Abstract
Background
The etiology of altered consciousness in patients with high-grade aneurysmal subarachnoid hemorrhage (SAH) is not thoroughly understood. We hypothesized that decreased cerebral blood flow (CBF) in brain regions critical to consciousness may contribute.
Methods
We retrospectively evaluated arterial-spin labeled (ASL) perfusion magnetic resonance imaging (MRI) measurements of CBF in 12 patients with aneurysmal SAH admitted to our neurocritical care unit. CBF values were analyzed within gray matter nodes of the default mode network (DMN), whose functional integrity has been shown to be necessary for consciousness. DMN nodes studied were the bilateral medial prefrontal cortices, thalami, and posterior cingulate cortices. Correlations between nodal CBF and admission Glasgow Coma Scale (GCS) score, admission Hunt and Hess (HH) class, and GCS score at the time of MRI (MRI GCS) were tested.
Results
Spearman’s correlation coefficients were not significant when comparing admission GCS, admission HH, and MRI GCS versus nodal CBF (p > 0.05). However, inter-rater reliability for nodal CBF was high (r = 0.71, p = 0.01).
Conclusions
In this retrospective pilot study, we did not identify significant correlations between CBF and admission GCS, admission HH class, or MRI GCS for any DMN node. Potential explanations for these findings include small sample size, ASL data acquisition at variable times after SAH onset, and CBF analysis in DMN nodes that may not reflect the functional integrity of the entire network. High inter-rater reliability suggests ASL measurements of CBF within DMN nodes are reproducible. Larger prospective studies are needed to elucidate whether decreased cerebral perfusion contributes to altered consciousness in SAH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pathophysiologic basis of altered consciousness in patients with high-grade aneurysmal subarachnoid hemorrhage (SAH) is incompletely understood. Though increased intracranial pressure, hydrocephalus, and seizures may all contribute to the pathogenesis of decreased consciousness, one hypothesis is that hypoperfusion is the primary etiologic factor [1]. Several lines of evidence support this hypothesis. Cerebral ischemia in patients with SAH is associated with poor neurological examinations [2–5]. Cerebral blood flow (CBF) as measured by arterial injection of radioactive oxygen-15 and of xenon-133 has been demonstrated to be inversely associated with Hunt Hess (HH) class [1, 6, 7]. Decreased uptake of technetium-99 M labeled d,l-hexamethyl-propylene amine oxime, which reflects CBF, was associated with lower levels of consciousness in SAH patients [8]. In transcranial Doppler ultrasound studies performed within 24 h of SAH onset, middle cerebral artery blood flow velocities, which correlate with CBF [9], were inversely correlated with the World Federation of Neurologic Surgeons scale [10].
Yet despite these preliminary data suggesting an association between low cerebral perfusion and altered consciousness in patients with SAH, the perfusion hypothesis has been difficult to test because of challenges associated with obtaining perfusion data in this patient population. Perfusion computed tomography (CT) requires iodinated contrast, carries a risk of radiation exposure, is subject to inter-observer variability, and may over- or underestimate areas of ischemia [11]. Furthermore, a recent perfusion CT study identified correlations between the World Federation of Neurology Surgeons scale and the perfusion parameters time-to-peak and mean transit time but not with CBF [12]. Single-photon emission CT does not allow for quantification of CBF. Positron emission tomography may be time-consuming and not routinely available in clinical centers [13]. Optical imaging of CBF is a non-invasive bedside method but has limited spatial coverage [14]. Perfusion magnetic resonance imaging (MRI) sequences that use gadolinium-based contrast have limited use in patients with renal dysfunction and can lead to errors in perfusion parameters if contrast leaks into the extravascular space [15].
On the other hand, arterial-spin labeling (ASL) is an MRI technique that relies on an endogenous contrast to generate a perfusion map of CBF [16]. In an ASL perfusion study, the arterial blood is labeled using a radiofrequency pulse as it passes through the carotid and vertebral arteries, and the dissipation of labeled proton spins is then measured in the distal cerebral tissues to provide a measure of CBF. The ASL sequence has been shown to detect perfusion changes in SAH [17–19]. In addition to the benefit of not requiring an injected contrast agent, ASL measurements of CBF can be obtained rapidly [20] and have high inter-rater reliability [15, 21, 22].
In this study, we performed ASL measurements of CBF in patients with SAH to test the hypothesis that altered levels of consciousness are associated with decreased cortical CBF. We also strove to demonstrate that ASL-based measurements of CBF are reproducible. We focused our CBF measurements on the default mode network (DMN), a resting-state network whose connectivity has been shown to be necessary for consciousness [23–26]. The DMN comprises gray matter modes that include the medial prefrontal cortex, posterior cingulate/precuneus, and thalamus [27, 28]. DMN structural and functional connectivity appears to be decreased in proportion to degree of altered consciousness in patients with severe brain injury [23–25]. In addition, DMN connectivity may portend a higher likelihood of functional recovery in patients with acute disorders of consciousness due to cardiac arrest [26].
Methods
Patient Demographics and Clinical Characteristics
We performed a retrospective analysis of a pilot cohort (n = 12) of high-grade (Fisher 3–4) SAH patients admitted to our neurocritical care unit who underwent brain MRI with ASL from August 2013 to February 2015. All patients admitted to our neurocritical care unit with the diagnosis of SAH were eligible for inclusion. MRIs were performed for clinical purposes at the discretion of the treating clinician. SAH patients who underwent MRI with ASL during the study time period were identified retrospectively through a prospective database of all SAH patients approved by our hospital’s Institutional Review Board. ASL data were processed for offline analysis under a separate research protocol that was also approved by our hospital’s Institutional Review Board. Demographic and clinical data such as age, sex, aneurysm location, admission HH, and admission Glasgow Coma Scale (GCS) were derived from the prospective database. GCS at the time of MRI (MRI GCS) was ascertained from the neurologic examination described in the daily neurology note in the electronic medical record. Levels of consciousness were defined by each patient’s admission GCS score, MRI GCS, and admission HH class.
ASL Data Acquisition
Patients were scanned on a 3-Tesla Siemens Skyra MRI system (Siemens Medical Solutions, Erlangen, Germany) located in the neurocritical care unit. Perfusion data were obtained using a pulsed ASL sequence (PICORE Q2T) and bolus duration of 700 ms using the following acquisition parameters: 45 control-tag pairs (for one patient there were 5.5 control-tag pairs), echo time = 12 ms, repetition time = 2500 ms, TI = 1800 ms, in-plane spatial resolution = 4.0 × 4.0 mm, slice thickness = 8.0 mm, inter-slice gap = 2.0 mm, acquisition matri× 64 × 64, flip angle = 90˚, field-of-view = 256 mm, 9 slices (for two patients there were 11 slices), and a total acquisition time of 3 min and 52 s. The limited number of slices was a trade-off between minimizing acquisition time and obtaining sufficient brain coverage.
DMN Region of Interest Analysis
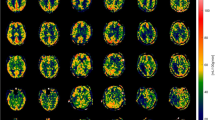
Circular regions-of-interest (ROIs) with a diameter of 1 mm were manually placed on DMN nodes to measure absolute CBF. The bilateral thalami, medial prefrontal cortices, and posterior cingulate cortices were chosen as DMN nodes of interest for the following reasons: (1) they are believed to be the most widely connected nodes within the DMN [27, 28]; (2) ASL-based measurements of CBF within these nodes has been shown to correlate with level of consciousness [22]; and (3) neuroanatomic localization of these network nodes was feasible on the ASL perfusion maps obtained in this study (Fig. 1). Two researchers (B.L.E. and G.R.) independently placed ROIs in the DMN nodes for four of the patients to derive an inter-rater reliability, and one (B.L.E.) placed ROIs for all 12 patients; both researchers were blinded to clinical data.
Statistical Analysis
For each patient, we calculated the mean CBF of the right and left sides of each node. We then tested for associations between mean nodal CBF and admission GCS, admission HH, and MRI GCS using Spearman’s correlation coefficient. Inter-rater reliability was evaluated using Spearman’s correlation coefficient for 12 mean CBF values (3 nodes for 4 patients). GraphPad Prism version 6.05 (GraphPad Software; La Jolla, CA) was used for these statistical analyses. Statistical significance was set at p < 0.05; Bonferroni correction was not applied given that this was a pilot study with a small cohort (n = 12).
Results
Twelve patients met the study inclusion criteria. Patient ages ranged from 40 to 74 years and 8 were female. Patients demonstrated a wide range of GCS and HH scores. Primary aneurysms were located in various areas of the cerebral vasculature, though for one patient (patient 11) no aneurysm was found. All patients were classified as Fisher 3–4. See Table 1 for a complete summary of demographic and clinical data.
The CBF values [in ml/100 g/min] and the mean CBF between the right- and left-sided CBF measurements for each node (thalami, medial prefrontal cortices, and posterior cingulate cortices) are listed in Table 2 for each patient. Table 3 provides the Spearman’s correlation coefficients and p values for each comparison; notably, no p values were significant (p > 0.05). Inter-rater reliability was r = 0.71 (p = 0.01).
Discussion
In this pilot study of 12 patients with SAH, ASL measurements of CBF did not correlate with level of consciousness in patients with SAH. Nonetheless, our study demonstrates that ASL measurements of CBF in a clinical cohort are reproducible and feasibly can be applied to future prospective investigations of cerebral CBF and its role in altered consciousness.
The absence of a correlation between DMN nodal CBF and level of consciousness in this study may be attributable to several different factors that relate to our study design or to the pathophysiology of altered consciousness in patients with SAH. From the standpoint of study design, our data were obtained retrospectively from a small cohort at a single institution. In addition, since the MRIs were performed for clinical indications, the timing of ASL data acquisition could not be standardized. Moreover, some patients with SAH admitted to our neurocritical care unit during the study time period were unable to tolerate MRI (e.g., inability to lie flat for a prolonged period of time, such as in patients with elevated intracranial pressure). As a result, not all SAH patients in our neurocritical care unit were included in the study. In addition, with its high reproducibility [21], pseudo-continuous ASL may provide better results than pulsed ASL; however, the MRIs in our study were performed for clinical reasons and only the pulsed ASL sequence is clinically available on our neurocritical care unit’s scanner. Finally, since the ASL sequence is known to be sensitive to artifacts, it is possible that patient instrumentation including electroencephalogram leads and external ventricular drains could have contributed to our non-significant results.
From a pathophysiologic standpoint, we may not have identified correlations between DMN nodal CBF and level of consciousness because cerebral hypoperfusion may be transient and may have resolved by the time MRI was performed. For instance, a period of intracranial circulatory arrest that decreases intracerebral circulation and is associated with increased intracranial pressure seems to occur shortly after aneurysmal rupture in SAH [29]. Focal vasoconstriction and accumulation of platelet aggregates in blood vessels may contribute to this episode of decreased perfusion [30]. Because these processes appear to last only minutes to hours [29, 30], the resulting perfusion deficits may not have been captured on our ASL sequences. Furthermore, it is possible that CBF within individual DMN nodes may not reflect the functional integrity of the entire network. Accordingly, functional connectivity analysis of the DMN using resting-state functional MRI or structural connectivity analysis of the DMN using diffusion tensor tractography may provide more clinically relevant data regarding the potential of the DMN to permit consciousness. Other variables such as systemic blood pressure, intracranial pressure, and presence or absence of vasospasm may have affected CBF data in our study; however, not all patients in the study had these variables or in a consistent format amenable to analysis and so could not be included as regressors. Nonetheless, these variables would be useful in future studies. Finally, it is possible that other pathophysiologic processes (e.g., increased intracranial pressure, hydrocephalus, and/or seizures) are stronger determinants than hypoperfusion in causing low levels of consciousness in high-grade SAH patients.
In considering how to further elucidate the potential role of hypoperfusion in altered consciousness, it is important to consider not only the study design limitations addressed above, but also the role of the DMN in consciousness and the importance of the specific DMN nodes analyzed in this study. Although an intact DMN has been shown to be necessary for consciousness, it may not be sufficient [25]. Thus, our CBF measurements may not have yielded significant correlations with levels of consciousness because we did not investigate CBF within the nodes of other brain networks that may be critical to consciousness, such as the executive control network and/or the salience network [31]. Similarly, it is possible that measuring CBF within other DMN nodes, in particular the inferior parietal lobule [27], would have increased the sensitivity of our analyses for detecting CBF correlations with consciousness. We limited our analyses to a small number of central DMN nodes primarily because these nodes were readily identifiable on our patients’ ASL perfusion maps. Due to the limited slice coverage (90 mm) in our intensive care population and the retrospective nature of our study, ASL slices were not always positioned to include other nodes such as the inferior parietal lobule since the slices were positioned for clinical purposes. Future studies involving fast whole head ASL acquisition protocols [32] will allow us to better interrogate additional DMN nodes, and hence, enable us to perform a more comprehensive analysis of the role of CBF changes in altered consciousness. Finally, it is possible that global cortical CBF may be the most robust predictor of level of consciousness and might provide a more relevant perfusion biomarker to test the hypothesis that altered consciousness in SAH is associated with decreased CBF [19, 22]; we did not analyze global cortical CBF because the spatial resolution of the ASL sequence was insufficient and because of artifacts related to intracranial hardware.
Conclusion
Overall, our findings do not support the hypothesis that perfusion deficits within central nodes of the DMN are associated with low levels of consciousness in SAH patients. While it is possible that hypoperfusion may not be an important contributor to altered consciousness in high-grade SAH patients, our study had several limitations, suggesting that larger prospective studies are needed to elucidate the potential pathophysiologic role of hypoperfusion in patients with SAH. The acquisition of ASL data on a clinical MRI scanner and the high inter-rater reliability of ASL-based CBF measurements demonstrated here suggest that ASL is a feasible and reliable tool for assessing CBF in patients with high-grade SAH.
References
Kobayashi K, Ishii R, Koike T, Ihara I, Kameyama S. Cerebral blood flow and metabolism in patients with ruptured aneurysms. Acta Neurol Scand Suppl. 1979;60:492–3.
Hadeishi H, Suzuki A, Yasui N, Hatazawa J, Shimosegawa E. Diffusion-weighted magnetic resonance imaging in patients with subarachnoid hemorrhage. Neurosurgery. 2002;50:741–8.
Sato K, Shimizu H, Fujimura M, Inoue T, Matsumoto Y, Tominaga T. Acute-stage diffusion-weighted magnetic resonance imaging for predicting outcome of poor-grade aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2010;30:1110–20.
Wartenberg KE, Sheth SJ, Schmidt JM, et al. Acute ischemic injury on diffusion-weighted magnetic resonance imaging after poor grade subarachnoid hemorrhage. Neurocrit Care. 2011;14:407–15.
Frontera JA, Ahmed W, Zach V, et al. Acute ischaemia after subarachnoid hemorrhage, relationship with early brain injury and impact on outcome: a prospective quantitative MRI study. J Neurol Neurosurg Psychiatry. 2015;86:71–8.
Grubb RL Jr, Raichle ME, Eichling JO, Gado MH. Effects of subarachnoid hemorrhage on cerebral blood volume, blood flow, and oxygen utilization in humans. J Neurosurg. 1977;46:446–53.
Ishii R. Regional cerebral blood flow in patients with ruptured intracranial aneurysms. J Neurosurg. 1979;50:587–94.
Hasan D, van Peski J, Loeve I, Krenning EP, Vermeulen M. Single photon emission computed tomography in patients with acute hydrocephalus or with cerebral ischaemia after subarachnoid hemorrhage. J Neurol Neurosurg Psychiatry. 1991;54:490–3.
Bishop CC, Powell S, Rutt D, Browse NL. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke. 1986;17:913–5.
Miranda P, Lagares A, Alen J, Perez-Nunez A, Arrese I, Lobato RD. Early transcranial Doppler after subarachnoid hemorrhage: clinical and radiological correlations. Surg Neurol. 2006;65:247–52.
Lui YW, Tang ER, Allmendinger AM, Spektor V. Evaluation of CT perfusion in the setting of cerebral ischemia: patterns and pitfalls. AJNR Am J Neuroradiol. 2010;31:1552–63.
Lagares A, Cicuendez M, Ramos A, et al. Acute perfusion changes after spontaneous SAH: a perfusion CT study. Acta Neurochir. 2012;154:405–12.
Hirano T. Searching for salvageable brain: the detection of ischemic penumbra using various imaging modalities? J Stroke Cerebrovasc Dis. 2014;23:795–8.
Kim MN, Edlow BL, Durduran T, et al. Continuous optical monitoring of cerebral hemodynamics during head-of-bed manipulation in brain-injured adults. Neurocrit Care. 2014;20:443–53.
Gunther M. Perfusion imaging. J Magn Reson Imaging. 2014;40:269–79.
Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45.
Aoyama K, Fushimi Y, Okada T, et al. Detection of symptomatic vasospasm after subarachnoid haemorrhage: initial findings from single time-point and serial measurements with arterial spin labeling. Eur Radiol. 2012;22:2382–91.
Kelly ME, Rowland MJ, Okell TW, et al. Pseudo-continuous arterial spin labelling MRI for non-invasive, whole-brain, serial quantification of cerebral blood flow following aneurysmal subarachnoid hemorrhage. Transl Stroke Res. 2013;4:710–8.
Labriffe M, Ter Minassian A, Pasco-Papon A, N’Guyen S, Aube C. Feasibility and validity of monitoring subarachnoid hemorrhage by a noninvasive MRI imaging perfusion technique: pulsed arterial spin labelling (PASL). J Neuroradiol. 2015;. doi:10.1016/j.neurad.2015.04.001.
Fernandez-Seara MA, Edlow BL, Hoang A, Wang J, Feinberg DA, Detre JA. Minimizing acquisition time of arterial spin labeling at 3T. Magn Reson Med. 2008;59:1467–71.
Chen Y, Wang DJ, Detre JA. Test-retest reliability of arterial spin labeling with common labeling strategies. J Magn Reson Imaging. 2011;33:47–57.
Liu AA, Voss HU, Dyke JP, Heier LA, Schiff ND. Arterial spin labeling and altered cerebral blood flow patterns in the minimally conscious state. Neurology. 2011;77:1518–23.
Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133:161–71.
Fernandez-Espejo D, Soddu A, Cruse D, et al. A role for the default mode network in the bases of disorders of consciousness. Ann Neurol. 2012;72:335–43.
Norton L, Hutchison RM, Young GB, Lee DH, Sharpe MD, Mirsattari SM. Disruptions of functional connectivity in the default mode network of comatose patients. Neurology. 2012;78:175–81.
Koenig MA, Holt JL, Ernst T, et al. MRI default mode network connectivity is associated with functional outcome after cardiopulmonary arrest. Neurocrit Care. 2014;20:348–57.
Buckner RL, Andrews-Hanna JR, Schacter DL, et al. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38.
Demertzi A, Soddu A, Laureys S. Consciousness supporting networks. Curr Opin Neurobiol. 2013;23:239–44.
Grote E, Hassler W. The critical first minutes after subarachnoid hemorrhage. Neurosurgery. 1988;22:654–61.
Friedrich V, Flores R, Muller A, Sehba FA. Luminal platelet aggregates in functional deficits in parenchymal vessels after subarachnoid hemorrhage. Brain Res. 2010;1354:179–87.
Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:1249–56.
Gunther M, Oshio K, Feinberg DA. Single-shot 3D imaging techniques improve arterial spin labeling perfusion measurements. Magn Reson Med. 2005;54:491–8.
Acknowledgments
This work was conducted with support from Harvard Catalyst and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. The authors also acknowledge Ms. Camille A. Spencer, who assisted with the statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Westover is supported by the following grants: NIH-NINDS 1K23NS090900, The Rappaport Foundation, and the Andrew David Heitman Neuroendovascular Research Fund. Drs. Nelson, Edlow, Wu, Rosenthal, and Rordorf have no conflict of interest.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institution and with the 1964 Helsinki declaration and its later amendments.
Rights and permissions
About this article
Cite this article
Nelson, S., Edlow, B.L., Wu, O. et al. Default Mode Network Perfusion in Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 25, 237–242 (2016). https://doi.org/10.1007/s12028-016-0244-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-016-0244-z