, Lemale CL, Kola V, Friedman A, Schoknecht K. Spreading depolarization is not an epiphenomenon but the principal mechanism of the cytotoxic edema in various gray matter structures of the brain during stroke. Neuropharmacology. 2018;134:189–207.
Mestre H, Du T, Sweeney AM, Liu G, Samson AJ, Peng W, et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science. 2020;367(6483).
Ullah G, Wei Y, Dahlem MA, Wechselberger M, Schiff SJ. The role of cell volume in the dynamics of seizure, spreading depression, and anoxic depolarization. PLoS computational biology. 2015;11(8):e1004414.
Takizawa T, Qin T, Lopes de Morais A, Sugimoto K, Chung JY, Morsett L, et al. Non-invasively triggered spreading depolarizations induce a rapid pro-inflammatory response in cerebral cortex. Journal of Cerebral Blood Flow & Metabolism. 2020;40(5):1117–31.
Claassen J, Albers D, Schmidt JM, De Marchis GM, Pugin D, Falo CM, et al. Nonconvulsive seizures in subarachnoid hemorrhage link inflammation and outcome. Ann Neurol. 2014;75(5):771–81.
Winkler MK, Chassidim Y, Lublinsky S, Revankar GS, Major S, Kang EJ, et al. Impaired neurovascular coupling to ictal epileptic activity and spreading depolarization in a patient with subarachnoid hemorrhage: possible link to blood–brain barrier dysfunction. Epilepsia. 2012;53:22–30.
Maslarova A, Alam M, Reiffurth C, Lapilover E, Gorji A, Dreier JP. Chronically epileptic human and rat neocortex display a similar resistance against spreading depolarization in vitro. Stroke. 2011;42(10):2917–22.
Tomkins O, Friedman O, Ivens S, Reiffurth C, Major S, Dreier J, et al. Blood–brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol Dis. 2007;25(2):367–77.
Wei Y, Ullah G, Schiff SJ. Unification of neuronal spikes, seizures, and spreading depression. J Neurosci. 2014;34(35):11733–43.
Launey Y, Fryer TD, Hong YT, Steiner LA, Nortje J, Veenith TV, et al. Spatial and Temporal Pattern of Ischemia and Abnormal Vascular Function Following Traumatic Brain Injury. JAMA Neurol. 2020;77(3):339–49.
Hartings JA, Li C, Hinzman JM, Shuttleworth CW, Ernst GL, Dreier JP, et al. Direct current electrocorticography for clinical neuromonitoring of spreading depolarizations. J Cereb Blood Flow Metab. 2017;37(5):1857–70.
Funding
This study was funded by the US Army Congressionally Directed Medical Research Program Psychological Health/Traumatic Brain Injury Research Program (W81XWH-08–2-0016). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Defense.
Author information
Authors and Affiliations
Contributions
Brandon Foreman conceived and designed the analysis, contributed data or analysis tools, performed the analysis, and wrote the article. Hyunjo Lee contributed data or analysis tools and performed the analysis. David O. Okonkwo collected and contributed data and contributed to critical revisions of the manuscript. Anthony J. Strong collected and contributed data and contributed to critical revisions of the manuscript. Clemens Pahl collected and contributed data. Lori A. Shutter collected and contributed data and contributed to critical revisions of the manuscript. Jens P. Dreier conceived and designed the analysis, contributed data or analysis tools, and contributed to critical revisions of the manuscript. Laura B Ngwenya contributed to critical revisions of the manuscript. Jed A. Hartings conceived and designed the study and the analysis, contributed data or analysis tools, and wrote the article. The final manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Foreman reports grant funding from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (K23NS101123), the US Department of Defense (W81XWH1620020, W81XWH1920013), and the National Science Foundation (IIS1838730) and personal fees from UCB Pharma and the Micromed Group outside the submitted work. Dr. Dreier reports grants from the DFG Deutsche Forschungsgemeinschaft (German Research Council) (DFG DR 323/5–1 and DFG DR 323/10–1) and BMBF Bundesministerium fuer Bildung und Forschung (Era-Net Neuron EBio2 with funds from BMBF 0101EW2004).
Ethical approval and informed consent
This study adheres to ethical guidelines, and ethical approval is reported in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the collection “Spreading Cortical Depolarization”.
Rights and permissions
About this article
Cite this article
Foreman, B., Lee, H., Okonkwo, D.O. et al. The Relationship Between Seizures and Spreading Depolarizations in Patients with Severe Traumatic Brain Injury. Neurocrit Care 37 (Suppl 1), 31–48 (2022). https://doi.org/10.1007/s12028-022-01441-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01441-2