Abstract
Background
We describe a case of convulsive status epilepticus caused by intracranial hypotension, a complication of spinal surgery. Intracranial hypotension (IH) is typically characterized by an orthostatic headache. There have been limited case reports describing surgery-associated IH presenting with seizures.
Methods
Case report and review of the literature.
Results
A 71-year-old woman with chronic back pain developed convulsive status epilepticus, characterized by generalized clonic seizures, immediately following scoliosis surgery. She had no history of seizures or other seizure risk factors. Despite treatment with intravenous midazolam, phenytoin, and lacosamide, seizures recurred five times over three hours. Thus, propofol and midazolam infusions were initiated. An electroencephalogram revealed burst suppression and bilateral hemispheric epileptiform discharges. Magnetic resonance imaging of the brain was consistent with IH without cortical vein thrombosis. Fluid from the surgical drains was positive for Beta-2 transferrin, consistent with cerebral spinal fluid. Her IH was likely due to an intraoperative dural tear causing status epilepticus. Over two weeks, she remained on bed rest, sedation was weaned, and phenytoin and lacosamide were tapered and discontinued. She had no further seizures.
Conclusions
IH is an under-recognized cause of seizure following the spinal or cranial surgery, lumbar puncture, or spinal anesthesia. Proposed mechanisms include traction on cortical structures, increased cerebral blood flow, and cortical irritation secondary to subdural hygromas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case Description
A 71-year-old woman developed convulsive status epilepticus (SE) in the operating room during emergence from anesthesia. Due to persistent chronic back pain, she underwent an elective redo-scoliosis surgery, which included removal of instrumentation from T4 to ilium, posterolateral decompression from L3 to L5, pedicle subtraction osteotomy at L4, and revision of scoliosis fusion from T11 to ilium.
Her past medical history was significant for breast cancer diagnosed 5 years prior, treated with bilateral mastectomies and chemotherapy, hypertension, osteoporosis, anxiety, and depression. She had no previous seizures, nor any seizure risk factors. Medication list included citalopram, trazodone, hydrochlorothiazide, fosamax, and percocet.
Anesthesia was induced with propofol, fentanyl, and rocuronium and maintained with lidocaine, sufentanil, and ketamine infusions. At the start of the procedure, she received cefazolin, tranexamic acid, dexamethasone, and ondansetron. During the six-hour procedure, she intermittently required norepinephrine infusion for maintenance of blood pressure. Repair of a small dural tear took place with no persistent leak. Blood loss was as expected. Following skin closure, when she was moved from a prone to supine position while still intubated, a generalized clonic seizure was noted. It was characterized by bilateral clonic movements of her upper and lower extremities, with eyes open and a vertical upward tonic gaze deviation. Profound bradycardia and hypotension accompanied the spell. The seizure was aborted transiently with a bolus of IV propofol, selected due to its availability in the operating theater and based on the fact that the patient was already intubated. However, the seizure recurred five times over the following three hours, despite further treatment with boluses of midazolam and propofol, and intravenous phenytoin loading. She also received 3000 mL of crystalloid and two units of packed red blood cells. Each spell resulted in severe hypotension, without prolonged pulselessness, delaying transfer to the intensive care unit (ICU). The increase in intrathoracic pressure, which occurred during each seizure, coupled with relative hypovolemia and sedation, reduced her cardiac output and led to ictal hypotension. This was supported by transesophageal echocardiogram performed during one seizure. Lacosamide was added due to ongoing seizures, and ultimately propofol and midazolam infusions were used to control the SE successfully.
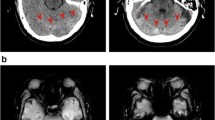
Emergent electroencephalography (EEG) revealed burst suppression, with left and occasionally right hemispheric epileptiform discharges. An early computed tomography scan showed absent basal cisterns and a crowded posterior fossa, but no cortical abnormality (Fig. 1). At this stage, the most likely etiology for the cerebral edema was felt to be early global ischemia related to intraoperative hypotension. Consideration was also made for bacterial meningitis, although this was felt to be less likely due to the early occurrence of seizures postoperatively. Nevertheless, she was started on empiric antibiotics, although lumbar puncture was not immediately possible. A low-grade serotonin syndrome was considered as a precipitant with exposure to a selective serotonin receptor antagonist and fentanyl, but she had no supportive clinical features. Magnetic resonance imaging (MRI) and MR angiogram of her brain was ordered to identify the cause for the seizures and assess for watershed infarction.
MRI of her brain revealed evidence of intracranial hypotension (IH), with mild, diffuse dural thickening and pachymeningeal enhancement (Fig. 2a), thin bilateral subdural effusions (Fig. 2b), and prominent dural sinuses and pituitary gland (Fig. 2c). Fluid collected from two drains left in intraoperatively was positive for beta-2 transferrin (13.6 and 18.1 mg/L; normal range < 1.0), indicating that cerebral spinal fluid (CSF) was present. Thus, IH causing SE secondary to an intraoperative dural tear was diagnosed. Upon transfer to the ICU, seizures recurred when placed upright and subsided promptly when nursed supine. Over the following days, she was kept on strict bed rest, her sedation was weaned, and her level of consciousness gradually improved, allowing for extubation. She gradually moved into an upright position without significant orthostatic symptoms emerging. Her phenytoin and lacosamide were tapered and discontinued over the following week. She had no further seizure recurrence, nor neurologic deficits. No immediate spinal imaging was obtained.
Cranial MRI revealing evidence of intracranial hypotension. a Axial T1 post-gadolinium showing diffuse, mild pachymeningeal thickening and enhancement (arrow). b Axial T2 revealing bilateral subdural effusions (arrows). c Sagittal T1 post-gadolinium showing prominent dural sinuses (black arrow) and pituitary hyperemia (white arrow)
She convalesced on the ward and recovered well. When she developed discharge from her lumbar incision in a delayed fashion, a large CSF collection was diagnosed and repaired (Fig. 3).
Discussion
IH is typically characterized by an orthostatic headache and may be associated with a number of other symptoms including altered mental status, neck stiffness, nausea, emesis, vertigo, muffled and distorted hearing, horizontal diplopia, superior binasal visual field defects, visual blurring, and photophobia [1]. Symptoms are thought to arise from a decrease in intracranial CSF volume, rather than CSF hypotension itself [2]. IH occurs most frequently following procedures such as lumbar punctures, spinal anesthesia, and cranial or spinal surgeries [3]. It may also develop “spontaneously,” as a result of structural weakness of the spinal meninges [4], disk herniation resulting in ventral dural tears, CSF venous fistulas, and dural weakness at the nerve root sleeves [2].
IH is generally treated using a stepwise approach [5]. Typically, headaches resolve with conservative management, which includes hydration and strict bed rest, allowing for relief of CSF pressure at the site of leakage and thus healing of the underlying defect. Caffeine administered intravenously or orally is also effective for post-lumbar puncture IH. If a conservative approach is not effective, epidural blood patches, epidural saline infusion, and surgical correction should be considered [5].
This patient had a contained leak and eventually required surgical correction. A conservative approach was initially taken due to the concern for a technically difficult repair of the tear, given her anatomy and multiple previous surgeries. Strict bed rest allowed for improvement in her IH, without any orthostatic headache or recurrent seizures developing. The delayed repair was uneventful, and she has not had IH symptom recurrence or further seizures.
We hypothesize that this patient developed SE as a result of IH. The traction on the meninges resulted in transient cortical irritability and led to seizure generation. This hypothesis is supported by the fact that her seizures occurred when placed upright and subsided promptly when nursed supine. Cerebral ischemia and cortical damage were ruled out by MRI.
It remains controversial whether IH has a causal relationship with subsequent seizure development, with only a limited number of reports describe seizures secondary to IH (Table 1). Lin et al. [6] describe a patient who developed multiple seizures in the post-anesthesia care unit following a thoracic laminectomy, who was found to have imaging findings consistent with IH. This patient went on to have an exploratory thoracic laminotomy, with a dural tear identified and repaired. Two other reports describe patients with hydrocephalus and ventriculo-peritoneal shunts experiencing intractable epilepsy presumed as a result of over-drainage [7, 8]. Interestingly, in one of the cases, seizures were provoked by the patient moving into an upright position and resolved when supine [8]. Chaudhary et al. [3] describe two patients who developed focal onset seizures with impaired awareness due to spontaneous intracranial hypotension.
The mechanisms by which IH may cause seizure development and convulsive SE have not been fully elucidated. It has been proposed that IH may cause meningeal irritation or traction on cortical structures of the brain, resulting in seizures [3, 6]. Others have hypothesized that hygromas may act as cortical irritants, although typically when much larger than those observed in our patient [1, 4]. Finally, alterations in ICP may result in acute changes in cerebral blood flow, leading to seizure generation [7].
Postoperative IH has also been reported to cause dysregulation of cerebral blood flow [9]. Postoperative intracranial hypotension-associated venous congestion (PIHV) is associated with distinct radiographic changes in the basal ganglia, thalamus, cerebellum, and brainstem. PIHV has been associated with the development of seizures and non-convulsive SE. The presented patient’s MRI did not have any of these findings. Seizures in IH may also develop due to cerebral venous thrombosis [10] and posterior reversible encephalopathy syndrome [11].
Convulsive syncope was also considered as an alternate mechanism. Traction on the ventral medulla from IH and secondary brainstem dysfunction has been associated with syncopal events [12]. This is similar to syncope precipitated by neck flexion occasionally seen in patients with craniovertebral subluxation [12]. Convulsive syncope could have caused the profound hypotension and bradycardia, but not explain the post-ictal EEG changes. This patient had clear clonic movements prior to hemodynamic changes. The findings of an intraoperative transesophageal echocardiogram, during which a seizure occurred, support this hypothesis.
This case illustrates a rare cause of convulsive SE. It should have been suspected in the context of spinal surgery. Recognizing the trigger altered the treatment plan and resulted in a good patient outcome. To date, only a small number of reports have described seizures in patients with intracranial hypotension. Better awareness of this association may improve recognition of the syndrome.
References
Mokri B. Spontaneous intracranial hypotension. Curr Neurol Neurosci Rep. 2001;1(2):109–17.
Kranz PG, Malinzak MD, Amrhein TJ, Gray L. Update on the diagnosis and treatment of spontaneous intracranial hypotension. Curr Pain Headache Rep. 2017;21(8):1–8.
Chaudhary N, Cooper P, Lownie SP, Ng W, Duggal N. Spontaneous intracranial hypotension: case series of rare clinical presentations. Can J Neurol Sci. 2011;38:54–8.
Schievink WI. Spontaneous spinal cerebrospinal fluid and ongoing investigations in this area. JAMA. 2006;295(19):2286–96.
Paldino M, Mogilner AY, Tenner MS. Intracranial hypotension syndrome: a comprehensive review. Neurosurg Focus. 2003;15(6):1–8.
Lin S-M, Tsou M-Y, Chan K-H, et al. Myoclonic seizure in the postanesthesia care unit after thoracic laminectomy. Anesth Analg. 2002;95(3):777.
Uchida D, Fujimoto A, Yamazoe T, Yamamoto T, Enoki H. Seizure frequency can be reduced by changing intracranial pressure: a case report of intractable epilepsy. Epilepsy Behav Case Reports 2018.
Agrawal D, Durity FA. Seizure as a manifestation of intracranial hypotension in a shunted patient. Pediatr Neurosurg. 2006;42(3):165–7.
Snyder KA, Clarke MJ, Gilbertson JR, Hocker SE. Prompt recognition and management of postoperative intracranial hypotension-associated venous congestion: a case report. Neurocrit Care. 2016;24(3):448–53.
Honig A, Eliahou R, Pikkel YY, Leker RR. Iatrogenic intracranial hypotension and cerebral venous thrombosis. J Neurol Sci. 2016;366:191–4.
Shields LBE, Johnson JR, Shields CB. Posterior reversible encephalopathy syndrome following a thoracic discectomy-induced dural leak: case report. J Neurosurg Spine. 2016;25(November):1–5.
de Carvalho M, Swash M. Neurologic complications of craniovertebral dislocation. Handb Clin Neurol. 2014;119:435–48.
Pabaney AH, Mirza FA, Syed NA, Ahsan H. Spontaneous dural tear leading to intracranial hypotension and tonsillar herniation in Marfan syndrome: a case report. BMC Neurol. 2010;10:54.
Hedna VS, Kumar A, Miller B, et al. Intracranial hypotension masquerading as nonconvulsive status epilepticus. J Neurosurg. 2014;120(3):624–7.
Funding
This manuscript received no funding.
Author information
Authors and Affiliations
Contributions
The authorship requirements have been met, and the final manuscript was approved by all authors. Dr. Gilmour was involved in the conceptual idea, data collection, and writing of the manuscript. Dr. Couillard was involved in the conceptual idea and critical revision of the manuscript. Dr. Scott was involved in the data collection.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This manuscript adheres to ethical guidelines. No approval by an Institutional Review Board was required. Patient consent was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gilmour, G.S., Scott, J. & Couillard, P. Leaking the Diagnosis: A Case of Convulsive Status Epilepticus Due to Intracranial Hypotension. Neurocrit Care 31, 562–566 (2019). https://doi.org/10.1007/s12028-019-00788-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00788-3