Abstract
Background
There is increasing evidence for the role of inflammation in clinical outcome after subarachnoid hemorrhage (SAH). Specifically, the TNF-alfa(α) pathway seems to be relevant after SAH. Although the TNF-α main receptor, TNF-R1 is associated with aneurysm growth and rupture, its relation to prognosis is unknown.
We sought to compare TNF-R1 levels in peripheral venous blood and arterial blood closer to the ruptured aneurysm to study the association of TNF-R1 blood levels with poor prognosis (modified Rankin Scale > 2 at discharge, 3 and 6 months) and complications (hydrocephalus or delayed cerebral ischemia/DCI) following SAH.
Methods
We included consecutive SAH patients admitted in the first 72 h of symptoms. Blood samples were simultaneously collected from a peripheral vein and from the main parent artery of the aneurysm. Levels of TNF-R1 were measured using enzyme-linked immunosorbent assays.
Results
We analyzed 58 patients. Arterial and venous levels of TNF-R1 were correlated (R = 0.706, p < 0.001). In multivariate regression analysis, venous TNF-R1 was an independent predictor of poor outcome at 6 months after adjusting by age and sex [odds ratio (OR) 11.63; 95% CI 2.09–64.7, p = 0.005] and after adjusting by Glasgow Coma Scale and Fisher scales (OR 8.74; 95% CI 1.45–52.7, p = 0.018). There was no association of TNF-R1 with DCI. A cut-off for arterial TNF-R1 of 1523.7 pg/mL had 75% sensitivity/66% specificity for the prediction of hydrocephalus.
Conclusion
Levels of venous TNF-R1 are associated with poor outcome in SAH. A specific association was found between levels of arterial TNF-R1 and hydrocephalus. These results are consistent with the role of TNF-α pathway in SAH and need to be validated in larger cohorts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous subarachnoid hemorrhage (SAH) is associated with high morbidity and mortality, with a case fatality rate reaching 25–50% [1]. Main independent determinants of poor outcome after SAH are age, poor clinical grade on admission, high blood burden on admission computed tomography (CT), angiographic vasospasm, neurological worsening of any cause, and cerebral infarction [2]. Delayed cerebral ischemia (DCI) occurs in around 30% of patients, is a major determinant of prognosis, and has been associated with vasospasm [3]. However, not all patients with vasospasm develop DCI, and not all patients with DCI have documentation of vasospasm [4]. This means that available tools for screening of vasospasm cannot detect all patients that will evolve to DCI. Biomarkers might have the potential to identify patients at risk of vasospasm, DCI or poor prognosis early in the course of disease, to allow better clinical management.
A number of biomarkers, especially inflammatory biomarkers in serum and cerebrospinal fluid (CSF), have been studied as outcome markers in SAH [5, 6]. Inflammatory markers such as matrix metalloproteinase-9 [7, 8], endothelin [9], neuropeptide Y [10], caspase [11], and fibronectin [12] have been studied as markers of vasospasm, cerebral ischemia and clinical outcome.
Tumor necrosis factor (TNF)-α, an inflammatory cytokine, is known to rise in the first 72 h after SAH, and is associated with neuronal and blood–brain barrier damage [13], as well as with poor outcome in SAH [14]. TNF signaling through its main receptor, TNF-R1, has been implicated in formation and rupture of cerebral aneurysms [15]. Therefore, TNF-α pathway seems a relevant diagnostic and therapeutic target both in human and experimental SAH. TNF-R1 is known to persist in the circulation beyond TNF-α, which has a comparably shorter half-life and is a reliable marker of the TNF-α pathway [16].
In our study, we sought to compare levels of TNF-R1 at admission in peripheral venous blood versus arterial blood collected intra-angiographically closer to the aneurysm rupture, in a series of patients with spontaneous SAH. We also investigated the association of TNF-R1 levels with complications and long-term prognosis of SAH. These results might help close the gaps in the study of TNF-α pathway in SAH and give a global picture of the relevance of TNF-α in this disease.
Methods
Patients
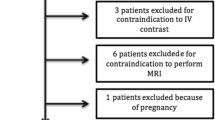
All patients with acute spontaneous SAH admitted at Centro Hospitalar de Lisboa Central between May 2013 and November 2014 were enrolled in a prospective cohort study. Inclusion criteria for the main study were: (1) age > 18 years; (2) acute non-traumatic SAH diagnosed by CT and/or lumbar puncture performed within the first 72 h of SAH; (3) imaging studies performed within the first 72 h of SAH; and (4) informed consent obtained from patient or legal representative. Patients in a very poor clinical condition (Glasgow Coma Scale [GCS] 3), pregnant women, patients with renal insufficiency, or with unknown time of onset of SAH were excluded. For the current analysis, we included all patients from whom peripheral venous blood and central arterial blood samples were collected.
Clinical and Imaging Data
Demographic data and clinical presentation were collected from the patients’ medical records. Neurological status at admission was evaluated using GCS, World Federation of Neurosurgeons scale (WFNS) and Hunt and Hess grade (HH). Other relevant variables such as the presence of hydrocephalus, presence and location of aneurysm, and surgical/endovascular treatment were recorded. The amount of blood on admission CT was assessed using the modified Fisher scale [17] and the Hijdra scale for cysternal and intraventricular hemorrhage [18].
Outcome
We aimed to evaluate the association of biomarkers with: (1) occurrence of complications and (2) modified Rankin Scale (mRS) at discharge, 3, and 6 months.
Two complications were assessed: occurrence of hydrocephalus and DCI. Hydrocephalus was defined as a bicaudate index above the 95th percentile for age, at any time between admission and discharge [19]. Patients were classified as having DCI if: (1) presenting with a new focal neurological deficit/decrease in level of consciousness non attributable to other causes (for example, hydrocephalus, seizures, metabolic derangement, infection, sedation), (2) there was a new infarct on follow-up imaging after 4 days post-ictus, or (3) both 1 and 2 [20, 21]. mRS was obtained at 3 and 6 months by stroke unit physicians, blinded to clinical/magnetic resonance imaging data, by in-person or telephone interview [22]. Functional outcome was dichotomized as good/independent (mRS 0–2) and poor/dependent (mRS > 2).
Laboratory Data
Blood samples were collected at the time of diagnostic digital subtraction angiography, at < 24 h from admission, and at < 72 after SAH onset. Two samples were collected simultaneously; one from peripheral venous access and one from the catheter used in the diagnostic angiography. Arterial blood was collected from the main parent artery of the aneurysm, if any, or from a vertebral artery in perimesencephalic hemorrhages. Blood was collected into EDTA tubes, centrifuged at 3000 rpm for 15 min, and plasma was frozen at − 80 °C until analysis. To determine TNF-R1, we used commercially available enzyme-linked immunosorbent assays (ELISA, R&D Systems Inc., USA), performed according to the manufacturer’s instructions at the Neurovascular Research Laboratory, at VHIR, Barcelona. All samples were tested in duplicate, and the valid coefficient of variation was < 20%. Results were expressed in pg/mL. Inter-assay variation was determined by testing two times in every plate a commercial internal control (Human serum type AB, male, from clotted, Sigma-Aldrich.USA). Staffs analyzing the samples were blind to clinical information.
Statistical Analysis
Characteristics of study patients were described using the mean (standard deviation [SD]) or median (interquartile range [IQR]) for continuous variables, and frequencies (percentages) for categorical variables. As TNF-R1 levels were not normally distributed neither in arterial nor in venous samples, nonparametric tests were further used. Arterial and venous TNF-R1 levels were compared with Wilcoxon test and correlations were assessed by Spearman test. Intergroup comparisons of TNF-R1 levels were assessed with Mann–Whitney U test. To assess the predictive value of TNF-R1 on functional outcome after SAH, logistic regression models were developed taking 3-month and 6-month poor functional outcome (mRS > 2) as dependent variables, with the forward-stepwise method. For inclusion in the logistic regression models, TNF-R1 levels were dichotomized based on the cutoff with the highest accuracy, identified through receiver operating characteristics (ROC) curves. A first predictive model was developed by adjusting TNF-R1 levels by age and sex. Then, a second predictive model was developed by including in the first step all covariates associated with 3-month and 6-month poor functional outcome in univariate analysis, removing the nonsignificant covariates in each step. The final model shows only the significant covariates. Independent models were developed for arterial and venous TNF-R1. All analyses were performed using SPSS statistical package, version 22.0 (Armonk, NY: IBM Corp).
Results
Demographic and Clinical Data
During the inclusion period, a total of 129 patients were admitted with non-traumatic SAH. Eighty patients fulfilled inclusion criteria for the main prospective cohort; however, only 58 patients had both arterial and venous blood samples obtained in adequate conditions for analysis (Supplemental Figure 1). The mean age of the patients was 56.7 years (± 16.1), and 23 (39.7%) were male. The median HH grade was 2 (1–3), and 33 patients were WFNS grade I; median GCS was 14 (12–15). A total of 16 patients (27.6%) developed DCI, and eight (14%) developed hydrocephalus. Mean hospital stay was 24.6 ± 19.9 days. Outcome data were available for 57 patients. At 3 months, 18 patients (31.6%) had mRS > 2. Mortality at 3 months was 8.8% (n = 5). At 6 months, 17 patients (29.8%) had mRS > 2, and mortality was the same (Table 1).
Correlation Between Venous and Arterial Biomarkers and Clinical Variables
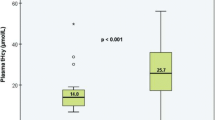
Levels of arterial TNF-R1 were lower than levels of venous TNF-R1 (1279.7[1056.3–1831.6] vs. 1388.6 [1078.3–1942.3] pg/mL, p = 0.013). However, both were strongly correlated (R = 0.706, p < 0.001) (Fig. 1). Regarding clinical variables (Table 2), arterial TNF-R1 levels were correlated with age (R = 0.528, p < 0.0001), WFNS (R = 0.292, p = 0.026) and HH grade (R = 0.268, p = 0.042) scales and inversely correlated with GCS score (R = − 0.294, p = 0.025). Venous TNF-R1 levels, however, were only correlated with age (R = 0.333, p = 0.011).
Association of TNF-R1 with Clinical Outcome
Patients with higher levels of arterial TNF-R1 were more likely to be dependent at discharge (p = 0.003), 3 months (p = 0.012) and 6 months (p = 0.006). Higher levels of venous TNF-R1 were associated with poor outcome at 3 (p = 0.027) and 6 months (p = 0.007), but not at discharge (p = 0.116) (Fig. 2).
Logistic regression models were performed for 3-month and 6-month outcome. A cutoff point of arterial TNF-R1 > 1581.8 had 66.7% sensitivity and 76.9% specificity for 3-month outcome. Regarding venous TNF-R1, a cutoff value of > 1754.3 (sensitivity 55.6%, specificity 84.6%) predicted poor outcome at 3 months. Patients that were dependent at 3 months were older (p = 0.022), had lower GCS score (p = 0.001), had higher HH (0.004), and higher Fisher (p = 0.008) grades. Results of univariate analysis are shown in Supplementary Table I. In logistic regression analysis, when adjusted by age and sex, both arterial and venous TNF-R1 levels resulted independent predictors of poor outcome. However, neither arterial nor venous TNF-R1 levels were independent predictors of poor outcome when GCS score was added to the models, being GCS the only independent predictor (Table 3).
Regarding 6-month outcome, patients with higher levels of arterial or venous TNF-R1 were more likely to be dependent (p = 0.006 and p = 0.007). For arterial TNF-R1, a cutoff level of > 1176.0 had 82.4% sensitivity and 45% specificity, and for venous TNF-R1, a cutoff level of > 1689.8 had 64.7% sensitivity and 85% specificity to predict dependency at 6 months. Patients that were dependent at 6 months were older (p = 0.033) had lower GCS score (p = 0.002) and had higher HH (0.008) and higher Fisher (p = 0.002) grades. Results of univariate analysis are shown in Supplementary Table II. In logistic regression analysis, arterial TNF-R1 was not an independent predictor of long-term poor outcome. However, venous TNF-R1 was an independent predictor of poor outcome after adjusting by age and sex (OR 11.63 [2.09–64.7], p = 0.005), and even after adjusting by GCS and Fisher scales (OR 8.74 [1.45–52.7], p = 0.018) (Table 3). We explored venous TNF for outcome at 6 months in both models would add predictive value in terms of accuracy—increase in AUC to the model with clinical predictors alone. AUCs were compared by the likelihood ratio test. In both cases, AUC was increased, as shown in Fig. 3.
Association of TNF-R1 with Complications
TNF-R1 levels were not associated with the occurrence of DCI. However, patients with higher levels of arterial TNF-R1 were more likely to have hydrocephalus (1925.3 [1360.6–2138.0] vs. 1251.4 [1050.6–1788.4] pg/mL, p = 0.031). In fact, a cutoff for arterial TNF-R1 of 1523.7 pg/mL had 75% sensitivity and 66% specificity for the prediction of hydrocephalus. Regarding venous TNF-R1, no differences were found regarding this complication (1738.6 [1283.6–2575.9] vs. 1283.4 [1056.8–1812.0] pg/mL, p = 0.191) (Fig. 4).
Discussion
In this prospective study, we demonstrated that blood TNF-R1 level is an independent predictor of long-term poor functional outcome. TNF-R1 signaling pathways have been related to the formation and rupture of cerebral aneurysms [15, 23]. However, the association of TNF-R1 to prognosis after SAH has not been previously described, to our knowledge. In a study by Gruber and colleagues [24], elevated serum levels of TNF-R1 were associated with multiple organ dysfunction after SAH. The rise of serum TNF-α in the first 3 days post-SAH is associated with poor outcome in SAH at 2 weeks [13], and at 3 and 6 months, but not with vasospasm [14]. Our results are consistent with these previous studies, since a rise in TNF-α probably implies increased expression or redistribution of its receptors [25, 26]. However, we could not demonstrate an association of TNF-R1 levels with DCI. The association of higher TNF-R1 levels with poor clinical prognosis might relate to its association to other complications, such as hydrocephalus and resulting intracranial hypertension, or systemic complications, as described by Gruber [24].
Pathophysiological mechanisms underlying cerebral and systemic injury after SAH are not fully understood, but recent evidence has shown that inflammation is central to many processes after SAH, such as vasospasm, DCI, and secondarily, poor outcome [27]. Inflammatory cascades are activated rapidly after extravasation of blood to the subarachnoid space and contribute both to early brain injury mechanisms and delayed complications such as cerebral ischemia [5, 28]. At a cellular level, TNF-α activates protein kinases and increases the synthesis of adhesion molecules and other cytokines, such as IL-6, that perpetuate the inflammatory response and in turn increase endothelial permeability and mediate brain injury [14, 29]. In previous studies, although there is a generalized rise in inflammatory cytokines after SAH, not all are related to clinical outcome like TNF-α is, which suggests there may be cytokine-specific mechanisms leading to poor outcome in SAH [14]. However, the source of circulating TNF-α and its receptor TNF-R1 remain to be determined, as well as the precise mechanisms of injury that ultimately affect prognosis.
Levels of inflammatory markers in CSF and plasma have a parallel course in SAH [24]. We hypothesized that there could be a local arterial production of cytokines, closer to the site of rupture, and indeed we found levels of TNF-R1 in the arterial samples we analyzed, although these levels were lower than in peripheral blood samples. Venous and arterial levels of TNF-R1 correlated well with each other. However, specific associations were found for arterial TNF-R1 with clinical status at admission and hydrocephalus. Our results show a correlation of arterial TNF-R1 with clinical severity at admission, but not venous TNF-R1. This could suggest that there is an initial local production of TNF-R1 more related to early injury. In fact, there is evidence that there are arterial to jugular concentration gradients for some cytokines in patients with brain injury, showing there is intracranial production of inflammatory cytokines, and a shift across the blood–brain barrier [30]. It could be argued that our sample has a large proportion of good clinical grade SAH patients, and it would be interesting to explore the association of TNF-R1 levels to clinical severity in a population with poor-grade SAH. Although both arterial and venous TNF-R1 were associated with poor outcome, only venous TNF-R1 remained an independent predictor of outcome at 6 months, after adjusting for confounding variables. This would further support the theory of brain cytokine production and shift to venous blood, that has been defended by other authors [30, 31].
Regarding the prediction of prognosis, our results show that maybe the use of peripheral blood samples is enough, and more simple in clinical practice, even in patients that do not undergo conventional angiography. The analysis of arterial samples might, however, be useful for predicting specific complications, although these preliminary results need to be confirmed in larger samples. There is no reference in the literature to the use of arterial blood close to the ruptured aneurysm for biomarker quantification, to our knowledge, but it might be an interesting path to investigate also for other markers.
The dynamic change in TNF-α levels along the first weeks after SAH has been studied by other authors (for example, Chou et al. [14]), that have found that TNF-α rises progressively in patients with worse long-term outcome. Future studies should focus in the analysis of temporal profile of TNF-R1 levels, in order to better define a time point to perform specific measures to predict outcome or complications.
At present, monitoring of SAH patients for complications is based on neurologic examination and imaging studies, mostly CT and transcranial Doppler. Regarding hydrocephalus, treatment decisions are sometimes delayed until symptoms of intracranial hypertension occur, further aggravating cerebral injury. There is no imaging marker that can reliably predict the occurrence of hydrocephalus, and therefore, our study highlights the importance that TNF-R1 might have in predicting this complication. Also, early intervention at the root of early brain injury could improve the prognosis in SAH, and therefore, TNF-R1 measured in the first days could have an impact in clinical decisions on monitoring and treatment, especially in poor-grade patients in whom neurological monitoring is difficult. Biomarkers could possibly complement imaging studies in diagnosis of complications and prediction of prognosis, but this remains to be studied.
Finally, since TNF pathway seems to relate to clinical severity of SAH, complications, and outcome, it would be interesting to explore therapeutic applications of anti-TNF agents in the treatment of early brain injury. There is already some evidence that TNF-α inhibitors improve endothelial dysfunction, and might therefore have the potential to modify the growth and rupture of intracranial aneurysms [15]. There are animal studies demonstrating the effect of TNF-α blocking drugs such as etanercept in reducing neuronal damage after ischemia [32]. However, the benefit of anti-TNF agents in SAH is still unclear.
Our study has some limitations. The main limitation is the small sample size; however, despite this, the consistent association of levels of TNF-R1 both arterial and venous with outcome at discharge, 3, and 6 months, suggests the potential role of TNF-R1 as a biomarker of outcome in SAH. These results need to be validated in larger cohorts. Also, the association of TNF-R1 levels to systemic infectious complications and the occurrence of systemic inflammation and organ dysfunction were not reported, as well as the association to other laboratorial parameters of inflammation, such as C-reactive protein and leukocyte count. The samples were collected at admission with a time window of 72 h; therefore in some patients, blood was collected in the first hours after rupture, while in others, blood was collected closer to 48–72 h. We recognize that there might be differences in TNF-R1 levels in the first days after SAH that were not detected in our study.
We acknowledge that in order to be clinically relevant, the use of biomarkers has to be integrated in clinical practice in a simple and rapid fashion, without sample preparation that is time-consuming and not available in all clinical settings. Therefore, a point-of-care based-test for TNF-R1 measurement, single or combined with other outcome markers in a panel, would be the ideal application of these results in clinical practice.
Conclusion
In this exploratory study, we found that venous TNF-R1 is an independent predictor of poor outcome in SAH. Arterial levels of TNF-R1, measured at the afferent artery of the ruptured aneurysm, were associated with the occurrence of hydrocephalus. Altogether, these results highlight the role of inflammatory cytokines in predicting complications and prognosis after SAH.
References
Hop JW, Rinkel GJE, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28(3):660–4.
Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38(8):2315–21.
Hijdra A, Van Gijn J, Nagelkerke NJD, Vermeulen M, Van Crevel H. Prediction of delayed cerebral ischemia, rebleeding, and outcome after aneurysmal subarachnoid hemorrhage. Stroke. 1988;19:1250–7.
Vergouwen MDI, Ilodigwe D, MacDonald RL. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke. 2011;42(4):924–9.
Hong CM, Tosun C, Kurland DB, Gerzanich V. Biomarkers as outcome predictors in subarachnoid hemorrhage–a systematic review. Biomarkers. 2014;19(2):95–108.
Jordan JD, Nyquist P. Biomarkers and Vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21(2):381–91.
Chou SH-Y, Feske SK, Simmons SL, et al. Elevated peripheral neutrophils and matrix metalloproteinase 9 as biomarkers of functional outcome following subarachnoid hemorrhage. Transl Stroke Res. 2011;2(4):600–7.
McGirt MJ, Lynch JR, Blessing R, Warner DS, Friedman AH, Laskowitz DT. Serum von Willebrand factor, matrix metalloproteinase-9, and vascular endothelial growth factor levels predict the onset of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2002;51(5):1125–8.
Seifert V, Loffler BM, Zimmermann M, Roux S, Stolke D. Endothelin concentrations in patients with aneurysmal subarachnoid hemorrhage. Correlation with cerebral vasospasm, delayed ischemic neurological deficits, and volume of hematoma. J Neurosurg. 1995;82(1):55–62.
Schebesch K-M, Brawanski A, Bele S, et al. Neuropeptide Y—an early biomarker for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurol Res. 2013;35(10):1038–43.
Wang J, Wang J-F, Hu X-M. Caspase-3 in serum predicts outcome after aneurysmal subarachnoid hemorrhage. Clin Chim Acta. 2016;460:196–202.
Frijns CJM, Rinkel GJE, Castigliego D, Van Gijn J, Sixma JJ, Fijnheer R. Endothelial cell activation after subarachnoid hemorrhage. Neurosurgery. 2002;50(6):1223–30.
Mathiesen T, Edner G, Ulfarsson E, Andersson B. Cerebrospinal fluid interleukin-1 receptor antagonist and tumor necrosis factor-alpha following subarachnoid hemorrhage. J Neurosurg. 1997;87(2):215–20.
Chou SH, Feske SK, Atherton J, et al. Early elevation of serum TNFα is associated with poor outcome in subarachnoid hemorrhage. J Investig Med. 2012;60(7):1054–8.
Young AMH, Karri SK, You W, Ogilvy CS. Specific TNF-alpha inhibition in cerebral aneurysm formation and subarachnoid hemorrhage. Curr Drug Saf. 2012;7(3):190–6.
Rogy MA, Coyle SM, Oldenburg HS, et al. Persistently elevated soluble tumor necrosis factor receptor and interleukin-1 receptor antagonist levels in critically ill patients. J Am Coll Surg. 1994;178(2):132–8.
Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59(1):21–7.
Hijdra A, Brouwers PJ, Vermeulen M, van Gijn J. Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke. 1990;21(8):1156–61.
van Gijn J, Hijdra A, Wijdicks EF, Vermeulen M, van Crevel H. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg. 1985;63(3):355–62.
Frontera JA, Fernandez A, Schmidt JM, et al. Defining vasospasm after subarachnoid hemorrhage: What is the most clinically relevant definition? Stroke. 2009;40(6):1963–8.
Vergouwen MDI, Vermeulen M, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5.
Janssen PM, Visser NA, Dorhout Mees SM, Klijn CJM, Algra A, Rinkel GJE. Comparison of telephone and face-to-face assessment of the modified Rankin Scale. Cerebrovasc Dis. 2010;29(2):137–9.
Aoki T, Fukuda M, Nishimura M, Nozaki K, Narumiya S. Critical role of TNF-alpha-TNFR1 signaling in intracranial aneurysm formation. Acta Neuropathol Commun. 2014;2(1):34.
Gruber A, Rössler K, Graninger W, Donner A, Illievich MU, Czech T. Ventricular cerebrospinal fluid and serum concentrations of sTNFR-I, IL-1ra, and IL-6 after aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2000;12(4):297–306.
Sedger LM, McDermott MF. TNF and TNF-receptors: from mediators of cell death and inflammation to therapeutic giants—past, present and future. Cytokine Growth Factor Rev. 2014;25(4):453–72.
Lotocki G, Alonso OF, Dietrich WD, Keane RW. Tumor necrosis factor receptor 1 and its signaling intermediates are recruited to lipid rafts in the traumatized brain. J Neurosci. 2004;24(49):11010–6.
Lucke-Wold BP, Logsdon AF, Manoranjan B, et al. Aneurysmal subarachnoid hemorrhage and neuroinflammation: a comprehensive review. Int J Mol Sci. 2016;17(4):1–17.
Sehba FA, Pluta RM, Zhang JH. Metamorphosis of subarachnoid hemorrhage research: from delayed vasospasm to early brain injury. Mol Neurobiol. 2011;43(1):27–40.
Pantoni L, Sarti C, Inzitari D. Cytokines and cell adhesion molecules in cerebral ischemia: experimental bases and therapeutic perspectives. Arterioscler Thromb Vasc Biol. 1998;18(4):503–13.
McKeating EG, Andrews PJ, Signorini DF, Mascia L. Transcranial cytokine gradients in patients requiring intensive care after acute brain injury. Br J Anaesth. 1997;78(5):520–3.
Hirashima Y, Nakamura S, Endo S, Kuwayama N, Naruse Y, Takaku A. Elevation of platelet activating factor, inflammatory cytokines, and coagulation factors in the internal jugular vein of patients with subarachnoid hemorrhage. Neurochem Res. 1997;22(10):1249–55.
Tuttolomondo A, Pecoraro R, Pinto A. Studies of selective TNF inhibitors in the treatment of brain injury from stroke and trauma: a review of the evidence to date. Drug Des Dev Ther. 2014;8:2221–39.
Acknowledgments
We thank the Clinical Pathology Laboratory of Hospital São José, CHLC, for careful preparation of the blood samples.
Funding
Dr Fragata was supported by Sociedade Portuguesa de AVC/Tecnifar. Dr Bustamante is supported by a Juan Rodes research contract (JR16/00008) from Instituto de Salud Carlos III.
Author information
Authors and Affiliations
Contributions
IF, AB, PC, JM contributed to project design; IF, AB, AP, PF, APN contributed to data collection; AB, IF performed data analysis; IF, AB, JM, PC contributed to Writing. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Institutional review board approval was obtained for this study. Informed consent obtained from patient or legal representative.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure 1
Flowchart depicting patients enrolled, included, and excluded in the study (TIFF 80095 kb)
Rights and permissions
About this article
Cite this article
Fragata, I., Bustamante, A., Penalba, A. et al. Venous and arterial TNF-R1 predicts outcome and complications in acute subarachnoid hemorrhage. Neurocrit Care 31, 107–115 (2019). https://doi.org/10.1007/s12028-019-00669-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00669-9