Abstract
Introduction
Early identification of delayed cerebral ischemia (DCI) in patients with aneurysmal subarachnoid hemorrhage (aSAH) is a major challenge. The aim of this study was to investigate whether quantitative EEG (qEEG) features can detect DCI prior to clinical or radiographic findings.
Methods
A prospective cohort study was performed in aSAH patients in whom continuous EEG (cEEG) was recorded. We studied 12 qEEG features. We compared the time point at which qEEG changed with the time point that clinical deterioration occurred or new ischemia was noted on CT scan.
Results
Twenty aSAH patients were included of whom 11 developed DCI. The alpha/delta ratio (ADR) was the most promising feature that showed a significant difference in change over time in the DCI group (median −62 % with IQR −87 to −39 %) compared to the control group (median +27 % with IQR −32 to +104 %, p = 0.013). Based on the ROC curve, a threshold was chosen for a combined measure of ADR and alpha variability (AUC: 91.7, 95 % CI 74.2–100). The median time that elapsed between change of qEEG and clinical DCI diagnosis was seven hours (IQR −11–25). Delay between qEEG and CT scan changes was 44 h (median, IQR 14–117).
Conclusion
In this study, ADR and alpha variability could detect DCI development before ischemic changes on CT scan was apparent and before clinical deterioration was noted. Implementation of cEEG in aSAH patients can probably improve early detection of DCI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Subarachnoid hemorrhage (SAH) has a worldwide incidence of 10.5 per 100.000. Approximately 80 % is caused by a rupture of an intracranial aneurysm [1]. Delayed cerebral ischemia (DCI) is the major cause of mortality and morbidity following aneurysmal SAH (aSAH) [2]. DCI typically occurs at day 3–14 following aSAH. DCI complicates approximately 30 % of the patients, often resulting in deterioration of consciousness and/or focal neurological deficits [3–7]. A major challenge in the management of aSAH is early identification of DCI, thereby enabling therapeutic interventions. However, clinical assessment of DCI is subjective and unreliable in patients who are comatose, sedated, or paralyzed [8]. A diagnostic tool with improved sensitivity to detect DCI is needed. Ideally, such a tool could detect DCI prior to start of clinical symptoms, enabling early treatment, to prevent irreversible brain damage and improve outcome. Electroencephalography (EEG) provides real-time information about cortical activity. When neuronal function is compromised due to insufficient cortical perfusion, various EEG changes occur, such as depression of fast activity or slowing of rhythms [9]. This deterioration of neuronal function is potentially reversible, providing a window of opportunity for therapeutic interventions [10–12]. The EEG might be suitable to detect DCI at an early stage and monitor effects of therapeutic interventions.
Compared to imaging techniques as computed tomography (CT) or magnetic resonance imaging (MRI), EEG allows continuous bedside monitoring. Some studies investigating the relation between DCI and continuous EEG (cEEG) monitoring have suggested that cEEG can assist in early detection of DCI [13–17]. Only two studies elaborated on the possibility to detect compromised neuronal function prior to clinical deterioration [16, 17]. One study only included patients at high risk for DCI, based on the modified Fisher scale. It was found that in three out of eight patients, changes in cEEG occurred prior to clinical changes [16]. The second study used cEEG monitoring in six DCI patients and was able to detect DCI prior to changes in transcranial Doppler (TCD) [17]. In this study, we explore if cEEG can be used as a diagnostic tool for detection of DCI prior to onset of clinical symptoms and/or ischemia development on CT. Furthermore, we investigate which quantitative EEG features are most sensitive for the detection of ischemia.
Methods
A prospective cohort study was performed from December 2012 until June 2013 in the mixed medical surgical intensive care unit (ICU) of two Dutch academic medical centers (Academic Medical Center Amsterdam and Radboud University Medical Centre, Nijmegen). Adult patients with aSAH admitted to the ICU were included after aneurysm treatment (coiling or clipping as decided by the neurosurgeon). Exclusion criteria were as follows: life expectancy < 48 h, severe additional traumatic brain injury, or cardiac arrest or DCI onset prior to start of EEG recording. When diagnosis of DCI was based solely on ischemia on CT, EEG data had to be available of > 48 h prior to ischemia development, otherwise the patient was excluded as well. The Institutional review board waived the need for informed consent for both participating centers, as cEEG monitoring is standard care and no other interventions were performed.
Clinical Data
Baseline demographics (age, gender), World Federation of Neurosurgeon (WFNS) classification, Glasgow Coma Scale (GCS), and focal neurological deficits as described on admission were recorded [18]. Also listed were severity of hemorrhage based on the Fisher classification [19], type of aneurysm, and mode of aneurysm treatment (coiling or clipping). After inclusion, minimum and maximum GCS, the absence/presence of focal neurological deficits, and relevant changes on CT/CT-angiography were reported. Type and dosage of administered sedatives, anti-epileptics, and vasopressors were listed daily. At hospital discharge, the modified Ranking Scale (mRS) was assessed [20]. DCI was defined as (1) clinical neurological deterioration such as a decrease in consciousness and/or development of new focal neurological deficits, otherwise unexplained and/or (2) new ischemic lesion on cerebral CT scan (not described on admission CT) [6]. DCI was diagnosed by the treating physicians (neurosurgeon and intensivist). In both study centers, DCI was treated by induction of hypertension on the ICU. The patient had to be normovolemic and the aneurysm had to be secured. Hypertension was induced by intravenous administration of norepinephrine, increasing mean arterial pressure (MAP), guided by clinical performance. Baseline MAP, cardiac function and age were taken into account. Treatment was not changed based on the results of the cEEG monitoring.
EEG Monitoring
CEEG monitoring was started as soon as possible after securing of the aneurysm by coiling or clipping, but at least within 5 days of aSAH. Eight electrodes were used, placed according to the international 10–20 system: F4, T4, P4, O2, F3, T3, P3, O1. Additionally, one ground electrode (Fz) and one reference electrode (Cz) were placed. The recording lasted as long as the patient was admitted to the ICU, but was ended whenever the patient developed scalp irritations, or otherwise did not tolerate the electrodes. When a patient was sedated, the first EEG epoch recorded at least 2 h after cessation of sedative drugs was used. After ICU discharge to the ward, short recordings (30 min) were made regularly. When a patient was readmitted to the ICU the cEEG recording was restarted. Results of cEEG registrations were not available to the treating physicians, and no therapeutic strategies were based on the results. Recordings were performed with a ViaSys Nicolet system (Natus Europe GmbH, Planegg, Germany) using a sample frequency of 1000 Hz. Analog filter settings were set between 0.053 and 500 Hz. The EEG data were saved as EDF+ files.
Data Analysis
Post hoc EEG analysis was performed, as 24-h analysis of EEG was not available. The EEG data were filtered with high-pass cut-off frequency of 0.5 Hz and low-pass of 35 Hz and fragmented into epochs of maximum 1 h each. Of each recording of 1 h of EEG data, five consecutive minutes containing the least amount of artifacts were selected. This was performed automatically by calculating the maximum height of amplitude compared to standard deviation, and the occurrence of flat lines in the EEG and the amount of EMG artifacts (>30 Hz) for each epoch [21]. The resulting epoch was manually inspected for residual artifacts.
Power spectral densities (PSD) were estimated using Welch’s method, using MATLAB (MATLAB R2012b, the MathWorks, inc. MA, USA) from which 12 quantitative EEG (qEEG) features were calculated (Table 1). The total power calculated for all individual EEG channels represents the power of the signal between 1 and 30 Hz. The frequency bands (delta, theta, alpha, beta) were calculated as a ratio of the total power. The alpha/delta ratio (ADR) and the alpha–beta/delta-theta ratio (ABDTR) were calculated from the previously mentioned frequency bands. The spectral edge frequency x (SEFx) is a measure which represents the frequency below which x percent of the total power of a given signal are located. SEF75 and SEF90 were used as quantitative EEG features. Asymmetry of the EEG was calculated by comparing powers of channels on the left hemisphere to the channels on the right hemisphere. The bispectral index (BSI) was calculated in the frequency range 1–7 Hz (BSI slow) and 7–25 Hz (BSI fast) [22]. Additional details are presented in the Appendix.
Statistical Analysis
Statistical analyses were performed using R (R 3.0.0, R Foundation for Statistical Computing, Vienna, Austria). Differences between individual features were evaluated with a paired t test, if data were normally distributed; otherwise a Wilcoxon Rank-Sum test for paired analysis was used, and data were considered significant if p < 0.05.
The percentage of change in qEEG after DCI development was compared to the percentage of change in qEEG of the non-DCI patients (control group). This enabled us to select the qEEG features that performed best for detection of DCI. In control patients, the percentage of change between the first EEG and the last EEG was calculated. In DCI patients, the first EEG was compared with the first EEG that was made following diagnosis of DCI based on clinical or radiographic criteria. In each patient, the electrode pair showing the largest change was selected. The time that elapsed between the two time moments (Δt) was calculated for all patients. Data from control patients were used for this analysis when Δt was >6 h. DCI patients were used when EEG data were available of >48 h prior to DCI diagnosis to ensure a sufficient window for detection of early changes. The two groups were compared using the student’s t test for normally distributed data and the Mann–Whitney U for not normally distributed data. The features that showed a significantly larger change in DCI patients compared to control patients were selected. A cut-off value for the selected qEEG features was determined with a receiver operating curve (ROC) curve. When more than one qEEG feature for detection of DCI was identified, a combined measure was used by selecting the feature that first exceeded the cut-off value per individual patient.
In individual DCI patients, we determined when the selected qEEG features first became aberrant. This yielded the interval in time between aSAH and exceeding of the threshold value based on the ROC curve. This interval was compared to the time interval that elapsed between aSAH and diagnosis DCI clinically or radiographically. Subsequently, we evaluated the potential of these EEG features to detect DCI prior clinical or radiographic diagnosis.
Results
Patients
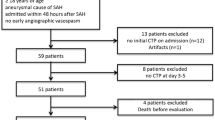
A total of 58 aSAH patients were assessed for eligibility of whom 21 were included in this study, see Fig. 1. In one patient, qualitatively sufficient EEG segments were unavailable due to severe agitation. Of the 20 remaining patients, 11 developed DCI. Table 2 shows the baseline characteristics and outcome data in the DCI group and control group.
Selection of EEG Features
Three patients who developed DCI were not included in this analysis, because EEG data were unavailable (two patients) or because of continuous sedation (one patient). Figure 2 schematically shows the moment that EEG recording was started and the moment that DCI was diagnosed. The selection of qEEG features for DCI detection was based on the eight remaining DCI patients (Table 3). A representative example of the EEG changes due to DCI development is depicted in Fig. 3.
A schematic overview of the moment that EEG was started compared to the moment of DCI diagnosis with each horizontal line representing a patient. Patient 2 had no EEG registration after DCI diagnosis due to severe agitation. EEG registration of patient 5 was started soon after clinical deterioration and was therefore also not taken into account for further analysis. Patient 14 was continuously sedated with different dosages and was therefore also not used for further analysis
Example of changes that occurred as a result of DCI (patient 3). Initially, the patient showed good recovery after aSAH with a GCS of E4M6V5 and no paresis. Panel A shows a fragment of the unprocessed EEG data on day 2 after aSAH. Panel B shows a topoplot that was made based on the alpha–delta ratio of the same EEG epoch. Panel C shows the CT scan that was made on day 1 after aSAH. On day 10 after aSAH, the patient showed a decrease of consciousness (E2M5V2) and a hemiparesis left, after which the patient was readmitted to the ICU and EEG monitoring was restarted. Panel D shows a fragment of EEG data made on day 11 after a panel E represents the corresponding topoplot. Panel F shows the CT scan made on day 12 after aSAH (the CT scan on day 10 did not show any changes compared to the scans made earlier)
First, a comparison was made between EEG signals in the DCI group versus the control group. Of the control group, six patients had two EEG recordings with a minimal interval of >6 h, the other patients were discharged from the ICU soon after admission. Delta, alpha, ADR, ABDTR, and BSI slow all showed a significant difference between DCI and control patients (p < 0.05), see Fig. 4. Of these, ADR was selected, as it showed the largest and most significant difference over time in the DCI group (median −62 % with IQR −87 to −39 %) compared to the control group (median +27 % with IQR −32 to +104 %, p = 0.013). As ADR encompasses alpha and delta frequencies and overlaps with ABDTR, the latter were not selected for further analysis. Alpha variability with a difference over time in the DCI group of −31 % (IQR −50 to −1 %) and in the control group of −4 % (IQR −11 to +6 %, p = 0.181) was also selected for analysis.
Boxplots of EEG features in the DCI (n = 8) and control group (n = 6). The y-axis represents the percentage of change of the qEEG feature between T1 (first available EEG epoch) and T2 (first epoch after DCI development for the DCI group or the last available epoch for the control group). The two groups were compared for each of the features using the Mann–Whitney U test. Delta, alpha, ADR, ABDTR, and the BSI slow showed a statistically significant difference (p < 0.05)
Early Detection of DCI
From the ROC curves (Fig. 5) a threshold value of −38 % (100 % sensitivity and 83.3 % specificity) in either ADR or alpha variability was chosen. This value was used to designate the moment at which the cEEG recording showed a change in cortical activity. All eight DCI patients showed a decrease of >38 % of either ADR or alpha variability. DCI was clinically diagnosed in eight patients, seven also developed ischemic lesions on CT scan. In five patients, the DCI compatible EEG changes were found prior to clinical DCI diagnosis. In seven patients, qEEG changes compatible with DCI developed before ischemia was identified on CT. The median time that elapsed between change of qEEG and clinical DCI diagnosis was 7 h (IQR −11 to −25), and a median of 44 h (IQR 14–117) delay between qEEG and CT changes. The location of the lesions on CT scan was compared to topoplots (Fig. 3). In four patients, widespread hypodensities in both hemispheres were found. This corresponded to the deterioration of EEG patterns in all of the EEG channels. In one patient with a focal lesion on CT scan, the EEG channels that showed the most severe deterioration corresponded to the area of hypodensity. In a patient with infarction of the thalamus and internal capsule, the EEG deterioration was most prominent in the frontal and temporal electrodes. Finally, a focal ischemic lesion basofrontally on CT scan yielded EEG deterioration on all channels, although only marginally in the occipital areas.
ROC curves of ADR, alpha variability, and a combined measure that encompasses either ADR or alpha variability, which is selected per patient on the basis of determination of the one showing the maximum change. These features were selected as the ones that were best able to distinct the DCI group from the control group based on the performed statistical tests
Discussion
The aim of this study was to find specific quantitative EEG features capable of early detection of DCI after aSAH. We found that the ADR and a combined measure of ADR and alpha variability allow early detection of DCI, at a time point when DCI is potentially reversible. EEG was able to detect ischemia several hours prior to clinical deterioration and 44 h prior to detection of ischemic lesions on CT scan.
Other small studies have investigated qEEG features for detection of DCI in 19 [14], 9 [15], and 6 [17] aSAH patients. The relative alpha variability, the ADR, and alpha power, respectively, were described as the most useful qEEG features for detection of DCI. The composite alpha index (a combined measure of alpha power and alpha variability) in anterior brain regions was also suggested to be a useful marker for DCI [16]. The total power did not differentiate between DCI and control patients in our study, which is In contrast to a study in 11 aSAH patients that reported the total EEG power to be the best indicator of DCI [13]. We investigated all features reported so far separately and assessed their additional value. ADR, alpha power and alpha variability were found to be the most promising.
The ADR is a composite feature of the alpha band and the delta band power spectra densities. The appearance of high-voltage delta oscillations and attenuation of alpha activity are the most commonly described phenomena in cerebral ischemia [9]. It is therefore not surprising that the ADR performs well in detecting DCI, although the degree to which alpha and delta are related in their underlying physiological mechanism has not been clarified. Other features besides ADR, alpha, and delta that showed good correlation with DCI in this study were ABDTR, SEF75, SEF90, and alpha variability. The ABDTR considerably overlaps with the ADR since it is constructed of alpha, beta, delta, and theta. Beta power is known to decrease as a result of ischemia, although this effect is less frequently documented than the change in delta and alpha activity [28]. Our findings are consistent with these observations as beta activity diminished in patients developing DCI. In our study, theta activity did not seem to be affected by DCI development at all, as was described earlier [29]. Theta activity alone has shown to be an unreliable measure for pathophysiological electrical activity of the cortex [29]. Alpha variability and associated qEEG features (such as the composite alpha index) have been investigated less than previously mentioned features. Beside the two studies on DCI detection, alpha variability was studied in traumatic brain injury patients [14, 16, 30]. A decrease of alpha variability was indicative for a poor outcome. It has been suggested that rhythmic fluctuations of alpha activity may change as a result of reduced brain perfusion through impairment of thalamic firing thereby affecting the cortico-thalamic loop [14].
None of the previous studies investigating the diagnostic ability of EEG compared EEG of aSAH patients that developed DCI to EEG of patients that did not develop DCI. Other reasons for changes in qEEG features can therefore not be excluded. We compared the percentage of change that occurred in the DCI group to a control group, thereby increasing likelihood that the observed change is related to DCI development. Secondly, in our study, an algorithm was used to select the EEG epoch that contained the least amount of artifacts [21]. Previous studies performed this epoch selection manually, except for one recent study that also used an automatic algorithm for artifact rejection [17]. Manual selection might impede the objectivity of the data analysis.
Two recent studies focused on the subset of aSAH patients with either a poor Hunt–Hess grade or a poor radiographic grade [16, 23]. This leads to exclusion of a large group of patients at risk for developing DCI. We enrolled patients regardless of their initial radiographic or clinical score. It is known that patients with an initial poor clinical score are more likely to develop DCI. Our results expand on this observation, demonstrating the importance of early detection in DCI patients with a less severe aSAH. Four out of 11 patients that developed DCI had a WFNS ≤3. Two other studies limited their analysis to awake patients which restricts extrapolation of results to all aSAH patients [13, 14]. One study mainly focused on radiographic vasospasm as a primary outcome. This is known to have a high false positive rate for diagnosis of DCI [14]. The most recently performed similar study included patients regardless of their initial clinical state or radiographic findings. They related changes in qEEG features mainly to DCI detection by TCD and were able to predict DCI >2 days in advance [17].
The use of cEEG monitoring was recently recommended for detection of DCI in poor grade aSAH patients [24]. Based on our results, we suggest that further research should be performed on cEEG monitoring in all aSAH patients. Especially, patients who primarily improve are at risk of secondary deterioration. A method that identifies the onset of DCI early might provide a window for interventions and therefore may improve outcome.
Since EEG is a reflection of synaptic activity in the cortex, synaptic silencing due to energy depletion can result in very early changes in EEG [11, 27]. To detect these changes, cEEG recording should start as soon as possible. It is possible that in some of our patients, a change in cortical activity had already occurred before EEG monitoring was started. As a cut-off for DCI development, we used a decrease of >38 % in qEEG in comparison to the baseline registration. Often, patients with an aSAH first recover from the initial hemorrhage. This recovery is also reflected on the selected qEEG features, so that an improvement first has to be overcome before a deterioration compared to baseline can be noticed. When the change would have been calculated in relation to the last available EEG the deterioration might be noticed even earlier. The drawback of this approach is that it is much more susceptible to daily fluctuations. EEG as a monitoring tool for DCI has some important advantages compared to other diagnostic tests such as transcranial Doppler and CT scans. EEG is a non-invasive, bedside available tool, which can continuously monitor brain function. A drawback of EEG is the fact that activities from deep parts of the brain such as the thalamus and basal ganglia are hardly detectable by scalp electrodes. It should be regarded as a monitor of the cortex. Automated quantitative analyses offer outcome parameters that are easy to interpret. When combined with repeated clinical examination and CT scans on indication a careful monitoring is possible.
The relevance of our study results for improving outcome in aSAH patients greatly depends on effective and safe treatments for DCI. Traditionally, DCI is treated with a combination of hypertension, hypervolemia, and hemodilution in order to improve cerebral perfusion. A recent review demonstrated that hemodilution and hypervolemia did not contribute to improvement of outcome and that induction of hypertension alone was as effective [25]. However, studies on therapeutic hypertension so far did not report a clear beneficial effect on outcome [26]. This ineffectiveness of therapeutic interventions may be due to the delay in diagnosis of DCI. For further research, we propose a study validating the relevant qEEG parameters using automatic EEG analysis software. Live qEEG feedback can be compared to clinical performance and more detailed information regarding the gain in therapeutic window can be obtained. Furthermore, if qEEG monitoring and automatic analyses provide a reliable indication of DCI development, a study in which therapeutic interventions are guided by qEEG parameters would be of great interest.
The strength of this study is our study design, using control patients to compare to DCI patients, thereby minimizing the probability that EEG changes can be attributed to other factors than DCI development. The study population is a heterogeneous group of aSAH patients admitted to the ICU of two medical centers, with no in- or exclusion criteria based on the severity of bleeding of clinical performance. By following up on patients after ICU discharge to the ward, we were able to stress the relevance of monitoring aSAH patients after primary clinical improvement.
An important limitation of our study is the small number of included patients, which was insufficient to create a subset of patients for validation of the threshold. Secondly, by selecting those features with the best ability for detection of DCI, the analysis that was subsequently performed may have been over-fitted. We suggested a cut-off value of −38 %, while the interquartile range of ADR in the control patients was −32 to +104 %. The clinical utility of this cut-off value as a single tool for diagnosing DCI may be limited. However, it does give an indication of impaired cerebral perfusion and should therefore be taken into account in assessment of the patient’s recovery. External data validation might improve the strength of this diagnostic tool. This study primarily focuses on the potential use of EEG for early detection of DCI. Although other parameters like hemodynamic data and the use of medication may add additional diagnostic information, they were left out in our study design.
Conclusion
The results of this pilot study show that cEEG monitoring, using the quantitative features ADR and alpha variability, is a promising tool for early detection of DCI. A median of 7 h elapsed between change seen in cEEG and clinical symptoms and there was a median of 44 h delay between cEEG and radiographic findings of DCI. Our results need additional validation in a larger patient cohort admitted after aSAH.
References
Suarez J, Tarr R, Selman W. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354:387–96.
Koenig M. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Continuum (Minneap Minn). 2012;18:579–97.
Dorsch N, King M. A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage. J Clin Neurosci. 1994;1:19–26.
Neil-Dwyer G, Lang D, Doshi B, Gerber C, Smith P. Delayed cerebral ischaemia: the pathological substrate. Acta Neurochir. 1994;131:137–45.
Rabinstein A, Weigand S, Atkinson J, Wijdicks E. Patterns of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2005;36:992–7.
Claassen J, Bernardini G, Kreiter K, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke. 2001;32:2012–20.
Murayama Y, Malisch T, Guglielmi G, et al. Incidence of cerebral vasospasm after endovascular treatment of acutely ruptured aneurysms: report on 69 cases. J Neurosurg. 1997;87:830–5.
Bederson J, Connolly E, Batjer H, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025.
Finnigan S, Walsh M, Rose S, Chalk J. Quantitative EEG indices of sub-acute ischaemic stroke correlate with clinical outcomes. Clin Neurophysiol. 2007;118:2525–32.
Sharbrough F, Messick J, Sundt T. Correlation of continuous electroencephalograms with cerebral blood flow measurements during carotid endarterectomy. Stroke. 1973;4:674–83.
Sundt T, Sharbrough F, Anderson R, Michenfelder J. Cerebral blood flow measurements and electroencephalograms during carotid endarterectomy. J Neurosurg. 1974;107:887–97.
Baron J-C. Perfusion thresholds in human cerebral ischemia : historical perspective and therapeutic implications. Cerebrovasc Dis. 2001;11:2–8.
Labar D, Fisch B, Pedley T, Fink M, Solomon R. Quantitative EEG monitoring for patients with subarachnoid hemorrhage. Electroencephalogr Clin Neurophysiol. 1991;78:325–32.
Vespa P, Nuwer M, Juhász C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol. 1997;103:607–15.
Claassen J, Hirsch L, Kreiter K, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004;115:2699–710.
Rathakrishnan R, Gotman J, Dubeau F, Angle M. Using continuous electroencephalography in the management of delayed cerebral ischemia following subarachnoid hemorrhage. Neurocrit Care. 2011;14:152–61.
Gollwitzer S, Groemer T, Rampp S, et al. Early prediction of delayed cerebral ischemia in subarachnoid hemorrhage based on quantitative EEG : a prospective study in adults. Clin Neurophysiol. 2015;126:1514–23.
Drake C, Hunt W, Kassell N, Sano K, Pertuiset B, De Villiers J. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry. 1988;51:1457.
Fisher C, Kistler J, Davis J. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9.
Van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–7.
Cloostermans M, de Vos C, van Putten M. A novel approach for computer assisted EEG monitoring in the adult ICU. Clin Neurophysiol. 2011;122:2100–9.
Van Putten M, Tavy D. Continuous quantitative EEG monitoring in hemispheric stroke patients using the brain symmetry index. Stroke. 2004;35:2489–92.
Claassen J, Hirsch L, Frontera J, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006;4:103–12.
Claassen J, Taccone F, Horn P, Holtkamp M, Stocchetti N, Oddo M. Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensiv Care Med. 2013;39:1337–51.
Dankbaar J, Slooter A, Rinkel G, van der Schaaf I. Effect of different components of triple-H therapy on cerebral perfusion in patients with aneurysmal subarachnoid haemorrhage: a systematic review. Crit Care. 2010;14:R23.
Gathier CS, van den Bergh WM, Slooter AJC, et al. HIMALAIA (Hypertension Induction in the Management of AneurysmaL subArachnoid haemorrhage with secondary IschaemiA): a randomized single-blind controlled trial of induced hypertension vs. no induced hypertension in the treatment of delayed cerebral ischem. Int J Stroke. 2014;9:375–80.
Hofmeijer J, van Putten M. Ischemic cerebral damage: an appraisal of synaptic failure. Stroke. 2012;43:607–15.
Leon-Carrion J, Martin-Rodriguez J, Damas-Lopez J, y Martin JB, Dominguez-Morales M. Delta-alpha ratio correlates with level of recovery after neurorehabilitation in patients with acquired brain injury. Clin Neurophysiol. 2009;120:1039–45.
Nuwer MR, Jordan SE, Ahn SS. Evaluation of stroke using EEG frequency analysis and topographic mapping. Neurology. 1987;37:11–53.
Vespa P, Boscardin J, Hovda D, et al. Early and persistent impaired percent alpha variability on continuous electroencephalography monitoring as predictive of poor outcome after traumatic brain injury. J Neurosurg. 2002;97:84–92.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Michel van Putten is co-founder of Clinical Science Systems. Marjolijn Rots, Astrid Hoedemaekers, and Janneke Horn declare that they have no conflict of interest.
Appendix: Used Equations
Appendix: Used Equations
with n the number of available data points
with M the number of channel pairs and N the number of Fourier coefficients
with M the number of channel pairs and N the number of Fourier coefficients.
Rights and permissions
About this article
Cite this article
Rots, M.L., van Putten, M.J.A.M., Hoedemaekers, C.W.E. et al. Continuous EEG Monitoring for Early Detection of Delayed Cerebral Ischemia in Subarachnoid Hemorrhage: A Pilot Study. Neurocrit Care 24, 207–216 (2016). https://doi.org/10.1007/s12028-015-0205-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-015-0205-y