Abstract
Background
In medically refractory vasospasm, invasive intervention may be required. A commonly used approach is intra-arterial (IA) drug infusion. Although calcium channel blockers (CCBs) have been widely applied in this setting, studies comparing their efficacies and durations of action have been few. This study was performed to compare attributes of three CCBs (nicardipine, nimodipine, and verapamil), focusing on duration of the vasodilatory action based on angiography.
Methods
Vasospasm was produced in New Zealand white rabbits (N = 22) through experimentally induced subarachnoid hemorrhage and confirmed in each via conventional angiography, grouping them by IA-infused drug. After chemoangioplasty, angiography was performed hourly for 5 h to compare dilated and vasospastic arterial diameters. Drug efficacy, duration of action, and changes in mean arterial pressure (relative to baseline) were analyzed by group.
Results
Effective vasodilation was evident in all three groups immediately after IA drug infusion. The vasodilative effects of nimodipine and nicardipine peaked at 1 h and were sustained at 2 h, returning to initial vasospastic states at 3 h. In verapamil recipients, effects were more transient by comparison, entirely dissipating at 1 h. Only the nicardipine group showed a significant 3-h period of lowered blood pressure.
Conclusions
Although nimodipine and nicardipine proved longer acting than verapamil in terms of vasodilation, their effects were not sustained beyond 2 h after IA infusion. Further study is required to confirm the vasodilatory duration of IA CCB based on perfusion status, and an effort should be made to find new alternative to extend the duration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vasospasm after rupture of cerebral aneurysms is the primary cause of heightened patient morbidity and mortality and is a relatively common complication of subarachnoid hemorrhage (SAH) seen by angiogram in two-thirds of patients with rupture and one-third of those with clinical symptoms [1, 2]. Despite rapid advancements made in surgical and endovascular treatments of cerebral aneurysms, mortality/morbidity due to vasospasm has remained unchanged. The etiology of vasospasm is thus poorly understood, leaving a void in definitive treatments for severely symptomatic patients who are then facing delayed cerebral ischemia.
Conservative treatment is a priority in instances of vasospasm, but oral nimodipine is the only agent to date with known benefits [1,2,3]. Other interventions, including intra-arterial (IA) chemoangioplasty, intrathecal drug infusion, balloon angioplasty, or angioplasty by retrievable stent, have also been performed in severely symptomatic patients refractory to medical treatment [2,3,4]. However, mechanical angioplasty via balloon and/or retrievable stent has very limited application if proximal arterial segments are involved [3, 5, 6]. Although recent use of intrathecal drug infusion is aimed at boosting intracerebral drug concentrations while reducing systemic hypotension [7], cerebrospinal fluid infection may be a related risk.
There are ample studies of chemoangioplasty by IA infusion of drugs, particularly calcium channel blockers (CCBs) and phosphodiesterase inhibitors (e.g., papaverine or milrinone) [3, 5, 6, 8,9,10,11,12,13,14,15,16,17,18,19,20,21]. Whereas most studies attest to their therapeutic efficacy, papaverine use is declining, given its paradoxical vasospasm and adverse reactions [21]. CCBs are otherwise relatively well known and are frequently used for their positive effects [2, 3, 5, 6, 8, 10,11,12,13, 22,23,24,25,26,27], although seldom directly compared. Moreover, there was no consensus that how long the vasodilatory action of IA chemoangioplasty is maintained and how many times a day should the chemoangioplasty be performed to avoid cerebral ischemia. The present investigation was launched to examine characteristics of the three most popular CCBs used for chemoangioplasty: nicardipine, nimodipine, and verapamil. A rabbit animal model enabled side-by-side comparisons, focusing on duration of the vasodilatory action based on angiography.
Materials and Methods
Preparation of Experimental Animals
This study was approved by the Committee for Ethical Animal Experimentation at our institution and was in compliance with all recommendations made. New Zealand white rabbits were selected for testing.
A total of 36 experimental rabbits weighing 2.5–3.0 kg were commercially obtained, without regard to gender. Each fasted for 6 h prior to actual testing, injecting a combination of ketamine (35 mg/kg; Huons Co, Seoul, South Korea) and xylazine (5 mg/kg; Bayer AG, Leverkusen, Germany) intramuscularly at the thigh for anesthesia. Supplemental injections took place every 20 min during experiments, as dictated by animal conditions. Once anesthetized, depilatory cream was applied to inguinal and occipital areas for hair removal, and each animal was secured to the examination table in supine position. All rabbits were skin tested and given intramuscular injections (50 mg/day) of a first-generation cephalosporin (cefazolin; Yuhan Co, Seoul, South Korea) until 3 days after the experiment.
Puncture of Femoral Artery and Insertion of Femoral Introducer
Each anesthetized animal was kept in supine position, using 75% alcohol to sterilize the inguinal area. Femoral artery was first palpated and then exposed within the femoral neurovascular bundle by inguinal incision (~ 20 mm) made under local anesthesia (1% lidocaine; Daihan Pharm Co, Ansan, South Korea). After puncturing the femoral artery (Micropuncture Introducer Set; Cook Medical, Bloomington, IN, USA), a 4-Fr introducer (Fast-Cath; St. Jude Medical, St Paul, MN, USA) was installed via Seldinger technique, secured to femoral artery, and filled with heparin/normal saline solution for subsequent transducer passage (IntelliVue MX700; Philips, Amsterdam, the Netherlands). Blood pressure readings were thus achieved. Once cerebral angiography was completed, the introducer was positioned within subcutaneous space for later follow-up angiography, using 5-0 nylon suture (Ethicon LLC, Guaynabo, Puerto Rico) for skin closure. Three days after inducing SAH of the brain, angiography was again conducted, expedited by the introducer still in place.
SAH Induction in Animal Model
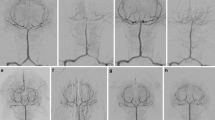
Each anesthetized animal was kept supine, with head rotated 90° to the left, for sterilization at occipital region (75% alcohol). Adjoining occipital–cervical areas were epilated and the head bent for puncture of dura mater under local anesthesia (1% lidocaine), using a 23-G spinal needle under fluoroscopy to pierce occipito-atlantal membrane. Upon first appearance of cerebrospinal fluid, a mixture of a contrast medium and a normal saline (1:1 ratio) was delivered to the cistern to identify subarachnoid space (Fig. 1a). Approximately 2 cc of cerebrospinal fluid was then removed, and 2 cc of arterial blood taken from femoral artery was introduced into subarachnoid space via spinal needle. The animal’s head was thereafter lowered about 30° and stabilized for 20 min [24, 26]. Ultimately, CT (Xper Computed Tomography) was performed to confirm SAH, using an angiography equipment (Fig. 1b).
Cerebral Angiography and IA CCB Infusion
Iso-osmolar contrast (Visipaque 270; GE Healthcare, Chicago, IL, USA) was utilized for cerebral angiography (Allura Xper FD20/10; Philips, Eindhoven, the Netherlands). Both microcatheters (Excelsior SL-10; Stryker Neurovascular, Fremont, CA, USA) and microguidewires (Synchro14; Stryker Neurovascular, Fremont, CA, USA) were engaged for IA drug infusion.
Prior to induction of SAH, cerebral angiography was routinely performed to determine vertebral and basilar arterial calibers at baseline for later referencing (Fig. 2a). On Day 3 of the SAH animal model, angiography was repeated to verify vasospasm of basilar artery (Fig. 2b), defined as > 30% luminal compromise relative to baseline status. Any animals with < 30% arterial narrowing were ineligible for further participation.
Digital subtraction angiogram of vertebrobasilar artery in rabbit: a angiographic image prior to hemorrhage; b follow-up angiographic image 3 days after subarachnoid hemorrhage (black arrow at most severe point of vasospasm); and c angiographic image immediately after intra-arterial (IA) drug infusion
Rabbits showing acceptable vasospasm were randomly assigned to IA drug infusion as follows: group C, nicardipine (0.06 mg/kg; Huons Co, Ansan, South Korea); group M, nimodipine (0.05 mg/kg; Reyon Pharm Co, Seoul, South Korea); or V group, verapamil (0.1 mg/kg; Ilsung Pharm Co, Seoul, South Korea). Each CCB capacity previously determined to have a maximum physiologic effect on arterial vasodilation was selected for this study [28,29,30,31]. The various drugs were individually mixed with normal saline (total volume of 5 cc) for IA infusion by microcatheter over a 10-min period. If blood pressure fell by 20% (relative to baseline), infusion was reduced by 50%. Infusion was stopped if blood pressure decline continued. After administration, initial drug efficacies were assessed by comparing degrees of vasospastic easing at points of severest luminal compromise (see Fig. 2b). Follow-up angiography was performed hourly for 5 h after IA infusion, measuring luminal calibers (Fig. 2c) to confirm that original vasospastic states had returned.
Measuring Arterial Diameters
Arterial diameters were determined via single-blind computer application (Vascular Quantification Software, Philips) for cerebral angiography. Images were selected in arterial phases of angiography with the least motional artifacts, equating diameter with magnitude in transverse plane. Diameters of normal arteries and arteries most affected (i.e., severest vasospasm) were each measured twice within specific time intervals without any information, recording average values. Luminal spasms were graded, based on percentage of baseline arterial diameter lost, whereas vasodilative effects after IA infusion represented percentages of vasospastic reversal.
Statistical Analysis
Continuous variables were each shown as median value (range). For group-wise analyses of vasodilative effects and blood pressure change after IA infusion, the Wilcoxon signed-rank test was used. The Kruskal–Wallis test was applied to group comparisons of vasodilatory degree, duration of action, and blood pressure change after IA infusion. If statistically significant, the Bonferroni correction for multiple comparisons was invoked. All computations were driven by standard software (SPSS v21.0; IBM Corp, Armonk, NY, USA) setting significance at p < 0.05.
Results
Data Summary
Of the 36 available rabbits, 22 served for experimentation (M group, n = 8; C group, n = 7; V group, n = 7). Fourteen rabbits were excluded owing to six deaths and eight induction failures (insufficient vasospasm). No animals required interruption of drug infusion due to blood pressure declines. Compared with basilar artery at baseline (prior to hemorrhage), the mean degree of vasoconstriction overall in the animal model of vasospasm animal was 41.6% (range 31–55%) and the median was 40%. Separately, the medians were as follows: group C, 37% (range 32–55%); group M, 41% (range 31–55%); and group V, 38% (range 37–53%) (see Table 1).
Efficacy of IA CCBs Infusion
Once vasospasm was induced, changes in basilar artery diameters (relative to baseline values) were measured immediately and at hourly intervals after IA infusion, as summarized in Table 1. Immediate vasodilatory effects were regularly observed (group C, 27.0%; group M, 27.1%; group V, 16.1%) and proved statistically significant (group C, p = 0.017; group M, p = 0.012; group V, p = 0.018). One hour later, repeat angiograms showed that vasodilation peaked in groups C (41.3%; p = 0.018) and M (35.6%; p = 0.012) but dissipated in group V, returning to original vasospastic states. At 2 h, significant vasodilative effects were sustained in groups C and M (p = 0.046 and p = 0.051, respectively), only to disappear completely in both groups at the next interval, 3 h after IA infusions (Fig. 3).
Luminal diameters of arteries before and after IA drug infusion. \({\text{Diameter}}\,({\text{\%}}) = \frac{{{\text{Diameter}}\,{\text{of}}\,{\text{basilar}}\,{\text{artery}}\,{\text{in}}\,{\text{each}}\,{\text{time}}\,{\text{zone}}\,{\text{after}}\,{\text{vasospasm}}}}{{{\text{The}}\,{\text{normal}}\,{\text{diameter}}\,{\text{of}}\,{\text{basilar}}\,{\text{artery}}\,{\text{before }}\,{\text{vasospasm}}}}\, \times \, 100\), IA, intra-arterial; C-group, nicardipine; M-group, nimodipine; V-group, verapamil; IADI, immediately after IA drug infusion. *p < 0.05
In group-wise comparisons of maximum vasodilatory efficacy, groups C and M were similar at every periodic testing. Although groups C and M surpassed group V in terms of potency, the difference was not statistically significant (p = 0.193). Vasodilatation was also sustained for 2 h in groups C and M, compared with < 1 h in group V, constituting a substantial departure in duration of action.
Change in Mean Arterial Pressure During and After IA Infusion
Blood pressure changes during and after IA infusion were observed in C group, the average arterial pressure falling by 10 mmHg within 1 h after IA infusion (Table 2). However, there was gradual recovery after 2 h, with normalization of blood pressure after 3 h. Almost no change in blood pressure was observed in groups M and V groups after IA infusion.
Discussion
The therapeutic imperative for treating vasospasm after SAH is preservation of cerebral blood flow, ostensibly through acceptably sustained central perfusion pressures. However, our understanding of the mechanisms that lead to delayed cerebral ischemia in this setting is limited. Until recently, a number of factors have been implicated in vasospasm, but one very important issue reportedly is cellular influx of calcium [10, 32]. CCBs suppress this influx, which is key in preventing and treating vasospasm and explains why oral intake of nimodipine is strongly recommended [2]. Triple-H therapy (i.e., induced hypertension, hypervolemia, and hemodilution) and IA papaverine infusion have been commonly used in the past, but the benefits and side effects of doing so are still under debate [10, 16, 33,34,35]. Specifically, IA papaverine use has declined in recent years due to paradoxical vasospasm, systemic hypotension, and drug toxicity [10, 16, 35]. Still, other vasodilators have shown efficacy by this route in instances of medically intractable cerebral vasospasm [2].
Of the available vasodilators for IA infusion, CCBs are the most commonly used in treating vasospasm after SAH. Nimodipine, nicardipine, and verapamil have actually found broad clinical use in the treatment of vasospasm. Nimodipine is an L-type CCB that acts directly on smooth muscle of cerebral vessels [32,33,34,35,36]. Compared with similar drugs, nimodipine is characterized as follows: (1) it is lipophilic, readily crossing the blood–brain barrier (BBB), and is long-acting [32], (2) its vascular relaxation properties are more prominent in arterioles than in large arteries [37], (3) cerebral blood flow is enhanced, without loss of systemic blood pressure [25], and (4) there are neuroprotective effects, the mechanisms of which remain uncertain [38]. Nicardipine belongs to the same drug class as nimodipine and has likewise shown promise in treating vasospasm [13, 23, 35]. However, Huang et al. [23] found the efficacy of IA nicardipine infusion to be uncertain in terms of avoiding delayed cerebral ischemia. In our animal model, nicardipine triggered systemic hypotension during and after IA infusion, unlike the other CCBs. It may be thus inferior in maintaining cerebral perfusion. In the class of benzothiazepines, verapamil (amphiphilic nature) reacts selectively with myocardium and is useful for coronary vasospasm. The reason for its utility in the brain is that both coronary and cerebral arteries are structurally similar, and both are subject to vasospasm. On the other hand, each differs distinctly in the nature of vasospasm, which may be transient in coronary arteries but prolonged cerebral arteries, lasting for 2 weeks. Despite various reports of its usage for cerebral vasospasm [5, 11, 18, 39], verapamil has a short half-life compared with other drugs and may be better suited for treating coronary vasospasm [5].
In drugs given by IA infusion for vasospasm, the extent and duration of vasodilatory effects have been not well documented individually [24, 36, 40]. In the present study, all CCBs exerted vasodilatory effects immediately after IA infusions, but they did not differ overall in degrees of vascular relaxation (p = 0.466). Verapamil recipients showed maximum effects immediately after infusion, whereas the other agents (groups C and M) peaked 1 h after infusion. They also proved more potent than verapamil, falling short of significance (p = 0.193) (see Table 1). The vasodilatory effects observed in groups C and M were sustained for 2 h, as opposed to < 1 h in group V, corresponding with respective drug half-lives (8-9 h for nimodipine and nicardipine; 3-7 h for verapamil) [25, 34, 41]. In our study, the vasodilatory effects achieved by IA infusion using CCB were not sustained beyond 2 h in any of the CCB groups, and it may be a limitation of IA chemoangioplasty in vasospasm. Due to poor durability of the chemoangioplasty, repeated procedure may be required in severe vasospasm. Moreover, there has been no standard for how many procedures a day should be performed in order to relieve radiologic vasospasm or to stabilize clinical symptoms.
Recently, new alternatives to extend the vasodilatory effect in severe vasospasm were suggested. Hafeez et al. [7] reported that intrathecal nicardipine injections for a SAH-related cerebral vasospasm appear efficacious using systematic review. Administration of 4 mg of nicardipine every 12 h was the most commonly reported dosing regimen. Intrathecal nicardipine decreases mean flow velocities on transcranial Doppler (TCD) and reduces angiographic and clinical vasospasm. They insisted that duration of vasodilatory action in the intrathecal infusion seems to be longer than that in IA infusion. However, they did not present the concrete vasodilatory maintenance duration based on angiography or cerebral perfusion, and cerebrospinal fluid infection was demonstrated in 6%. Ortiz Torres et al. [42] and Majidi et al. [43] suggested that vasodilatory effect after IA dantrolene infusion maintained longer than 1 or 2 days, although the reports were case series.
According to related studies, various kinds of animals such as rats, rabbits, dog, and monkeys have been used as animal model of vasospasm [44]. The reason why we chose rabbits as experimental animals in our study was that the rabbit’s brain artery was large enough to measure its size and we had to experiment dozens of animals repeatedly and had advantages in terms of cost. Endo and Firat et al. also used rabbits as vasospasm model [29, 45]. Most of the rabbits used in our experiment were male, because it was not easy to find a female that weighed just as much as it could be used in the experiment. Mo et al. [46] used only male rabbit in animal vasospasm study, and Firat et al. [29] conducted the experiment regardless of the sex of the animal. It has not been well known about the equivalent dosage of each CCB drug. However, the dose of each drug used in our study was greater than the maximum vasodilatory dose in the dose–response curve of other studies [30, 31]. Therefore, we judged that the maximum relaxation effects of each CCB could be compared with each other. Nakajima et al. [47] argued that the more severe the vasospasm and the older the patient, the shorter the vasodilatory effects of CCBs will be.
As a final note, it must be emphasized that angiographic improvements in vasospasm do not necessarily mirror clinical improvements [6, 35]. Hoh et al. [6] showed that although vasospasm improved by 83% in follow-up angiograms, clinical improvement after IA infusion was much less (43%). Thus, angiographic findings in the setting of vasospasm may regularly correlate with the status of cerebral perfusion but not accurately align with clinical manifestations of cerebral ischemia. In addition, some authors insist that TCD is an inadvisable means of screening patients with potential cerebral vasospasm [48]. Brain perfusion testing and conventional angiography should accompany TCD [15, 35, 48].
This study has some limitations. Diagnosis and degree of vasospasm, as well as later improvement, were based on the most severely compromised arterial diameters. Because the vasodilatory effects of IA-infused drugs are expressed by both large arteries and small arterioles, such measurements may not reflect actual perfusion status. We must also acknowledge that the number of animals participating in this experiment was marginal for a three-group comparison. Furthermore, there are no established dosing equivalencies when treating cerebral blood vessels with these three CCBs, and studies focused on drug potencies have produced conflicting results. Hence, the IA drug dosages used herein may not be equivalent. Finally, we magnified photographs to determine basilar artery diameters, making it difficult to distinguish the boundaries of small vessels and perhaps introducing measurement errors. In aggregate, these drawbacks are considerable and should be appropriately resolved in future experimentation.
Conclusion
After IA infusion for vasospasm, all CCBs tested produced effective vasodilation based on angiography. Compared with verapamil, nimodipine and nicardipine exhibited longer durations of action, but only the nicardipine group displayed systemic hypotension during and after infusion. Unfortunately, the vasodilatory effects achieved by IA chemoangioplasty were not sustained for > 2 h in any of the treatment groups. Therefore, the new alternative should be found and verified to extend the duration of vasodilatory action in severe vasospasm, besides IA infusions of CCBs.
References
Allen GS, Ahn HS, Preziosi TJ, Battye R, Boone SC, Boone SC, et al. Cerebral arterial spasm–a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med. 1983;308(11):619–24.
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711–37.
Hejcl A, Cihlar F, Smolka V, Vachata P, Bartoš R, Procházka J, et al. Chemical angioplasty with spasmolytics for vasospasm after subarachnoid hemorrhage. Acta Neurochir (Wien). 2017;159(4):713–20.
Kwon HJ, Lim JW, Koh HS, Park B, Choi SW, Kim SH, et al. Stent-retriever angioplasty for recurrent post-subarachnoid hemorrhagic vasospasm—a single center experience with long-term follow-up. Clin Neuroradiol. 2019;29(4):751–61.
Feng L, Fitzsimmons BF, Young WL, Berman MF, Lin E, Aagaard BD, et al. Intraarterially administered verapamil as adjunct therapy for cerebral vasospasm: safety and 2-year experience. AJNR Am J Neuroradiol. 2002;23(8):1284–90.
Hoh BL, Ogilvy CS. Endovascular treatment of cerebral vasospasm: transluminal balloon angioplasty, intra-arterial papaverine, and intra-arterial nicardipine. Neurosurg Clin N Am. 2005;16(3):501–16.
Hafeez S, Grandhi R. Systematic review of intrathecal nicardipine for the treatment of cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2019;31(2):399–405.
Hanggi D, Turowski B, Beseoglu K, Yong M, Steiger HJ. Intra-arterial nimodipine for severe cerebral vasospasm after aneurysmal subarachnoid hemorrhage: influence on clinical course and cerebral perfusion. AJNR Am J Neuroradiol. 2008;29(6):1053–60.
Huang BR, Chang PC, Yeh WL, Lee CH, Tsai CF, Lin C, et al. Anti-neuroinflammatory effects of the calcium channel blocker nicardipine on microglial cells: implications for neuroprotection. PLoS ONE. 2014;9(3):e91167.
Kerz T, Boor S, Beyer C, Welschehold S, Schuessler A, Oertel J. Effect of intraarterial papaverine or nimodipine on vessel diameter in patients with cerebral vasospasm after subarachnoid hemorrhage. Br J Neurosurg. 2012;26(4):517–24.
Keuskamp J, Murali R, Chao KH. High-dose intraarterial verapamil in the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2008;108(3):458–63.
Kumar A, Phalak M. Is intra arterial nimodipine really beneficial in vasospasm following aneurysmal subarachnoid hemorrhage? Br J Neurosurg. 2017;31(3):290.
Lele A, Lyon T, Pollack A, Husmann K, Reeves A. Intra-arterial nicardipine for the treatment of cerebral vasospasm in postpartum cerebral angiopathy: a case study and review of literature. Int J Neurosci. 2011;121(10):537–42.
Marbacher S, Fandino J, Kitchen ND. Standard intracranial in vivo animal models of delayed cerebral vasospasm. Br J Neurosurg. 2010;24(4):415–34.
Nogueira RG, Lev MH, Roccatagliata L, Hirsch JA, Gonzalez RG, Ogilvy CS, et al. Intra-arterial nicardipine infusion improves CT perfusion-measured cerebral blood flow in patients with subarachnoid hemorrhage-induced vasospasm. AJNR Am J Neuroradiol. 2009;30(1):160–4.
Polin RS, Hansen CA, German P, Chadduck JB, Kassell NF. Intra-arterially administered papaverine for the treatment of symptomatic cerebral vasospasm. Neurosurgery. 1998;42(6):1256–64.
Rosenberg N, Lazzaro MA, Lopes DK, Prabhakaran S. High-dose intra-arterial nicardipine results in hypotension following vasospasm treatment in subarachnoid hemorrhage. Neurocrit Care. 2011;15(3):400–4.
Sehy JV, Holloway WE, Lin SP, Cross DT 3rd, Derdeyn CP, Moran CJ. Improvement in angiographic cerebral vasospasm after intra-arterial verapamil administration. AJNR Am J Neuroradiol. 2010;31(10):1923–8.
Stiefel MF, Spiotta AM, Udoetuk JD, et al. Intra-arterial papaverine used to treat cerebral vasospasm reduces brain oxygen. Neurocrit Care. 2006;4(2):113–8.
Stuart RM, Helbok R, Kurtz P, Maloney-Wilensky E, Weigele JB, Hurst RW, et al. High-dose intra-arterial verapamil for the treatment of cerebral vasospasm after subarachnoid hemorrhage: prolonged effects on hemodynamic parameters and brain metabolism. Neurosurgery. 2011;68(2):337–45.
Tejada JG, Taylor RA, Ugurel MS, Hayakawa M, Lee SK, Chaloupka JC. Safety and feasibility of intra-arterial nicardipine for the treatment of subarachnoid hemorrhage-associated vasospasm: initial clinical experience with high-dose infusions. AJNR Am J Neuroradiol. 2007;28(5):844–8.
Godfraind T, Miller RC. Specificity of action of Ca++ entry blockers. A comparison of their actions in rat arteries and in human coronary arteries. Circ Res. 1983;52(2 Pt 2):I81–91.
Huang RQ, Jiang FG, Feng ZM, Wang TY. Nicardipine in the treatment of aneurysmal subarachnoid haemorrhage: a meta-analysis of published data. Acta Neurol Belg. 2013;113(1):3–6.
Jansen I, Tfelt-Hansen P, Edvinsson L. Comparison of the calcium entry blockers nimodipine and flunarizine on human cerebral and temporal arteries: role in cerebrovascular disorders. Eur J Clin Pharmacol. 1991;40(1):7–15.
Katz AM, Leach NM. Differential effects of 1,4-dihydropyridine calcium channel blockers: therapeutic implications. J Clin Pharmacol. 1987;27(11):825–34.
Linfante I, Delgado-Mederos R, Andreone V, Gounis M, Hendricks L, Wakhloo AK. Angiographic and hemodynamic effect of high concentration of intra-arterial nicardipine in cerebral vasospasm. Neurosurgery. 2008;63(6):1080–6.
Liu Y, Lo YC, Qian L, Crews FT, Wilson B, Chen HL, et al. Verapamil protects dopaminergic neuron damage through a novel anti-inflammatory mechanism by inhibition of microglial activation. Neuropharmacology. 2011;60(2–3):373–80.
Yui H, Imaizumi U, Beppu H, Ito M, Furuya M, Arisaka H, et al. Comparative effects of verapamil, nicardipine, and nitroglycerin on myocardial ischemia/reperfusion injury. Anesthesiol Res Pract. 2011;2011:521084.
Firat MM, Gelebek V, Orer HS, Belen D, Firat AK, Balkanci F. Selective intraarterial nimodipine treatment in an experimental subarachnoid hemorrhage model. AJNR Am J Neuroradiol. 2005;26(6):1357–62.
Seker F, Hesser J, Neumaier-Probst E, Groden C, Brockmann MA, Schubert R, et al. Dose-response relationship of locally applied nimodipine in an ex vivo model of cerebral vasospasm. Neuroradiology. 2013;55(1):71–6.
Toshima M, Kassell NF, Sasaki T, Tanaka Y, Machi T. The effect of hemoglobin on vasodilatory effect of calcium antagonists in the isolated rabbit basilar artery. J Neurosurg. 1992;76(4):670–8.
Tallarico RT, Pizzi MA, Freeman WD. Investigational drugs for vasospasm after subarachnoid hemorrhage. Expert Opin Investig Drugs. 2018;27(4):313–24.
Loan JJM, Wiggins AN, Brennan PM. Medically induced hypertension, hypervolaemia and haemodilution for the treatment and prophylaxis of vasospasm following aneurysmal subarachnoid haemorrhage: systematic review. Br J Neurosurg. 2018;32(2):157–64.
Singh BN, Josephson MA. Clinical pharmacology, pharmacokinetics, and hemodynamic effects of nicardipine. Am Heart J. 1990;119(2 Pt 2):427–34.
Vajkoczy P, Horn P, Bauhuf C, Munch E, Hubner U, Ing D, et al. Effect of intra-arterial papaverine on regional cerebral blood flow in hemodynamically relevant cerebral vasospasm. Stroke. 2001;32(2):498–505.
Alborch E, Salom JB, Perales AJ, Torregrosa G, Miranda FJ, Alabadí JA, et al. Comparison of the anticonstrictor action of dihydropyridines (nimodipine and nicardipine) and Mg2+ in isolated human cerebral arteries. Eur J Pharmacol. 1992;229(1):83–9.
Tanaka K, Gotoh F, Muramatsu F, Fukuuchi Y, Okayasu H, Suzuki N, et al. Effect of nimodipine, a calcium antagonist, on cerebral vasospasm after subarachnoid hemorrhage in cats. Arzneimittelforschung. 1982;32(12):1529–34.
Pickard JD, Walker V, Vile J, Perry S, Smythe PJ, Hunt R. Oral nimodipine reduces prostaglandin and thromboxane production by arteries chronically exposed to a periarterial haematoma and the antifibrinolytic agent tranexamic acid. J Neurol Neurosurg Psychiatry. 1987;50(6):727–31.
Mazumdar A, Rivet DJ, Derdeyn CP, Cross DT 3rd, Moran CJ. Effect of intraarterial verapamil on the diameter of vasospastic intracranial arteries in patients with cerebral vasospasm. Neurosurg Focus. 2006;21(3):E15.
Takayasu M, Bassett JE, Dacey RG Jr. Effects of calcium antagonists on intracerebral penetrating arterioles in rats. J Neurosurg. 1988;69(1):104–9.
De Cicco M, Macor F, Robieux I, Zanette G, Fantin D, Fabiani F, et al. Pharmacokinetic and pharmacodynamic effects of high-dose continuous intravenous verapamil infusion: clinical experience in the intensive care unit. Crit Care Med. 1999;27(2):332–9.
Ortiz Torres MJ, Jahngir M, Qualls K, Litofsky NS, Litofsky NS, Nattanmai P, Qureshi AI. Intra-arterial dantrolene for refractory cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. World Neurosurg. 2019;125:247–52.
Majidi S, Grigoryan M, Tekle WG, Qureshi AI. Intra-arterial dantrolene for refractory cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2012;17(2):245–9.
Megyesi JF, Vollrath B, Cook DA, Findlay JM. In vivo animal models of cerebral vasospasm: a review. Neurosurgery. 2000;46(2):448–61.
Endo S, Branson PJ. Alksne JF Experimental model of symptomatic vasospasm in rabbits. Stroke. 1988;19:1420–5.
Mo Y, Huang L, Chen L, Veronesi M, Shi Y, Chen S, et al. An experimental rabbit model of symptomatic cerebral vasospasm with in vivo neuroimaging assessment and ex vivo histological validation. Exp Ther Med. 2018;15(3):2411–7.
Nakajima M, Date I, Takahashi K, Ninomiya Y, Asari S, Ohmoto T. Effects of aging on cerebral vasospasm after subarachnoid hemorrhage in rabbits. Stroke. 2001;32(3):620–8.
Washington CW, Zipfel GJ, Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid H. Detection and monitoring of vasospasm and delayed cerebral ischemia: a review and assessment of the literature. Neurocrit Care. 2011;15(2):312–7.
Acknowledgements
This work was supported by the New Faculty Startup Fund from Seoul National University and by Chungnam National University Hospital Research Fund (2018).
Author information
Authors and Affiliations
Contributions
JL, YDC drafted study conception and design. JL, HJK, SHB, HSK contributed to acquisition of data. JL, YDC, BP, SWC contributed to analysis and interpretation of data. JL, YDC drafting of manuscript. YDC, HSK, SWC contributed to critical revision.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
This study was conducted with the approval of the Chungnam National University Hospital, Chungnam National University.
Human and animal rights
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lim, J., Cho, Y.D., Kwon, HJ. et al. Duration of Vasodilatory Action After Intra-arterial Infusions of Calcium Channel Blockers in Animal Model of Cerebral Vasospasm. Neurocrit Care 34, 867–875 (2021). https://doi.org/10.1007/s12028-020-01112-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-020-01112-0