Abstract
Background
We present an exploratory analysis of the occurrence of early corticothalamic connectivity disruption after aneurysmal subarachnoid hemorrhage (SAH) and its correlation with clinical outcomes.
Methods
We conducted a retrospective study of patients with acute SAH who underwent continuous electroencephalography (EEG) for impairment of consciousness. Only patients undergoing endovascular aneurysm treatment were included. Continuous EEG tracings were reviewed to obtain artifact-free segments. Power spectral analyses were performed, and segments were classified as A (only delta power), B (predominant delta and theta), C (predominant theta and beta), or D (predominant alpha and beta). Each incremental category from A to D implies greater preservation of corticothalamic connectivity. We dichotomized categories as AB for poor connectivity and CD for good connectivity. The modified Rankin Scale score at follow-up and in-hospital mortality were used as outcome measures.
Results
Sixty-nine patients were included, of whom 58 had good quality EEG segments for classification: 28 were AB and 30 were CD. Hunt and Hess and World Federation of Neurological Surgeons grades were higher and the initial Glasgow Coma Scale score was lower in the AB group compared with the CD group. AB classification was associated with an adjusted odds ratio of 5.71 (95% confidence interval 1.61–20.30; p < 0.01) for poor outcome (modified Rankin Scale score 4–6) at a median follow-up of 4 months (interquartile range 2–6) and an odds ratio of 5.6 (95% confidence interval 0.98–31.95; p = 0.03) for in-hospital mortality, compared with CD.
Conclusions
EEG spectral-power-based classification demonstrates early corticothalamic connectivity disruption following aneurysmal SAH and may be a mechanism involved in early brain injury. Furthermore, the extent of this disruption appears to be associated with functional outcome and in-hospital mortality in patients with aneurysmal SAH and appears to be a potentially useful predictive tool that must be validated prospectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) causes devastating neurological injury and has a 30-day mortality of 21–44% [1]. Improvement in outcomes have occurred in patients with SAH who receive early diagnosis, early aneurysm occlusion, and monitoring and effective treatment of hydrocephalus and delayed cerebral ischemia [2,3,4,5,6]. As part of the next leap in improving outcomes after SAH, there is a need to identify the substrate of disorders of consciousness such that they can be targets for intervention.
Our understanding of underlying structural and functional mechanisms affecting consciousness in SAH remains poor; few biomarkers exist for evaluating severity of brain injury, monitoring effects of treatment, or serving as surrogate end points for treatments [7]. Currently, clinical grades are used as predictors of outcome, but within these scales, determining those who will recover versus those who will not is limited [8, 9].
Continuous electroencephalography (cEEG) serves as a tool to evaluate background cerebral electrophysiological function, seizures, and cerebral ischemia [10,11,12]. Offline analysis of cEEG provides a powerful tool for studying brain network connectivity. Electroencephalographic (EEG) power spectra are efficient indices in deciphering correlation with levels of consciousness and show monotonic gradation with different severities of arousal dysfunction [13]. The anterior forebrain mesocircuit has been a focus in disorders of consciousness characterized by disruption of corticothalamic excitation, and measures linked to this model have shown evidence of prediction of outcomes [14, 15].
Connectivity between thalamic and cortical structures is extensive and reciprocal; up to 826 cortico-cortical connections and 651 thalamocortical connections have been identified in cats [16]. Although there may exist interindividual variability in intercortical connections, this is less true for thalamic connections [17]. Because of rich interconnections, injuries to thalamic nuclei are associated with widespread cell death and involve extensive disruption in hemispheric network connectivity [14]; similarly, widespread multifocal cerebral injuries exert disproportionate impact of thalamic cell loss, concentrating within the central thalamus [18, 19]. Within central thalamic nuclei, intralaminar and midline nuclei are associated with functions of arousal, having strong connections from the brainstem arousal system [20]. At times, even focal injuries to these nuclei in a variety of pathologies cause disorders of arousal proportional to severity of injury [21]. Additionally, disruption of inhibitory input to the striatum can further increase inhibition of thalamic nuclei and result in hypofunction of corticothalamic projections, causing diminished arousal [14, 22, 23]. Functional magnetic resonance imaging (MRI) studies have revealed disconnection between the default mode network and anterior forebrain structures, rather than disruption within the default mode network, in patients who are unconscious after hemorrhagic stroke [24].
Following brain injuries, disassociation between motor and cognitive function may at times occur and confound level of consciousness determined solely by motor response. Deafferentation leading to cognitive motor dissociation has been described in several studies of patients with chronic brain injury [25], whereas in acute brain injury, it has only recently been demonstrated, and data remain sparse [14, 26,27,28]. In patients with preserved command-following on functional MRI, EEG-based corticothalamic integrity is preserved and may serve as a reasonable alternative for assessing cognitive motor dissociation in the intensive care setting after an acute brain injury [29, 30]. This is a bedside tool that is objective and can potentially be further developed into a more sophisticated automated system. Furthermore, it compares favorably with alternative tests for arousal evaluation, such as functional MRI, positron emission tomography, and nonquantitative EEG, which may have lower interrater reliability, especially with respect to frequency assessment [31].
In this study, we used quantitative cEEG to evaluate early forebrain corticothalamic integrity in patients with SAH and studied its association with functional outcome and mortality. This study has mechanistic as well as prognostic implications and may potentially be developed for (automated) assessment of arousal levels in patients with SAH.
Methods
Data Availability
Anonymized data will be shared at the request of any qualified investigator: (1) raw EEG clips from which spectra were derived and (2) spectra for each patient.
Study Population
This was a retrospective study of all adult patients (> 18 years) with SAH admitted to the neurosciences intensive care unit (neuro-ICU) of Weill Cornell Medical Center/NewYork-Presbyterian Hospital between January 1, 2010, and October 31, 2015. Patients were included in the study if they had confirmed aneurysmal SAH and were monitored with 24-h cEEG within 14 days from ictus. This included approximately 25% of our aneurysmal SAH population for this period. Only patients who underwent endovascular aneurysm treatment were included to prevent confounding by surgical complications, such as brain handling, retractions, ischemia, and contusions. Patients were excluded if they were deemed unable to survive either because of comorbid conditions or because of SAH severity or if withdrawal of life support occurred within 14 days of the primary SAH diagnosis. The institutional review board for research integrity approved the study.
Clinical Variables
Medical records were reviewed retrospectively by using twoperson review. Demographic data, SAH clinical severity grades (World Federation of Neurological Surgeons [WFNS] and Hunt and Hess [HH]), and a radiological severity grade (modified Fisher Scale) on admission were collected for all patients. Both WFNS and HH grades were divided into good (1–3) and poor (4–5) clinical grades. The Glasgow Coma Scale (GCS) score was recorded at admission, on the day of the EEG, and at discharge [32].
Sedation is routinely stopped for all patients at 6 a.m. for clinical evaluation by the intensive care unit (ICU) team, and from 8 to 10 a.m., the neurological wake-up test is conducted. cEEG clips were taken from this period for every patient. For multiple segments, clips were taken from multiple days from the same time period in the morning. This allowed for sedative washout, although complete washout could not be verified, but this method is routinely used in all neurocritical care units as a wake-up test for the best neurological assessment. Use of sedative agents preceding the wake-up test was recorded, as was the neurological examination from the same period, all of which were documented in the hourly ICU record. Additional clinical data on the day of the cEEG were recorded, including concurrent mean arterial pressure, any occurrence of elevated intracranial pressure, concurrent antiepileptic drug use, hydrocephalus, concomitant cerebral vasospasm, and concurrent fever. ‘ABCD’ categories were also compared by using these variables. We used the modified Rankin Scale (mRS) as a functional outcome measure, as it is one of the most often used outcome measures in clinical research of SAH outcomes [33]. The mRS was retrospectively computed at a single time point by using twoperson blinded review of follow-up outpatient clinical notes. Other outcomes measures included were mortality and hospital length of stay. Dichotomized mRS scores were used to classify good (0–2) and poor outcome (3–6).
Clinical Management
All patients were admitted to the neuro-ICU and underwent computed tomographic (CT) angiography on admission to identify aneurysm(s), location(s), and morphology. Aneurysms were treated by endovascular coiling based on location, size, morphology, and consensus between neurosurgeon and endovascular surgeon and discussion with the patient and/or surrogate decision-maker. Patients remained in the neuro-ICU for at least 14 days and were treated according to standard clinical treatment protocols based on American Heart Association/American Stroke Association guidelines [34]. This included treatment with nimodipine and treatment of delayed cerebral ischemia with hypertensive hyperdynamic therapy as indicated. Intraarterial calcium channel antagonists, angioplasty, and stenting were used to treat refractory vasospasm. Hydrocephalus was treated with ventriculostomy, if needed. All patients with unexplained fluctuating or prolonged altered mental status or suspected seizures were monitored with cEEG for 24–48 h, regardless of hemorrhagic grade. Antiepileptic prophylaxis was minimized to the period prior to aneurysm occlusion, and levetiracetam was the preferred agent.
cEEG Recordings
cEEG was recorded digitally by using 21 electrodes placed per the international 10–20 system. This was performed by using an XLTEK 32channel computerized video EEG system (Natus Medical Incorporated, Pleasanton, CA) with digital analysis of EEG for spike detections, topographic and occurrence analysis, computerized seizure detection algorithms, compressed spectral array for quantification of EEG frequency content, and a patient event marker. Recordings were interpreted by a board-certified electroencephalographer and were reviewed periodically. EEG reports were written once daily, and the clinical team was updated over the course of the day when seizures were detected. The presence of slowing, epileptiform discharges, reactivity to stimulation, and any seizure recorded on cEEG was recorded for our analysis.
Spectral Classification
For each patient on day 1 of cEEG recording, segments were cut around identical times of maximal arousal during morning examination rounds between 8 and 10 a.m., with sedation held for 2 h prior to hourly clinical examinations, documented in the nursing charts, and detailed examination at this period by the ICU team, documented in the doctors’ notes. All available channels were inspected; however, given the electrically noisy environment in the ICU, which frequently resulted in abundance of artifacts in frontal and temporal channels, midline and parasagittal centroparietal leads (Cz, C4, C3, Pz, P3, P4) were typically the most useful for determination of spectral categories. After visual assessment of artifacts (e.g., eye blinks, major movement artifacts, or possible artifacts from other equipment), an average of 70–80 (range ~ 10–300) 3-s-long artifact-free segments were selected for quantitative analysis. Power spectra were calculated for each channel by using Laplacian montage and Thomson’s multitaper method [35] with five tapers plotted between 2 and 24 Hz.
Power spectral analyses of EEG potentials recorded from scalp electrodes were used to classify corticothalamic integrity [36]. The categories were based on presence of activity in delta (< 4 Hz), theta (5–7 Hz), alpha (8–12 Hz) and beta (15–40 Hz) frequencies [14]. On one end of the spectrum, category A implies complete loss of thalamocortical circuit functional integrity, B relates to severe loss with spontaneous cortical oscillations and no thalamic output, and C relates to local cortical disinhibition with thalamic burst firing, whereas on the other end of the spectrum, category D illustrates normal activity pattern with preserved circuit functional integrity [14, 15] (Fig. 1). Accordingly, category A consists of sole delta-range activity, category B consists of predominant delta- and theta-range activity, category C consists of predominant theta and beta frequencies, and category D consists of predominant alpha and beta frequencies [15]. Background EEG pattern was also examined to confirm concordance with ‘ABCD’ spectral categorization.
An investigator blinded to all clinical details analyzed and classified all EEG segments into ‘ABCD’ spectral categories. Only patients meeting ‘ABCD’ classification were included in the primary analysis. Types A and B were grouped together (AB) as poor corticothalamic connectivity, and types C and D were grouped together (CD) as good corticothalamic connectivity for outcomes analyses. Background EEG activity was examined to ensure there was consistency between clipped segments and ongoing EEG activity.
Imaging Analysis
CT perfusion studies with baseline noncontrast brain CT were performed on the basis of clinical indications and protocols to evaluate for regional hypoperfusion and stroke and were independently interpreted by a certified neuroradiologist. Follow-up imaging, including noncontrast head CT, CT angiography, and CT perfusion, was performed as clinically indicated. The report filed in the clinical record was used for assessing presence of cerebral hypoperfusion and stroke. Brain MRI was not performed routinely; however, when done, images were examined for the presence of nonprocedural stroke (Supplemental Table 1).
Statistical Analysis
Characteristics of study patients are described as means (standard deviations) for continuous variables and frequencies (percentages) for categorical variables. Clinical variables, EEG characteristics, and outcome measures were compared between AB and CD groups by using the two-sample t-test and χ2 test. All statistical tests were two-sided, with a significance level of p < 0.05. Logistic regression models were created by using variables that showed certainty of association in univariate analyses (p < 0.10). Because this was an exploratory analysis, we used a cutoff of p < 0.10 to ensure inclusion of variables that may demonstrate a reasonable difference between the two groups. Correlations among spectral categories and clinical severity grades were assessed by using the Spearman correlation test. All statistical analyses were conducted with SAS version 9.4 (SAS Institute, Inc., Cary, NC). Univariate receiver operating characteristic curves were constructed for ‘ABCD’ classification for comparison between HH and WFNS grades by using Stata 13.1 (StataCorp, College Station, TX). Because this was an exploratory retrospective study, we did not perform a power analysis. For sensitivity analysis, we generated multivariate receiver operating characteristic curves for the outcome model with and without ‘ABCD’ classification using known predictors of outcome, including age, the HH (or WFNS) grade, and presence of stroke.
Results
Baseline Characteristics
The cohort included 69 patients with SAH, with patient selection summarized in Fig. 2. Baseline characteristics are summarized in Table 1. Seventy percent of patients were female, and the mean age was 58 years (± 15.7). EEG was performed at a median of 3 days after SAH diagnosis, with an interquartile range of 2–8 days. Follow-up examinations occurred at a median of 4 months (interquartile range 2–6) from admission. Mortality was 17.4%; 11 of 12 deaths occurred during the initial hospitalization, and 9 of 12 deaths occurred in the setting of withdrawal of life-sustaining therapies.
‘ABCD’ Spectral Classification
Of 69 patients in the cohort, it was possible to obtain EEG segments from which definite spectral categorization could be obtained in 58 (84%) patients. Distribution of categories for these patients was as follows: six patients had spectral features consistent with A type only, 22 patients had B type, 22 patients had C type, and eight patients had D type; four additional patients had a predominant alpha feature, one patient had a broad spectral band at 11–14 Hz, and the six remaining patients had no specific predominant features.
Association Between ‘ABCD’ Classification and Clinical Characteristics
Data on ‘ABCD’ classification and distribution of clinical variables are shown in Table 1. The mean age was not different between AB and CD groups. There was strong evidence of differences in clinical (HH and WFNS) grades between AB and CD classifications. There was weak inverse correlation between clinical grades and ‘ABCD’ classification (Spearman coefficient of correlation − 0.44 [WFNS] and − 0.41 [HH]). The best GCS score on the day of the EEG was lower in the AB group. However, the mean modified Fisher Scale score was similar between groups.
There was no evidence of differences between spectral categories with respect to anytime high intracranial pressure, hypotension, hypoperfusion on CT perfusion, or preceding sedation. However, concurrent fever was more common in the AB group compared with the CD group.
Univariate receiver operating characteristic curves demonstrated similar areas under the curve (AUCs) for ‘ABCD’ classification and clinical severity grades for predicting functional outcome (ABCD 0.7562, HH 0.7630, WFNS 0.7469) but significantly greater AUCs for mortality (ABCD 0.7585, HH 0.6989, WFNS 0.5872) (Fig. 3a, b).
Mortality and functional outcome by ‘ABCD’ classification and clinical severity and multivariate modeling for mortality and functional outcome. Univariate ROC for mRS score (a) and mortality (b) by ‘ABCD’ classification and SAH clinical severity grades. Multivariate ROC for dichotomized mRS score (c) and mortality (d) comparing logistic regression models with (model 1) and without (model 2) ‘ABCD’ categorization on an ordinal scale. Other variables included were age, HH score, and stroke. HH Hunt and Hess, mRS modified Rankin Scale, ROC receiver operating characteristic curve, SAH subarachnoid hemorrhage, WFNS World Federation of Neurological Surgeons
Association Between ‘ABCD’ Classification and EEG Characteristics
There was no evidence of association of concurrent antiepileptic drug use, epileptiform discharges, or reactivity seen on EEG between groups. One patient had a seizure in the CD group, as opposed to none in the AB group.
Outcomes Analyses
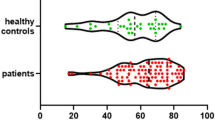
Summary data on outcomes are presented in Table 2. There was strong evidence of differences in GCS score at discharge, functional outcome by mRS score, and mortality between the AB and CD groups. In the CD group, compared with the AB group, the GCS score at discharge was higher, functional outcome by mean mRS score was higher, the mortality rate was lower, and good outcome by dichotomized mRS score was more likely (Fig. 4). Length of stay and presence of stroke on final in-hospital imaging were not different between groups. Nonsurvivors had significantly worse ‘ABCD’ categorization than survivors.
Functional Outcome
Functional outcome on an ordinal mRS was significantly better in the CD group compared with the AB group (p < 0.01). Univariate and multivariate analyses of clinical variables associated with functional outcomes, as determined by dichotomized mRS scores, are shown in Table 3. In univariate analyses for functional outcome, by using dichotomized mRS scores, unadjusted odds for poor outcome were 0.38 (95% confidence interval [CI] 0.15–0.96) for good WFNS grade vs. 2.18 (95% CI 1.06–4.45) for poor grade, 0.43 (95% CI 0.20–0.94) for good HH grade vs. 3.50 (95% CI 1.41–8.67) for poor grade, 1.15 (95% CI 0.55–2.42) for absence of fever vs. 1.07 (95% CI 0.52–2.22) for presence of fever, 0.50 (95% CI 0.23–1.07) for CD category vs. 2.86 (95% CI 1.21–6.76) for AB category.
In multivariate analyses, in model 1 (with WFNS grade, fever, and ‘ABCD’ category) and model 2 (with HH grade, fever, and ‘ABCD’ category), fever was not significantly associated with functional outcome. Adjusted odds ratios (ORs) for poor outcome for poor WFNS grade were 5.47 (95% CI 1.43–20.76) and for poor HH grade were 7.25 (95% CI 2.0–26.7); adjusted OR for poor outcome for AB group were 5.45 (95% CI 1.46–20.36) in the model with WFNS, and 5.52 (1.41–21.70) in the model with HH. Strata-specific adjusted ORs are shown in Table 4. In the linear regression model using WFNS grade for clinical severity, with CD classification with a good WFNS clinical grade as the reference, AB classification with a poor WFNS grade had the highest adjusted OR for poor outcome (45.2 [95% CI 5.6–365.6]), whereas poor clinical grade with CD classification had a lower adjusted OR (16.7 [95% CI 2.2–127.4]) for poor outcome compared with good WFNS grade and AB categorization (29.0 [95% CI 2.3–362.9]). Similarly, in the model with HH grade included and CD classification with a good HH grade as the reference, poor clinical grade with AB categorization had the highest adjusted OR for poor outcome. There was no evidence of significant interaction between clinical grades and ABCD classification.
Mortality
Univariate and multivariate analyses of variables associated with mortality are shown in Table 3. In the univariate analysis, death was associated with poor clinical grades and ‘ABCD’ classification but not with fever; unadjusted odds for death were 0.10 (95% CI 0.02–0.42) for good WFNS grade vs. 0.28 (95% CI 0.13–0.63) for poor grade, 0.07 (95% CI 0.01–0.30) for good HH grade vs. 0.42 (95% CI 0.18–0.96) for poor grade, 0.26 (95% CI 0.11–0.64) for presence of fever vs. 0.16 (95% CI 0.05–0.45) for absence of fever, and 0.40 (95% CI 0.17–0.90) for AB category vs. 0.07 (95% CI 0.02–0.30) for CD category. In multivariate analyses using WFNS clinical grade, ‘ABCD’ classification had the highest adjusted OR for death (6.3 [95% CI 1.02–39.16]). However, in the model using HH grade, clinical grade was significantly associated with death, and ‘ABCD’ classification showed a trend of association (p = 0.06). In strata-specific analyses with both WFNS and HH grades, poor clinical grade with AB category had the highest mortality rate (Table 4). There was evidence of some interaction of ‘ABCD’ classification with WFNS grade (p = 0.03) but not HH grade (p = 0.21) in these analyses.
In the sensitivity analysis using a logistic regression model with age, HH grade, and evidence of stroke, addition of ‘ABCD’ classification significantly improved the model for both functional outcome and mortality. For the mRS score, the AUC improved from 0.7652 (good) to 0.8141 (excellent), and for mortality, the AUC improved from 0.7894 (good) to 0.8383 (excellent) (Fig. 3c, d).
Details of justification for withdrawal of life-sustaining therapy were as follows: clinical deterioration and loss of pupillary function despite maximal therapy, multifocal cerebral infarctions and status epilepticus, multiorgan failure and dependence on continuous renal replacement therapy, bilateral middle cerebral artery infarctions, bilateral hemispheric infarctions, persistent coma at 23 days post injury and dependence on continuous renal replacement therapy, poor examination results with right posterior middle cerebral artery infarction, multifocal cerebral infarctions, and poor examination results and advanced age.
Discussion
In this study, we have examined whether there is evidence of disruption in corticothalamic integrity of the anterior forebrain mesocircuit following SAH and whether this is associated with clinical outcomes. Results from our study suggest that broad corticothalamic functional denervation occurs following SAH in some patients and is associated with poor functional outcome and higher probability of death. Furthermore, this denervation occurs early after SAH, and its spectral signature may be used as an objective biomarker for early brain injury. Importantly, the spectral signature provided a statistically independent measure from clinical SAH grade.
Compared with patients with CD type spectral patterns on EEG, those with AB type patterns had lower GCS scores at discharge, higher mRS scores, a higher proportion of poor functional outcome by dichotomized mRS score, and higher mortality. When ‘ABCD’ classification is combined with clinical grade, there is a sequentially increased probability for poor functional outcome and death: from good grade with an intact corticothalamic network to poor grade with severely disrupted or absent connectivity.
Disruption in neuronal cells and pathways has been demonstrated in acute stages of SAH. Neurofilament-heavy (NfH) chains, related to axonal degeneration, have been described in cerebrospinal fluid following SAH and correlate with initial GCS score and WFNS grade and inversely with the Glasgow Outcome Scale score [37]. Patients with poor functional outcome had higher NfH chain levels early, which increased at around day 7, followed by a secondary increase in levels at around 2 weeks related to delayed cerebral ischemia. Although the majority of patients in this particular study underwent surgical clipping, the ninefold higher concentration of NfH chains in patients with poor outcome seems to suggest worse axonal injury in those patients. Furthermore, as demonstrated by the partially delayed time course, axonal damage that leads to network interruption may be independent of perfusion [38, 39]. Similar findings were found with both cerebrospinal fluid and plasma concentrations of neurofilament-light chains [40, 41].
It is therefore relevant to be able to identify and validate an objective bedside marker for structural injury in arousal networks based solely on passive measures; thus, D type spectrum in a patient with SAH with low clinical grade may identify an important dissociation. Equally, it is important to distinguish whether there is widespread thalamic or neocortical neuronal death, thalamocortical deafferentation, or subset network (circuit) dysfunction [14]. A mixture of these might be possibly seen following SAH and account for degrees of arousal disturbances. A specific signature, as demonstrated within the ‘ABCD’ model, may be helpful in discerning severe deafferentation versus preserved connectivity, which can help assess early neurological status.
Our data illustrate intermediate association between clinical severity grades on initial neurological assessment following SAH and ‘ABCD’ classification and may explain why presenting clinical grades do not always relate absolutely with neurological outcome. There are likely other factors that play a role in the initial neurological condition after hemorrhage, such as hydrocephalus, increased intracranial pressure, hypoperfusion, etc. Moreover, clinical grades are static and graded at admission, whereas EEG classification is dynamic.
This study has several implications. Firstly, it provides evidence of corticothalamic disruption following SAH; the severity is related to severity of hemorrhage at presentation. However, given several confounders at initial ictus, the spectral signature provides an objective measure of injury severity and can serve as a mechanistic biomarker for early brain injury. The spectral signature can potentially be visualized via a simplified automated spectral display in real time and can serve as an additional and useful objective bedside tool.
Secondly, ‘ABCD’ pattern is associated with, and predicts, outcomes with good discrimination. This may aid early decision-making, especially when unknown confounders may make prognostication incomplete or uncertain. Withdrawal of life-support treatment occurs often in patients with SAH with poor neurological examination results yet may be on unconvincing grounds for the family and physician.
Thirdly, spectral pattern may be used to measure secondary deterioration or effect of therapies that may be deemed beneficial. Several drugs, including zolpidem, amantadine, and L-dopa, have been shown to rebalance excitatory–inhibitory balance in the forebrain mesocircuit and result in changes in consciousness following acute brain injury [42]. These can be tested and monitored by using connectivity studies in real time in acute settings. If such a real-time tool is developed, it may also be beneficial in demonstrating whether other interventions, such as those aimed at decreasing intracranial pressure or increasing cerebral perfusion, impact an otherwise unconscious patient. Additionally, because cEEG was generally performed several days after initial injury, it may be useful to evaluate changes in spectra over time rather than in a single, static time point.
Lastly, understanding the mechanisms of loss of consciousness networks following SAH is key to developing new-generation therapies that may modify outcomes following this devastating brain injury. Understanding network connectivity at bedside without the use of functional MRI is certainly helpful and opens up a new field of investigation.
This is a retrospective study; therefore, biases in treatment of patients cannot be ruled out. Certainly, patient selection is biased toward those who underwent cEEG because of clinical indications, as patients with both excellent and dismal clinical examination findings might not have had a clinically appropriate reason for the test to be performed. We have not examined concordance between cognitive function and EEG spectra in individual patients. However, in the pooled cohort, there is a tendency for worse outcomes in patients with poor GCS scores and poor category spectra.
The median time to EEG was 3 days from ictus. The assumption is that most of our findings are secondary to ictal events; however, it cannot be ruled out that other events after SAH may also contribute to corticothalamic disruption. The incidence of strokes was low in our cohort, but hypoperfusion seen on CT perfusion was not uncommon; although these occurred later in the stay, there may be some relationship between them. Therefore, these findings, as all others in complex critically ill patients, must be viewed with the knowledge that in such patients, residual confounding may remain.
It is well known that multiple physiologic and pharmacologic factors can affect EEG background, including sedating medication, which can cause both diffuse delta activity as well as excess alpha and beta activity. To minimize the potential effects of sedating medications, EEG segments were reviewed during morning wake-up neurological examinations, for which sedation holidays were always performed; however, a washout period bias may exist.
Withdrawal of life-sustaining therapy significantly limits interpretation of mortality data. In our study, 9 of 12 deaths occurred in this setting and 11 of 12 deaths occurred during the initial hospitalization. After examining in detail the degree of injury for these patients at the time of death, we concluded that they would not have an impact on functional outcomes as evaluated in this study. And it remains rather likely that long-term survival in these patients was doubtful. The mRS score is retrospectively abstracted and may carry a bias, although retrospective evaluation directly from patients has been validated as accurate for use in stroke research [43]. Lastly, small sample size limited our ability to examine each category of the ABCD model separately, necessitating combination of AB and CD spectral types.
Conclusions
Injury to corticothalamic connectivity, as determined by power spectra on EEG, occurs early after aneurysmal SAH. These findings provide a novel basis for understanding mechanisms of early brain injury after SAH, and the categorized spectral signatures can serve as a useful biomarker. Furthermore, ‘ABCD’ classification significantly improved accuracy of prediction of functional outcome when combined with known predictors. Further prospective studies are needed to validate this approach, along with using it to measure effects of interventions in improving outcomes after SAH.
References
Pobereskin LH. Incidence and outcome of subarachnoid haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry. 2001;70(3):340–3.
Cesarini KG, Hårdemark HG, Persson L. Improved survival after aneurysmal subarachnoid hemorrhage: review of case management during a 12-year period. J Neurosurg. 1999;90(4):664–72.
Luo YC, Shen CS, Mao JL, Liang CY, Zhang Q, He ZJ. Ultra-early versus delayed coil treatment for ruptured poor-grade aneurysm. Neuroradiology. 2015;57(2):205–10.
Dhandapani S, Singh A, Singla N, et al. Has outcome of subarachnoid hemorrhage changed with improvements in neurosurgical services? Stroke. 2018;49(12):2890–5.
Naval NS, Chang T, Caserta F, Kowalski RG, Carhuapoma JR, Tamargo RJ. Improved aneurysmal subarachnoid hemorrhage outcomes: a comparison of 2 decades at an academic center. J Crit Care. 2013;28(2):182–8.
La Pira B, Singh TD, Rabinstein AA, Lanzino G. Time trends in outcomes after aneurysmal subarachnoid hemorrhage over the past 30 years. Mayo Clin Proc. 2018;93(12):1786–93.
Carpenter KL, Czosnyka M, Jalloh I, et al. Systemic, local, and imaging biomarkers of brain injury: more needed, and better use of those already established? Front Neurol. 2015;18(6):26.
Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28(1):14–20.
Dengler NF, Diesing D, Sarrafzadeh A, Wolf S, Vajkoczy P. The barrow neurological institute scale revisited: predictive capabilities for cerebral infarction and clinical outcome in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2017;81(2):341–9.
Rosenthal ES, Biswal S, Zafar SF, et al. Continuous electroencephalography predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective study of diagnostic accuracy. Ann Neurol. 2018;83(5):958–69.
Maciel CB, Gilmore EJ. Seizures and Epileptiform patterns in SAH and their relation to outcomes. J Clin Neurophysiol. 2016;33(3):183–95.
Allen BB, Forgacs PB, Fakhar MA, et al. Association of seizure occurrence with aneurysm treatment modality in aneurysmal subarachnoid hemorrhage patients. Neurocrit Care. 2018;29(1):62–8.
Sitt JD, King JR, El Karoui I, et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain. 2014;137(Pt 8):2258–70.
Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33(1):1–9.
Forgacs PB, Frey HP, Velazquez A, et al. Dynamic regimes of neocortical activity linked to corticothalamic integrity correlate with outcomes in acute anoxic brain injury after cardiac arrest. Ann Clin Transl Neurol. 2017;4(2):119–29.
Scannell JW, Burns GA, Hilgetag CC, O’Neil MA, Young MP. The connectional organization of the cortico-thalamic system of the cat. Cereb Cortex. 1999;9(3):277–99.
MacNeil MA, Lomber SG, Payne BR. Thalamic and cortical projections to middle suprasylvian cortex of cats: constancy and variation. Exp Brain Res. 1997;114(1):24–32.
Adams JH, Graham DI, Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain. 2000;123(Pt 7):1327–38.
Maxwell WL, MacKinnon MA, Smith DH, McIntosh TK, Graham DI. Thalamic nuclei after human blunt head injury. J Neuropathol Exp Neurol. 2006;65(5):478–88.
Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39(2–3):107–40.
Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129(1):105–18.
Fridman EA, Beattie BJ, Broft A, Laureys S, Schiff ND. Regional cerebral metabolic patterns demonstrate the role of anterior forebrain mesocircuit dysfunction in the severely injured brain. Proc Natl Acad Sci USA. 2014;111(17):6473–8.
Chen P, Xie Q, Wu X, et al. Abnormal effective connectivity of the anterior forebrain regions in disorders of consciousness. Neurosci Bull. 2018;34(4):647–58.
Mikell CB, Banks GP, Frey HP, et al. Frontal networks associated with command following after hemorrhagic stroke. Stroke. 2015;46(1):49–57.
Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. 2006;313(5792):1402.
Schiff ND. Cognitive motor dissociation following severe brain injuries. JAMA Neurol. 2015;72(12):1413–5.
Edlow BL, Chatelle C, Spencer CA, et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain. 2017;140(9):2399–414.
Claassen J, Velazquez A, Meyers E, et al. Bedside quantitative electroencephalography improves assessment of consciousness in comatose subarachnoid hemorrhage patients. Ann Neurol. 2016;80(4):541–53.
Claassen J, Doyle K, Matory A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med. 2019;380:2497–505.
Forgacs PB, Conte MM, Fridman EA, et al. Preservation of electroencephalographic organization in patients with impaired consciousness and imaging-based evidence of command-following. Ann Neurol. 2014;76(6):869–79.
Mani R, Arif H, Hirsch LJ, Gerard EE, LaRoche SM. Interrater reliability of ICU EEG research terminology. J Clin Neurophys. 2012;29(3):203–12.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;304(7872):81–4.
Andersen CR, Fitzgerald E, Delaney A, Finfer S. A systematic review of outcome measures employed in aneurysmal subarachnoid hemorrhage (aSAH) clinical research. Neurocrit Care. 2019;30(3):534–41.
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711–37.
Thomson DJ. Spectrum estimation and harmonic analysis. Proc IEEE. 1982;70(9):1055–96.
Schiff ND, Nauvel T, Victor JD. Large-scale brain dynamics in disorders of consciousness. Curr Opin Neurobiol. 2014;25:7–14.
Petzold A, Keir G, Kay A, Kerr M, Thompson EJ. Axonal damage and outcome in subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2006;77(6):753–9.
Nelson S, Edlow BL, Wu O, Rosenthal ES, Westover MB, Rordorf G. Default mode network perfusion in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2016;25(2):237–42.
Mangat H, Ivanidze J, Mao X, et al. Selective frontal lobe metabolic dysfunction after subarachnoid hemorrhage—evidence for flow-metabolism uncoupling? Neurology 2017; 88(suppl 16):P5.067.
Zanier ER, Refai D, Zipfel GJ, et al. Neurofilament light chain levels in ventricular cerebrospinal fluid after acute aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2011;82(2):157–9.
Hviid CVB, Lauridsen SV, Gyldenholm T, Sunde N, Parkner T, Hvas AM. Plasma neurofilament light chain is associated with poor functional outcome and mortality rate after spontaneous subarachnoid hemorrhage. Transl Stroke Res. 2020;11(4):671–7.
Fridman EA, Schiff ND. Neuromodulation of the conscious state following severe brain injuries. Curr Opin Neurobiol. 2014;29:172–7.
Wang M, Rajan SS, Jacob AP, et al. Retrospective collection of 90-day modified Rankin Scale is accurate. Clin Trials. 2020;17(6):637–43.
Funding
Dr. Forgacs reports grants from the National Institute of Neurological Disorders and Stroke (Grant No. K23-NS096222), grants from the Leon Levy Neuroscience Fellowship Award, grants from the National Center for Advancing Translational Sciences (Grant No. UL1-TR000043), grants from the Rockefeller Clinical and Translational Science Award Program, and grants from the Stavros Niarchos Foundation during the conduct of the study. Dr. Allen has nothing to disclose. Ms. Wu reports grants from the National Center for Advancing Translational Sciences. Dr. Gerber reports grants from the National Center for Advancing Translational Sciences. Dr. Boddu has nothing to disclose. Dr. Fakhar has nothing to disclose. Dr. Stieg has nothing to disclose. Dr. Schiff reports grants from the National Institute of Neurological Disorders and Stroke (Grant No. RO1-HD051912), grants from the James S. McDonnell Foundation, and grants from the Jerold B. Katz Foundation. Dr. Mangat has nothing to disclose.
Author information
Authors and Affiliations
Contributions
Peter B. Forgacs, MD, designed and conceptualized the study, analyzed and interpreted the data, and drafted the manuscript for intellectual content. Baxter B. Allen, MD, designed and conceptualized the study, analyzed and interpreted the data, and drafted and revised the manuscript for intellectual content. Xian Wu, MPH, analyzed the data and drafted the manuscript for intellectual content. Linda M. Gerber, PhD, analyzed the data and drafted the manuscript for intellectual content. Srikanth Boddu, MD, contributed a major role in the acquisition of data and revised the manuscript for intellectual content. Malik Fakhar, MD, contributed a major role in the acquisition of data. Phillip E. Stieg, PhD, MD, designed and conceptualized the study. Nicholas D. Schiff, MD, designed and conceptualized the study and revised the manuscript for intellectual content. Halinder S. Mangat, MD, designed and conceptualized the study, interpreted the data, and drafted and revised the manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval/informed consent
The institutional review board for research integrity at Weill Cornell Medicine approved the study, and all ethical guidelines were adhered to.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Forgacs, P.B., Allen, B.B., Wu, X. et al. Corticothalamic Connectivity in Aneurysmal Subarachnoid Hemorrhage: Relationship with Disordered Consciousness and Clinical Outcomes. Neurocrit Care 36, 760–771 (2022). https://doi.org/10.1007/s12028-021-01354-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01354-6