Abstract
Background and Purpose
Poor-grade subarachnoid hemorrhage (SAH) (World Federation of Neurosurgical Societies grade 4 and 5) is associated with high mortality rates and unfavorable functional outcomes. We report a single-center cohort of poor-grade SAH patients, combined with a systematic review of studies reporting functional outcome in the poor-grade SAH population.
Methods
Data on a cohort of poor-grade SAH patients treated between 2009 and 2013 were retrospectively collected and combined with a systematic review (from inception to November 2015; PubMed, Embase). Two reviewers assessed the studies independently based on predefined inclusion criteria: consecutive poor-grade SAH, functional outcome measured at least 3 months after hemorrhage, and the report of patients who died before aneurysm treatment.
Results
The search yielded 329 publications, and 23 met our inclusion criteria with 2713 subjects enrolled from 1977 to 2014 in 10 countries (including 179 poor-grade patients from our cohort). Mortality rate was 60 % (1683 patients), of which 806 (29 %) died before and 877 (31 %) died after aneurysm treatment, respectively. Treatment was undertaken in 1775 patients (1775/2826—63 %): 1347 by surgical clipping (1347/1775—76 %) and 428 (428/1775—24 %) by endovascular methods. Outcome was favorable in 794 patients (28 %) and unfavorable in 1867 (66 %). When the studies were grouped into decades, favorable outcome increased from 13 % in the late 1970s to early 1980s to 35 % in the late 1980s to early 1990s, and remained unchanged thereafter.
Conclusion
Although mortality remains high in poor-grade SAH patients, a favorable functional outcome can be achieved in approximately one-third of patients. The development of new diagnostic methods and implementation of therapeutic approaches were probably responsible for the decrease in mortality and improvement in the functional outcome from 1970 to the 1990s. The plateau in functional outcome seen thereafter might be explained by the treatment of sicker and older patients and by the lack of new therapeutic interventions specific for SAH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the landmark publication by Hunt and Hess (H&H) [1], the authors proposed a classification to estimate the perioperative risk of death in patients suffering from aneurysmal subarachnoid hemorrhage (SAH). Patients were grouped in five categories according to the severity of focal and global neurological deficits and the presence of preexisting comorbidities. The grading system ranges from asymptomatic patients with minimal headache and slight nuchal rigidity (Grade 1) to patients in “deep coma, decerebrate rigidity, moribund appearance” (Grade 5). Based on patients’ grade at admission and prior to operation, the mortality was 71 and 100 % in patients admitted in poor neurological condition, i.e., Grades 4 and 5. The authors then concluded “prompt surgical intervention is important for patients admitted in good condition. Conservative therapy until the patient’s condition improves is advisable for the more seriously ill” [1].
The admission neurological condition classified by H&H [1] or by the World Federation of Neurosurgical Societies (WFNS) [2] is one of the most powerful predictors of mortality and long-term disability after SAH [3]. Poor-grade SAH (i.e., H&H 4 or 5 or WFNS 4 or 5) has frequently been associated with poor outcome. However, the selection of patients for treatment based on admission neurological grade may result in withdrawing of life support from approximately one-third of the patients who could possibly have good functional recovery if aggressively treated [4].
We report our experience in poor-grade SAH patients treated aggressively in a high-volume academic center in Toronto, Canada. All patients admitted in poor neurological condition from 2009 to 2013 were included in the analysis, even those who died before angiography or aneurysm treatment. A systematic literature review addressing functional outcome after poor-grade SAH was performed and reported along with our results.
Methods
Part A: Single-Center Study
We retrospectively collected data from all SAH patients admitted between January 2009 and December 2013, including baseline demographics, initial and follow-up Glasgow Coma Scale (GCS), modality of aneurysm treatment, surgical interventions [e.g., craniectomy and external ventricular drain (EVD) insertion], and modified Rankin Scale (mRS) score at the patients’ last follow-up visit in our neurovascular clinic, occurring at least 3 months after hemorrhage. All patients were admitted to a dedicated Trauma and Neurosurgical Intensive Care Unit at St. Michael’s Hospital, a high-volume [5] academic center affiliated with the University of Toronto, Canada. During the study period, 568 SAH patients were admitted. From this cohort, we only selected patients with poor-grade SAH, defined as WFNS 4 or 5 [6].
The neurological examination was obtained immediately before EVD insertion and used to assess the initial neurological status according to the WFNS grade. Intracerebral hematoma (ICH) volume was measured using the ABC/2 method [7]. Cerebral infarction due to delayed cerebral ischemia (DCI) was defined as a new hypodensity on head computed tomography (CT) within 6 weeks after SAH, or the latest CT or magnetic resonance imaging (MRI) before death (when it occurred within 6 weeks and was not present on a CT or MRI done within 48 h of the aneurysm repair procedure). Hypodensities attributed to aneurysm treatment (e.g., surgical retractions), EVD placement, or ICH were not considered cerebral infarctions due to DCI [8]. The radiological images were all analyzed and reported by neuroradiologists. They were unaware of the study.
The primary outcome was in-hospital mortality. Secondary outcome included functional neurological recovery assessed at least 3 months after hemorrhage, defined by the mRS score. The patients were treated according to our institutional SAH protocol, which has been published elsewhere [9–11], and closely follows the guidelines published by the Neurocritical Care Consensus Conference [12] and the American Heart Association/American Stroke Association [13].
Because of the risk of aneurysm recanalization and late rebleeding [14], patients treated by endovascular coiling are seen routinely for a face-to-face follow-up in our neurovascular clinic to assess the need for late retreatment [15]. After endovascular coiling, the postoperative surveillance is performed by magnetic resonance angiography (MRA) [16] according to our institutional protocol: MRA in the first week after coiling followed by MRA at 8 weeks, 6 months, 1 year, and 3 years after treatment [17]. If there are concerns regarding recanalization (e.g., aneurysm >10 mm or incomplete occlusion) or if the patient is young (<40 years old), the surveillance is kept beyond the third year [17]. Patients who had their aneurysm clipped undergo computed tomography angiography (CTA) before discharge, at 8 weeks, 1 year, between 3 and 5 years, and every 5 years thereafter [17].
During the routine face-to-face follow-up, patients are assessed for motor and speech deficits and the ability to live and walk without assistance (ability to live alone means the patient is able to use the toilet, bathe, shop, prepare and get meals, and manage finances). The functional information was retrieved from the medical charts and classified according to the simplified mRS questionnaire algorithm [18].
Statistical Analysis
All the data were divided into continuous variables and discrete variables, which included categorical and dichotomous ones. Most of the continuous data were normally distributed, while some data had almost normal distribution with several outliers. The decision was made to compare the patient characteristics of the two groups (i.e., WFNS 4 and 5) that were presented as continuous variables using the Student t test with Welch’s correction for heteroscedasticity in order to correct for differences in the variances of the groups. Two-way proportion test with continuity correction was used for the comparison of dichotomous and categorical variables (Table 1). All the analyses were performed in the R {v3.0.2} statistical environment.
Part B: Systematic Literature Review
Our research question and the inclusion and exclusion criteria were established before the systematic review was performed. The protocol was prepared according to the PRISMA guidelines [19] and registered in the international prospective register of systematic reviews (PROSPERO–CRD42015029716). The literature search (see Electronic Supplementary Material) on outcomes after poor-grade SAH was performed through PubMed and Embase databases. Two reviewers (ALOM and AM) independently screened article titles and abstracts. Articles with at least an abstract in English were searched using the following combination: SAH and poor-grade or high-grade (for complete search strategy, see Electronic Supplementary Material; last search on November 20, 2015). When a given cohort generated multiple publications, the most recent published article was included. In case of disagreement, one additional author (MRG) was used to resolve the disagreement by consensus among the three reviewers. The reference list of all selected studies was searched for additional eligible articles. The authors own databases were also searched, and the expert opinion of one of the authors (RLM) supervised the process.
Articles met the inclusion criteria if they reported (1) patients with SAH, defined as the presence of subarachnoid blood in the noncontrast CT or lumbar puncture; (2) an abstract in English; (3) inclusion of consecutive patients; (4) functional outcome measure at least 3 months after hemorrhage; and (5) patients not treated or who died before aneurysm treatment. Articles were excluded if they were (1) conference abstracts, and (2) cohorts not reporting patients who died before initiation of treatment or who were not treated at all. A more detailed outline of the inclusion/exclusion criteria and search protocol can be found in the Electronic Supplementary Material.
Results
Part A: Single-Center Study
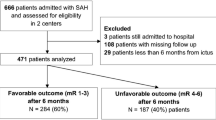
A total of 179 poor-grade SAH patients were included, which comprises 32 % of the total SAH population admitted during the study period (Fig. 1). Table 1 describes the baseline characteristics in the St. Michael's Hospital poor-grade cohort, including the patients who died before aneurysm treatment. Seventy-seven (77/179—43 %) and 102 patients (102/179—57 %) were WFNS 4 and 5, respectively. WFNS 5 patients were less likely to be female (82 vs. 58 % in WFNS 4 and 5, respectively, p < 0.01), and to undergo endovascular treatment (65 vs. 44 %, p < 0.01). However, WFNS 5 patients were more likely to have thick subarachnoid blood associated with bilateral ventricular hemorrhage (i.e., modified Fisher 4; 32 vs. 53 %, p = 0.01), larger ICH volume (17.7 ± 13.4 vs. 40.1 ± 36.5, p < 0.01), to die before aneurysm treatment (5 vs. 28 %, p < 0.01), and had higher percentages of unfavorable outcome (19 vs. 34 %, p < 0.05).
One hundred and forty-six patients (146/179—82 %) underwent digital subtraction angiography (DSA); and 137 (137/179—76 %) patients had their aneurysm treated, 95 by endovascular approach (95/179—53 %), and 42 by surgical clipping (42/179—23 %). Nine patients (9/179—5 %) had a diffuse SAH pattern and negative DSA.
Outcomes
Mortality (Fig. 1)
Thirty-three patients (33/179—18 %) died before aneurysm treatment, of which 22 (22/33—68 %) received an emergency EVD. The vast majority of patients who died before aneurysm treatment were WFNS 5 [29/33 patients (88 %)]. Twenty-five (25/179—14 %) patients died after aneurysm treatment, resulting in a total of 58 in-hospital deaths (58/179—32 %). The mortality among patients with WFNS 4 and 5 was 5 and 28 % before aneurysm treatment (p < 0.01), and 10 and 17 % after aneurysm treatment (p = 0.33—Table 1), respectively. In total, 12 patients with WFNS 4 (12/77—15 %) and 46 patients with WFNS 5 (46/102—45 %) died.
Functional Outcome
One hundred and twenty-one patients (121/179—68 %) were discharged alive. We were able to retrieve the outcome assessment of 105 patients. Sixteen patients (16/179—9 %) were lost in follow-up (Fig. 1). The mean time from hemorrhage to mRS assessment was 13 ± 13 months. Eighty patients (80/179—45 %) had a favorable functional outcome (mRS ≤ 2). They were more likely to be WFNS 4 than WFNS 5 (66 vs. 28 %, p < 0.01). Twenty-five patients who survived (25/179—14 %) had unfavorable outcomes (Fig. 1).
Part B: Systematic Review
The search yielded a total of 329 publications, 23 of which met our inclusion criteria (Fig. 2; Table 2). A total of 2713 patients were studied from 1977 to 2014, in 10 countries across Europe, Asia, and North America. One thousand one hundred and seventy-three patients were WFNS 4 (1173/2713—43 %), while 1347 (1347/2713—50 %) were WFNS 5. One publication with 166 poor-grade patients (166/2713—6 %) did not distinguish between WFNS 4 and 5 [20]. Sixty percent of patients died (1683/2826—60 %), of those, 806 patients (806/2713—29 %) before aneurysm treatment and 877 (877/2713—31 %) after angiography or aneurysm treatment. In total, 1775 patients underwent treatment (1775/2713—63 %), 1347 (1347/1775—76 %) by surgical clipping and 428 (24 %) by endovascular methods.
The definitions of favorable outcome differed slightly among the studies. All articles included in this systematic review reported functional outcome measured by Glasgow Outcome Scale (GOS) [21], extended GOS [22], neurological examinations, independence in activities of daily living (ADLs), or mRS [23].
In the majority of studies (17/24—70 %), favorable outcome was reported as a GOS score of 4 or 5. One study defined favorable outcome as an eGOS of 5–8 [24]. Four studies defined favorable outcome according to ADLs [25–28], which considered ability to return to work and lifestyle independency. One study defined favorable outcome as a mRS of 0–3 [29]. The last studies [30], including our cohort, were more conservative defining favorable outcome (i.e., mRS 0–2). In total, 794 patients (794/2826—28 %) achieved a favorable outcome (Table 3).
The studies were grouped according to the years of enrollment (Table 3). Newer studies (2000–2010) included a larger sample of WFNS 5 patients compared to early studies (69 vs. 42 %). The number of treated poor-grade SAH patients increased from approximately 50 % in the late 1970s–early 1980s to almost 80 % in the last two decades. Regarding modality of treatment, surgical clipping was the only modality of treatment until the mid 1990s, when some studies started to report the use of endovascular coiling to treat poor-grade SAH patients [28]. The newer cohorts have a higher number of patients managed by endovascular methods, with at least half of the poor-grade patients being treated by endovascular methods in the last 15–20 years [24, 30, 31].
The percentage of favorable outcome after severe SAH initially reported was very small (average of 13 %). A favorable outcome of 35 % has been reported by studies from the late 1980s–early 1990s and continued to be between 30 and 40 % thereafter (Table 3). Although the percentage of favorable outcome has not changed over the last 30 years, the number of WFNS 5 patients included and treated in the newer studies was larger when compared to early studies (43 vs. 69 %—Fig. 3).
Chart of favorable versus unfavorable functional outcome. This chart shows the functional outcome trends according to different decades of patients’ enrollment in the included articles. Also, the percentage of Grade 5 patients included was plotted, showing a trend to increase inclusion of Grade 5 patients. The definition of favorable outcome varies according to study, including GOS 4–5, mRS 0–2, ability to live independently (for full detail, please refer to Table 2)
Discussion
In this retrospective study, we report our experience with aggressive treatment of poor-grade SAH patients and combined our results with a systematic review, which includes 23 cohorts of unselected poor-grade SAH patients treated from 1977 to 2014 in 10 countries. We found that mortality remains high in this patient population (approximately 30 % before and 30 % after aneurysm treatment). However, favorable outcome is possible in approximately one-third of patients admitted in poor neurological condition. Unselected poor-grade SAH population was defined as cohorts that reported patients who were not treated or died before angiography or aneurysm treatment. Additionally, only studies that reported functional outcome at least 3 months after hemorrhage were included in the final qualitative analysis. There are a large number of studies reporting data on this patients’ population that excludes patients that died before aneurysm treatment, which could overestimate the percentage of patients with favorable outcome. Likewise, studies that report early outcome (<3 months) were also excluded to avoid the overestimation of unfavorable outcome.
Very early studies reported high mortality rates close to 100 % in the grade 5 patients [1]. These initial reports tended to use clinical grading scales to decide about eligibility for treatment. Hunt and Hess [1] suggested that “conservative therapy until the patient’s condition improves is advisable for the more seriously ill.” The vast majority of these patients admitted in poor neurological condition would die without surgical treatment [1]. However, the selection of patients for treatment based on admission neurological grade may result in withdrawing of life support from approximately one-third of the patients who could possibly have good functional recovery if aggressively treated [4].
We showed that the percentage of patients who died before aneurysm treatment decreased over time, from 46 % in the late 1970s–early 1980s to a current mortality rate of approximately 20 % (Table 3). This drop in mortality before aneurysm treatment was accompanied by an increase in the percentage of poor-grade patients treated (from 51 to 79 %—Fig. 3). This increase in the percentage of poor-grade patients treated may reflect a change in current practice, to aggressively treat poor-grade SAH patients [11], which probably explains the drop in the percentage of patients who die before aneurysm treatment. Additionally, this finding might also be explained by recent data showing a decrease of in-hospital death over time associated with the reduction of death from aneurysm rebleeding, probably because of earlier aneurysm treatment [32]. Rebleeding is common in the poor-grade population [24], and it is associated with high mortality rates [33] and poor functional outcome [24]. van den Berg et al. [24], in a cohort of WFNS 5 patients, reported a rebleeding rate of approximately 20 %. Among 26 patients who rebleed, only one had a favorable outcome (1/26—4 %). Therefore, the early diagnosis combined with early aneurysm treatment might explain this time-trend reduction of in-hospital mortality associated with rebleeding. Although poor-grade SAH patients may benefit from early aneurysm repair to prevent rebleeding [24], the ideal timing of treatment remains to be determined [34, 35].
SAH case fatality has decreased since the 1960s, as shown by several studies [36, 37]. Two high-quality meta-analyses previously published covering 5 decades, from 1960 to 1995 [37] and from 1995 to 2007 [36], have already shown a decrease in SAH case fatality. Hop et al. [37] performed a systematic review and meta-analysis, which included 21 studies between 1960 and 1992. Case fatality rates varied between 32 and 67 % and decreased by 0.5 % per year. The same group published a follow-up meta-analysis showing a 17 % case fatality drop over three decades (between 1973 and 2002) [36]. During this period, case fatality varied from 8.3 to 66.7 % between studies and decreased 0.8 % per year [36]. The authors justified this reduction in case fatality over time by the introduction of improved management strategies such as endovascular coiling, the use of calcium channel blockers, and the implementation of more accurate diagnostic tools [36].
Favorable Outcome
The first study included in the systematic review that reported long-term functional outcome after poor-grade SAH came from the late 1970s [38]. Hijdra et al. [38] prospectively included 70 poor-grade patients, of those only 2 (3 %) progressed to a favorable outcome. Favorable outcome improved to approximately 30 % in the mid 1980s–early 1990s and plateaued thereafter. However, this plateau in the last 3 decades was accompanied by an increase in the percentage of Grade 5 patients included and treated in more recent cohorts. Additionally, the mean age of patients in the general SAH population had increased in the period 1973–2002 from 52 to 62 years [36].
Several reasons may have contributed to the reduction in morbidity and mortality over the first decades assessed in this article: (1) development of accurate diagnostic tools [17]; (2) the use of nimodipine [39, 40]; (3) the surgical and endovascular treatment procedures developed to occlude a ruptured aneurysm [41]; (4) the introduction of stroke and dedicated neuroscience intensive care units [42]; and (5) the patients’ management in high-volume centers [5]. However, since the key trial by Pickard et al. [40], showing the improvement in functional outcome with the prophylactic use of nimodipine, and the introduction of detachable coils for the endovascular treatment of intracranial aneurysms by Guglielmi and colleagues in 1991 [43], no other treatment strategy developed specific for SAH has been shown to improve functional outcome.
Early and Accurate Diagnosis Probably Corroborated to Improve Outcome
From the mid 1960s to mid 1990s, several important diagnostic methods were developed for the management of patients suffering from acute brain injury, including SAH, which probably corroborated to improve favorable outcome. The institution of CT, MRI, and catheter angiography have greatly improved the accuracy of the diagnosis of SAH and also the detection of cerebral aneurysms and angiographic vasospasm [17]. Misdiagnosis of SAH is not uncommon, and it is associated with increased mortality and morbidity [44]. Currently, noncontrast CT sensitivity for SAH approaches 100 % if performed within 6 h of headache onset [45]. Therefore, the institution of CT greatly improved our ability to detect SAH, which is an important advance for the early diagnosis of SAH. Additionally, CTA and DSA are highly sensitive to detect cerebral aneurysms, even small ones (<3 mm), which permits the early detection, planning, and treatment of ruptured aneurysms [17].
CTA and DSA are also sensitive and have a high degree of correlation for diagnosing proximal angiographic cerebral vasospasm [17]. Approximately 50 % of patients with angiographic cerebral vasospasm will develop delayed cerebral ischemia, which is a major determinant of morbidity and mortality after aneurysmal SAH [46]. The introduction of these techniques allowed monitoring, detecting, and treating angiographic cerebral vasospasm, which have the potential benefit of reducing angiographic vasospasm and improving neurological deficits [46].
Aneurysm Treatment
Surgical clipping was the main modality of treatment compared to endovascular coiling (76 vs. 24 %). Surgical clipping was the exclusive modality of treatment in 14 studies, which represents 58 % of all included cohorts. From the mid 1990s on [28], an increased number of patients treated by endovascular means were included in the studies. One study did not report the modality of treatment [29].
The introduction of the surgical microscope in the 1970s advanced the microsurgical approach to intracranial aneurysms [47]. Surgical clipping remained the sole approach to treat intracranial aneurysms for decades, as shown in Table 3. In 1991, Guglielmi and colleagues [43] introduced the use of detachable coils for the endovascular treatment of intracranial aneurysms. Thereafter, the number of patients treated by endovascular means has gradually increased (Table 3). A large randomized clinical trial showed that the short- and long-term morbidity and mortality rates are decreased by the endovascular treatment of ruptured intracranial aneurysms compared with surgical clipping [14, 41, 48]. Additionally, the timing of treatment has shifted from late (>10 days) to early (0–3 days) after the SAH [49].
Medical Management, Dedicated Neurocritical Care, and High-Volume Centers
Several drugs have been tested for the management of SAH [11]; however, nimodipine remains the only one proven drug to reduce the risk of delayed cerebral ischemia and poor functional outcome and also to be approved in North America and Europe. The prophylactic use of nimodipine was shown to decrease by 40 % poor functional outcome [40], and it is currently considered the standard of care [12, 13, 50]. Additionally, patients’ outcomes are influenced by the center of admission [5]. Patients treated in high-volume centers (>60 cases per year) have a lower risk of death and a higher chance of favorable outcome [5, 51]. McNeill et al. [51] showed that the caseload of SAH is inversely related to 6-month mortality. According to the authors, each 100-patient increase in annual patient volume was associated with a 24 % reduction in mortality. Many factors may influence the better outcomes achieved at high-volume centers including (1) the patients admission to dedicated stroke and neurocritical care units; (2) the presence of neurointensivists; and (3) the endovascular coiling and intra-arterial rescue treatment performed by interventional neuroradiologists [5].
In our cohort, one hundred and twenty-one patients (121/179—68 %) were discharged alive, and 80 patients (80/179—45 %) were completely independent (mRS ≤ 2), including 51 patients admitted with WFNS 4 (51/77—66 %) and 29 patients with WFNS 5 (29/102—28 %). The high proportion of favorable outcomes in our cohort may have resulted from:
-
1.
Aggressive treatment of poor-grade patients, including external ventricular drain insertion (i.e., 79 % of patients underwent EVD insertion);
-
2.
The use of an institutional protocol for the management of patients suffering from SAH [9, 11];
-
3.
The treatment of culprit aneurysm by endovascular approach (i.e., only 23 % of patients were treated by surgical clipping);
-
4.
The access to dedicated neurocritical care and specialized multidisciplinary team (i.e., all patients were managed in a dedicated neuro ICU and were cared for by a multidisciplinary team, which includes specialized neuro ICU nurses, vascular neurosurgeons, interventional neuroradiologists, and neurointensivists) [9];
-
5.
The access to interventional neuroradiologists (i.e., according to a USA study, less than 37 % of SAH patients are treated at centers with access to interventional neuroradiology) [52];
-
6.
The province of Ontario regionalization of care, and St. Michael's Hospital—a high-volume center (i.e., between 180 and 200 SAH patients are treated in our hospital per year. Most patients are transferred from low-volume centers or hospitals without capability of neurovascular management. Transfer to high-volume centers appears to be cost-effective, and regionalization of care may be necessary [51, 53]).
Additionally, a percentage of approximately 40 % in favorable outcome has been previously described by several cohorts included in this systematic review [4, 26, 27, 54, 55]. The highest percentage of favorable outcome described in this systematic review was 68 % [56].
Study Limitations
First, studies included in this review enrolled patients treated from the late 1970s to now, making it difficult to explain the factors associated with changes in functional outcome in the last 3–4 decades. Second, the studies included in this systematic review used different definitions and different timing of assessment of favorable outcome. Most studies measured functional outcome by GOS; however, other scales were also used. Additionally, the timing of functional outcome was assessed in some studies at 3 months, while others studies assessed patients at 6–12 months.
Most importantly, the timing of neurological assessment and the grade scale applied to classify the patients may influence long-term functional outcome. For example, Ransom et al. [57] showed that poor-grade SAH patients, who respond to EVD insertion improving ≥one H&H grade, have long-term functional outcome similar to patients admitted with lower grade hemorrhages. Ideally patients should be graded after the initial clinical stabilization, which includes EVD insertion and cerebral spinal fluid drainage. Most studies included in this systematic review did not comment on the timing of neurological assessment.
Regarding clinical grading scales, the H&H scale is a well-known and widely used scale; however, the authors recognized its issues: “It is recognized that such classifications are arbitrary and that the margins between categories may be ill defined” [1]. Words such as drowsy, stupor, and deep coma, are ambiguous and subjective, which make this scale less precise. Some studies included in this review used the H&H scale. The WFNS scale is the most frequently used and recommended clinical grading scale. Its benefits over the H&H scale are (1) it uses less subjective terms (because it is primarily based on the GCS, and (2) it grades level of consciousness and focal deficits on two separate axes. Most studies included in this review used the WFNS scale.
The main limitation of the WFNS scale is the broad GCS range in the Grade 4 (GCS: 7–12), compromising patients that may have a very wide range of functional outcomes [58]. Additionally, WFNS 5 (GCS 3–6) with signs of brainstem compression (posturing and/or pupillary abnormalities) have worse functional outcome compared with WFNS 5 patients without brainstem dysfunction [59]. It has been proposed to classify the WFNS 5 patients without brainstem dysfunction as WFNS 4 instead [59].
Lastly, patients classified as having favorable outcome commonly experience deficits in memory, executive function, and language [60]. However, the functional outcome scales applied in the studies included in the systematic review might not be sensitive enough to detect these subtle deficits that may affect the patients’ day-to-day activities (ceiling effect).
Conclusion
Mortality remains high in poor-grade SAH population; however, a drop in mortality before aneurysm treatment has been described. It was accompanied by an increase in the percentage of poor-grade patients treated, which supports the early aneurysm treatment in this population. Surgical clipping has been the main reported modality of treatment; however, endovascular coiling has increasingly been used in the last two decades. If treated aggressively, one-third of poor-grade SAH patients can achieve independence and resume working. The development of new diagnostic methods (e.g., CT, MRA, and DSA) and implementation of therapeutic approaches, i.e., the use of nimodipine and endovascular coiling, were probably responsible for the decrease in mortality and improvement in functional outcome from 1970 to the 1990s. The plateau in functional outcome in the last 3 decades might be explained by the treatment of sicker and older patients and by the lack of new therapeutic interventions specific for SAH.
References
Hunt WEW, Hess RMR. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–20.
Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry. 1988;51:1457.
Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2315–21.
Le Roux PD, Elliott JP, Newell DW, Grady MS, Winn HR. Predicting outcome in poor-grade patients with subarachnoid hemorrhage: a retrospective review of 159 aggressively managed cases. J Neurosurg. 1996;85:39–49.
The participants in the international multi-disciplinary consensus conference on the critical care management of subarachnoid hemorrhage, Vespa P, Diringer MN. High-Volume Centers. Neurocrit Care. 2011;15:369–372.
Drake CG. Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J Neurosurg. 1988;68:985–6.
Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–5.
Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5.
de Oliveira Manoel AL, Turkel-Parrella D, Duggal A, Murphy A, McCredie V, et al. Managing aneurysmal subarachnoid hemorrhage: it takes a team. Clevel Clin J Med. 2015;82:177–92.
Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Publ Group. 2013;10:44–58.
de Oliveira Manoel AL, Goffi A, Marotta TR, Schweizer TA, Abrahamson S, Macdonald RL. The critical care management of poor-grade subarachnoid haemorrhage. Crit Care. 2016;20:21.
Diringer MN, Bleck TP, Claude Hemphill J, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the neurocritical care society’s multidisciplinary consensus conference. Neurocrit Care. 2011;15:211–40.
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:1711–37.
Molyneux AJ, Kerr RS, Yu L-M, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–17.
Campi A, Ramzi N, Molyneux AJ, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the international subarachnoid aneurysm trial (ISAT). Stroke. 2007;38:1538–44.
van Amerongen MJ, Boogaarts HD, de Vries J, et al. MRA versus DSA for follow-up of coiled intracranial aneurysms: a meta-analysis. AJNR. 2014;35:1655–61.
de Oliveira Manoel AL, Mansur A, Murphy A, et al. Aneurysmal subarachnoid haemorrhage from a neuroimaging perspective. Crit Care. 2014;18:557.
Bruno A, Shah N, Lin C, et al. Improving modified Rankin Scale assessment with a simplified Questionnaire. Stroke. 2010;41:1048–50.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41.
Wilby MJ, Sharp M, Whitfield PC, Hutchinson PJ, Menon DK, Kirkpatrick PJ. Cost-effective outcome for treating poor-grade subarachnoid hemorrhage. Stroke. 2003;34:2508–11.
Jennett B, Bond M. Assessment of outcome after severe brain damage: a practical scale. Lancet. 1975;305:480–4.
Teasdale GM, Pettigrew L. Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J Neurotrauma. 1998;15:587–97.
Wilson JT, Hareendran A, Grant M, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33:2243–6.
van den Berg R, Foumani M, Schröder RD, et al. Predictors of outcome in World Federation of Neurologic Surgeons grade V aneurysmal subarachnoid hemorrhage patients. Crit Care Med. 2011;39:1–2727.
Chyatte D, Fode NC, Sundt TM. Early versus late intracranial aneurysm surgery in subarachnoid hemorrhage. J Neurosurg. 1988;69:326–31.
Bailes JE, Spetzler RF, Hadley MN, Baldwin HZ. Management morbidity and mortality of poor-grade aneurysm patients. J Neurosurg. 1990;72:559–66.
Lee KC, Huh SK, Park HS, Shin YS, Lee KS. Management of poor-grade patients with ruptured intracranial aneurysm. Keio J Med. 1997;46:69–73.
Rordorf GG, Ogilvy CSC, Gress DRD, Crowell RMR, Choi ISI. Patients in poor neurological condition after subarachnoid hemorrhage: early management and long-term outcome. Acta Neurochir. 1997;139:1143–51.
Starke RM, Komotar RJ, Otten ML, et al. Predicting long-term outcome in poor grade aneurysmal subarachnoid haemorrhage patients utilising the Glasgow Coma Scale. J Clin Neurosci. 2009;16:26–31.
Schuss P, Hadjiathanasiou A, Borger V, Wispel C, Vatter H, Güresir E. Poor-grade aneurysmal subarachnoid hemorrhage: factors influencing functional outcome—a single-center series. World Neurosurg. 2016;85:125–9.
Ross J, O’Sullivan MG, Grant IS, Sellar R, Whittle IR. Impact of early endovascular aneurysmal occlusion on outcome of patients in poor grade after subarachnoid haemorrhage: a prospective, consecutive study. J Clin Neurosci. 2002;9:648–52.
Vergouwen M, Jong-Tjien-Fa AV, Algra A. Time trends in causes of death after aneurysmal subarachnoid hemorrhage: a hospital-based study. Neurology. 2016;86:59–63.
Ameen AA, Illingworth R. Anti-fibrinolytic treatment in the pre-operative management of subarachnoid haemorrhage caused by ruptured intracranial aneurysm. J Neurol Neurosurg Psychiatry. 1981;44:220–6.
Mitra D, Gregson B, Jayakrishnan V, et al. Treatment of poor-grade subarachnoid hemorrhage trial. AJNR. 2015;36:116–20.
Oudshoorn SC, Rinkel GJE, Molyneux AJ, et al. Aneurysm treatment <24 versus 24–72 h after subarachnoid hemorrhage. Neurocrit Care. 2014;21:4–13.
Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8:635–42.
Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28:660–4.
Hijdra A, Braakman R, van Gijn J, Vermeulen M, van Crevel H. Aneurysmal subarachnoid hemorrhage. Complications and outcome in a hospital population. Stroke. 1987;18:1061–7.
Petruk KC, West M, Mohr G, et al. Nimodipine treatment in poor-grade aneurysm patients. Results of a multicenter double-blind placebo-controlled trial. J Neurosurg. 1988;68:505–17.
Pickard JD, Murray GD, Illingworth R, et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ. 1989;298:636–42.
Molyneux AA, Kerr RR, Stratton II, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267–74.
Kramer AH, Zygun DA. Do neurocritical care units save lives? Measuring the impact of specialized ICUs. Neurocrit Care. 2011;14:329–33.
Guglielmi G, Viñuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: preliminary clinical experience. J Neurosurg. 1991;75:8–14.
Kowalski RG, Claassen J, Kreiter KT, et al. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA. 2004;291:866–9.
Perry JJ, Stiell IG, Sivilotti MLA, et al. Sensitivity of computed tomography performed within 6 h of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ. 2011;343:d4277.
Kimball MM, Velat GJ, Hoh BL. Participants in the international multi-disciplinary consensus conference on the critical care management of subarachnoid hemorrhage. Critical care guidelines on the endovascular management of cerebral vasospasm. Neurocrit Care. 2011;15:336–41.
Yasargil MG, Fox JL. The microsurgical approach to intracranial aneurysms. Surg Neurol. 1975;3:7–14.
Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RSC. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International subarachnoid aneurysm trial (ISAT). Lancet. 2015;385:691–7.
Ohman J, Heiskanen O. Timing of operation for ruptured supratentorial aneurysms: a prospective randomized study. J Neurosurg. 1989;70:55–60.
Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G. European stroke organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35:93–112.
McNeill L, English SW, Borg N, Matta BF, Menon DK. Effects of institutional caseload of subarachnoid hemorrhage on mortality: a secondary analysis of administrative data. Stroke. 2013;44(3):647–52.
Cross DTD, Tirschwell DLD, Clark MAM, et al. Mortality rates after subarachnoid hemorrhage: variations according to hospital case volume in 18 states. J Neurosurg. 2003;99(5):810–7.
Bardach NS, Olson SJ, Elkins JS, Smith WS, Lawton MT, Johnston SC. Regionalization of treatment for subarachnoid hemorrhage: a cost-utility analysis. Circulation. 2004;109:2207–12.
Quigley MR, Salary M. Defining survivorship after high-grade aneurysmal subarachnoid hemorrhage. Surg Neurol. 2008;69:261–5.
Barcelos GK, Tholance Y, Grousson S, et al. Outcome of poor-grade subarachnoid hemorrhage as determined by biomarkers of glucose cerebral metabolism. Neurocrit Care. 2013;18:234–44.
Taylor B, Harries P, Bullock R. Factors affecting outcome after surgery for intracranial aneurysm in Glasgow. Br J Neurosurg. 1991;5:591–600.
Ransom ER, Mocco J, Komotar RJ, et al. External ventricular drainage response in poor grade aneurysmal subarachnoid hemorrhage: effect on preoperative grading and prognosis. Neurocrit Care. 2007;6:174–80.
Rosen DS, Macdonald RL. Subarachnoid hemorrhage grading scales: a systematic review. Neurocrit Care. 2005;2:110–8.
Fung C, Inglin F, Murek M, et al. Reconsidering the logic of World Federation of Neurosurgical Societies grading in patients with severe subarachnoid hemorrhage. J Neurosurg. 2016;124:299–304.
Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41:e519–36.
Ohno K, Suzuki R, Masaoka H, Monma S, Matsushima Y, Inaba Y. A review of 102 consecutive patients with intracranial aneurysms in a community hospital in Japan. Acta Neurochir. 1988;94:23–7.
Inagawa T, Takahashi M, Aoki H, Ishikawa S, Yoshimoto H. Aneurysmal subarachnoid hemorrhage in Izumo City and Shimane Prefecture of Japan. Outcome Stroke. 1988;19:176–80.
Seifert V, Trost HA, Stolke D. Management morbidity and mortality in grade IV and V patients with aneurysmal subarachnoid haemorrhage. Acta Neurochir. 1990;103:5–10.
Nowak G, Schwachenwald R, Arnold H. Early management in poor grade aneurysm patients. Acta Neurochir. 1994;126:33–7.
Alberti O, Becker R, Benes L, Wallenfang T, Bertalanffy H. Initial hyperglycemia as an indicator of severity of the ictus in poor-grade patients with spontaneous subarachnoid hemorrhage. Clin Neurol Neurosurg. 2000;102:78–83.
Inamasu J, Nakamura Y, Saito R, Kuroshima Y, Mayanagi K, Ichikizaki K. Endovascular treatment for poorest-grade subarachnoid hemorrhage in the acute stage: Has the outcome been improved? Neurosurgery. 2002;50:1199–1205 (discussion 205–6).
Ritz R, Schwerdtfeger K, Strowitzki M, Donauer E, Koenig J, Steudel W-I. Prognostic value of SSEP in early aneurysm surgery after SAH in poor-grade patients. Neurol Res. 2002;24:756–64.
Laidlaw JD, Siu KH. Poor-grade aneurysmal subarachnoid hemorrhage: outcome after treatment with urgent surgery. Neurosurgery. 2003;53:1275–1280 (discussion 1280–2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Macdonald receives grant support from the Physicians Services Incorporated Foundation, Brain Aneurysm Foundation, Canadian Institutes for Health Research, and the Heart and Stroke Foundation of Canada and is Chief Scientific Officer of Edge Therapeutics, Inc.
Conflict of interest
The other authors report no conflict of interest.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Oliveira Manoel, A.L., Mansur, A., Silva, G.S. et al. Functional Outcome After Poor-Grade Subarachnoid Hemorrhage: A Single-Center Study and Systematic Literature Review. Neurocrit Care 25, 338–350 (2016). https://doi.org/10.1007/s12028-016-0305-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-016-0305-3