Abstract
Background
Using clinical parameters to identify and monitor treatment response in patients with delayed cerebral ischemia (DCI) following subarachnoid hemorrhage is challenging. We sought to determine whether continuous electroencephalography (CEEG) aids the prediction of the clinical course and response to treatment of DCI.
Methods
Patients deemed high-risk for DCI based on the modified Fisher scale were prospectively monitored. A novel CEEG parameter measuring relative alpha power and variability in the anterior brain quadrants termed composite alpha index (CAI) was graphically displayed. Predictions of the status of patients for the ensuing day were made by an independent reviewer, first using clinical data then repeated following the addition of CAI trends. These were compared to the actual clinical state. The reviewer was blinded to the presence and treatment of DCI. Patients with DCI were further studied by trending the daily mean alpha power against the modulation of treatment and clinical evolution.
Results
Fifty-nine predictions were made in 12 patients (mean age 54.3 years, range 35–70; nine females) with Hunt–Hess grades ranging I–V. Sensitivity of predicting clinical deterioration with CEEG improved from 40 to 67% and clinical improvement from 8 to 50%. In three patients, CEEG was predictive greater than 24 h prior to clinical change. Tracking the daily mean alpha power accurately identified DCI recurrence and poor responders to first-line therapy at pre-clinical stages.
Conclusion
CEEG is a useful non-invasive tool to supplement routine clinical parameters in the prediction of DCI. It can dynamically monitor the response to treatment and might aid pre-clinical management decisions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Subarachnoid hemorrhage (SAH) has a worldwide incidence of 10.5 per 100,000 [1]. A third of patients who survive need long-term care and only 20 to 35% make a good functional recovery. [1]. Vasospasm is a major complication of SAH. Angiographic vasospasm is observed in 70% of patients though not all are symptomatic [1–5]. Distinct from this, delayed cerebral ischemia (DCI) is a clinical phenomenon of vasospasm-related hypoperfusion and occurs in 22–40% of patients, resulting in morbidity and mortality rates of up to 30% [2, 5, 6].
A major challenge in the management of SAH is the early identification of patients at risk of developing DCI using a non-invasive method that has optimal spatial and temporal resolution. Serial clinical assessments may not detect its onset in patients who are comatose, sedated, or paralysed by neuromuscular blockade [2, 4]. Furthermore, even in awake patients, DCI restricted to the anterior brain region is not easy to diagnose acutely. Isolated neurobehavioral and cognitive deficits characteristic of frontal dysfunction are often evident only after permanent ischemic damage has occurred [7].
Continuous electroencephalography (CEEG) is a non-invasive tool that can provide real-time measurement of electrical brain activity [8, 9]. Pyramidal neurons in cortical layers 3, 5, and 6 contribute to the activity seen on scalp EEG and are particularly vulnerable to hypoxia and ischemia [9, 10]. EEG abnormalities arise when the normal cerebral blood flow (CBF) of 50 to 70 ml/100 g/min decreases to 25 to 30 ml/100 g/min. Neuronal death occurs when the CBF drops below 10 to 12 ml/100 g/min [11]. CEEG, therefore, provides a potential window of opportunity to therapeutically intervene by detecting changes in cerebral perfusion prior to the point of irreversible damage [9, 12, 13].
Using fast Fourier transformation (FFT), raw EEG data can be compressed into quantitative EEG (qCEEG) parameters. In three studies that used this technique, various features were shown to correlate with DCI such as the trending of the total EEG power, relative alpha variability, and alpha–delta ratios [4, 14, 15]. Changes on qCEEG were observed prior to Transcranial Doppler (TCD) abnormalities and preceded clinical deterioration up to 2 days [14, 15]. However, a significant limitation is that different quantitative parameters were studied in patient populations of selected Hunt–Hess grades. It has been shown to be particularly useful in patients with poor-grade SAH in whom clinical assessments are difficult [4]. The baseline EEG recordings are likely to be very different between grades [4]. Hence, limited conclusions can be made regarding the best quantitative parameter irrespective of clinical status at the time of presentation.
We aimed to further this body of work by determining the feasibility of using qCEEG in predicting DCI-related clinical changes. We decided not to restrict our study to patients with poor Hunt–Hess grades of SAH at the time of admission. Although clinical signs are easier to detect in patients who initially have minimal or no neurological deficits, we sought to prospectively determine whether qCEEG might also be predictive in this group. These patients have functionally more to lose from a relatively good baseline and early intervention would potentially be beneficial. We wished to also study if qCEEG could chart dynamic changes in brain perfusion during the course of treatment of the DCI that corresponded to the clinical evolution of the patients, particularly to ascertain if recurrence of DCI could be easily detected.
Methods
Subjects
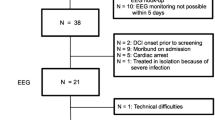
Between November 2008 and August 2009, patients admitted to the Neurological Intensive Care Unit (NICU) at the Montreal Neurological Institute and Hospital were prospectively studied. The attending neuro-intensivist selected patients with a high likelihood of developing symptomatic vasospasm based on a poor radiological grade which was defined as a modified Fisher scale ≥ 3. All Hunt–Hess grades at the time of admission were included. In order to allow generalization of the findings, the inclusion criteria were minimally restrictive so that a representative patient population with aneurysmal SAH could be sampled. The primary inclusion was a poor radiological grade of SAH in order to target patients with a higher likelihood of DCI. Eligible patients underwent CEEG if portable recording machines were available at the time of selection. Due to limitations in hardware availability, CEEG was performed on one patient at a time. CEEG was discontinued either at the point of discharge from the NICU or at the request of the treating physician. Selection was not made based on the nature of the primary treatment of the aneurysm (Guglielmi Detachable Coils or surgical clipping) nor its location. The CEEG findings were analyzed post hoc and were not used to influence clinical decision-making. No comparison was made between patients who underwent recording and those who did not. Thus, clinical data were recorded only for those who were studied. Patients with traumatic and non-aneurysmal SAH were excluded. The study was approved by the hospital’s Research Ethics Board. Informed consent was obtained from the patients or their next of kin in accordance with institutional requirements.
Definition of DCI
This was defined as (1) a new unexplained focal neurological deficit and/or a decreased level of consciousness or (2) a clinically silent infarction on follow-up CT. Other potential causes of the deterioration such as a new structural lesion on neuroimaging (for example, hydrocephalus or re-bleeding) or a metabolic disturbance (for example, an acute electrolyte imbalance or concomitant infection) were rigorously excluded. The diagnosis was independently confirmed by retrospective review of the clinical course of the patient. As the definition of DCI was a clinical one, the presence of radiological vasospasm was supportive but not mandatory. Recurrence was defined as clinical deterioration that was not attributable to other causes, after an initial response to treatment.
Management of DCI
At our institution, management of DCI consists of aggressive management of fluid balance, hemodynamic and temperature control in addition to the use of nimodipine. Based on previously published data [16–19], patients are also administered intravenous milrinone, a phosphodiesterase III inhibitor, initially as a bolus of 0.05–0.1 mg/kg followed by an intravenous infusion titrated stepwise to a maximum of 1.5 μg/kg/min according to clinical response. Those who do not improve despite maximal medical therapy undergo angiography and assessment for possible endovascular therapy.
CEEG Acquisition
CEEG was performed using a digital video bedside monitoring system with a 200 Hz digital sampling rate (Stellate, Montreal, Canada). A standard 10–20 longitudinal bipolar montage was applied using 20 conductive plastic electrodes compatible with neuroimaging (ScanFit Ives Conductive Plastic Electrodes). The high pass filter was set at 0.3 Hz and the low pass filter at 50 Hz. Recording was interrupted only when the patient was transported out of the NICU for angiography (CT was performed in the NICU).
After rigorous removal of artifact-contaminated sections by an experienced electroencephalographer, quantitative analysis was performed. The power within the alpha band (8–15 Hz) was determined in each channel using epochs of 30 s. The mean during a 30 min period was calculated. We restricted our analysis to the anterior quadrants of the brain that included the sensorimotor areas. The posterior quadrants were excluded to avoid misinterpreting physiological asymmetrical alpha activity in parieto-occipital regions as being abnormal. The sum of the alpha power in the following channels represented the left and right anterior regions, respectively: Left: Fp1-F7, Fp1-F3, F3-C3, F7-T3; Right: Fp2-F8, Fp2-F4, F4-C4, F8-T4.
The product of the standard deviation and mean alpha power was used as a composite measure of the variability and amplitude of alpha power. This composite alpha index (CAI) was calculated over 6 h duration and repeated along a window sliding by 30 min. CAI was not computed if a 6 h segment contained more than 90 min of unusable data. Measurements were graphically displayed to provide a continuous daily trend.
To evaluate the utility of CEEG in charting the response to treatment of DCI, daily mean alpha power was correlated with the clinical status. CAI was not used for this purpose as its derivation was based on a sliding time window to provide trends rather than absolute values at discrete time points. Also, 6 h of data interrupted by a maximum of 90 min of unusable data are required for the calculation of CAI. Using the daily mean alpha power allowed the incorporation of maximal artifact-free EEG over a 24 h period.
Prediction of Clinical Status
A neurologist who had no prior knowledge of the cases made predictions of the status of all patients for the ensuing 24 h based solely on the clinical data available from the time of admission to 0900 of the day in question. This included detailed history, serial clinical examinations, medication and sedation status, as well as results of neuroimaging. If clinical deterioration occurred, details regarding investigations performed during that period were provided. Patients were predicted to improve, deteriorate, or remain stable in terms of conscious level and/or neurological examination from the previous 24 h. This same reviewer was blinded to the time and treatment rendered for each episode of symptomatic vasospasm.
Once a prediction based on clinical data alone was made, CAI trends up to 0900 of the day in question were subsequently provided and another prediction made. These were compared to the actual clinical state that was determined from chart reviews, using the same classification, by an independent physician who was not involved in the care of the patient or in making the predictions. Results were plotted on a 3 × 3 table. Sensitivities, specificities, and likelihood ratios of clinical data alone and with additional CEEG data were then calculated, using the actual state as the gold standard.
Results
Thirteen patients were included in the study. Clinical and demographic data are presented on Table 1. Patient 6 was excluded due to technical problems with the CEEG recording. The mean age was 54.3 years (range 35–70 years). The mean duration of recording was 7.25 days (range 3–15). Eight patients developed DCI, in four of whom this was recurrent with two episodes each. Two patients developed frontal lobe syndromes that persisted to the time of discharge. One patient died from a malignant hemispheric infarction. One patient underwent balloon angioplasty as second-line therapy. Patients who underwent sedation were given infusions of propofol at 25 mcg/kg/min.
Prediction of Vasospasm
Fifty-nine days were predicted in 12 patients. Sensitivities, specificities, and likelihood ratios with and without supplemental CEEG data are highlighted in Table 2. An improving trend (in bold) was seen particularly in the ability of CEEG data to assist accurate predictions of deterioration and improvement. Agreement between predicted and observed clinical states was better with supplemental CEEG than clinical data alone (kappa value 0.312 and 0.069, respectively).
A review of the data revealed that in three of the eight DCI patients, changes in the CEEG trends were observed greater than 24 h in advance of any clinical changes (for example see Fig. 1). Predictions based on the CAI at these times were different from the ‘actual’ clinical status as the decrease in CAI would have been asymptomatic during that period. In Fig. 1, the actual clinical state of the patient was classified as stable on days 11 and 12. The decrease in CAI, however, prompted the prediction of deterioration, which did indeed occur on day 13.
Segment of qCEEG of patient 3. The patient is on a milrinone infusion and the CAI trend matched the clinical improvement between days 6 and 9 (higher values of the CAI denote improvement). After the first stepwise decrease of milrinone infusion on day 9, the CAI trend continues to improve. A decrease in the CAI was noted from day 11 onwards during the second reduction in the dose. The patient was clinically well at this point. Deterioration, however, occurred on the evening of day 13 and the patient developed a permanent frontal lobe syndrome. The patient was not sedated during this period
Monitoring the Response to Treatment of DCI
Changes in mean daily alpha power of patients with recurrent DCI were able to accurately predict the clinical evolution following modulation of treatment. Unfavorable responses manifested as decreases in the daily alpha power prior to the onset of clinical changes. Figures 2, 3, and 4 illustrate some of these findings.
Daily mean alpha power in patient 3 trended against the modulation of the dose of milrinone. Alpha power improved in tandem with an increase in the milrinone. During the reduction in the dosage from day 9 onwards, there was a parallel decrease in the alpha power which was clinically silent. Deterioration occurred on day 13 resulting in a permanent neurological deficit
Discussion
Continuous EEG is non-invasive, provides real-time data, and is sensitive to the early onset of ischemia. In this study, a single measurable qEEG parameter improved the ability to predict the evolution of patients with SAH a day in advance by detecting electroencephalographic changes at the pre-clinical stage. It augmented available clinical data in determining the likelihood of a deterioration, improvement, or status quo from the day before. Alterations in qCEEG can precede clinical change by more than 24 h [15]. In our study, this occurred in 3 of the 8 patients who developed DCI. This ultra-early detection suggests that in a proportion of patients, the therapeutic window of intervention to prevent permanent neurological damage may extend beyond 24 h.
The pragmatic design of the prediction exercise demonstrates the applicability of this technique in a simulated clinical scenario that is experienced daily in the NICU. Potentially, the physician would have predictive data for that particular day made available in the morning during rounds. This might provide the opportunity to either perform ancillary investigations to corroborate the findings or to very closely monitor the patient clinically, setting a lower threshold to institute treatment. Predictions were made using visual graphical displays as in Figs. 1 and 5. The reviewer did not have access to raw EEG data. Using this technique, the EEG trends may potentially be interpreted by non-electroencephalographer.
In order to remove any selection bias during the quantification of CEEG, we utilized all artifact-free data that were available rather than a predetermined duration of artifact-free epochs [4]. We devised the CAI following studies that individually demonstrated changes in alpha power and variability as markers of decreased perfusion [14, 15, 20]. By combining these features into a single value, a deviation from baseline was amplified. Hence, subtle changes in the alpha power and variability resulted in larger changes in CAI as opposed to evaluating each parameter on its own. A decrease in both variables would also provide a robust marker of possible DCI that would more readily be identified by the clinician. Performing a continuous analysis using a sliding window enabled a clear visual trend analysis to detect changes.
Selective non-invasive diagnostic tools such as computerized tomography (CT) angiography, CT perfusion, magnetic resonance (MR), single positron emission CT, and transcranial Doppler ultrasound (TCD) have been shown to be useful in the detection of disruptions of perfusion or flow due to vasospasm [2, 21–23]. With the exception of TCD, these methods are not performed by the bed-side and have not shown significant applicability in the pre-clinical detection of DCI [2, 23]. TCD detects high mean cerebral blood flow velocities in the major vessels from macrovascular vasospasm [24]. Up to 8% of the general population have absent acoustic windows and its reliability is dependent on the vessel that is insonated [4, 24]. The sensitivity and false-negative rates of detecting anterior cerebral artery vasospasm are 45 and 55% compared to that of the middle cerebral artery at 64 and 36%, respectively [24]. In addition, DCI may be present without radiological or angiographic evidence of macrovasular vasospasm and vice versa [23, 25]. TCD is insensitive to vasospasm affecting distal vasculature [26]. Detecting a deficit in perfusion rather than a change in vessel diameter or flow alone is more indicative of DCI [23]. However, perfusion studies do not provide continuous data and are initiated only at the point of clinical deterioration [23]. CEEG may be a useful supplemental tool particularly in situations where TCD acoustic windows are absent or anterior region DCI is a particular concern.
Studies of the correlation between clinical grade and symptomatic vasospasm have shown mixed results [27]. While the accuracy in determining high-risk patients is improved using a combination of clinical and investigative parameters, it is not foolproof [26, 28]. We found that CEEG is technically feasible and well tolerated in patients who have none or mild neurological deficits at the time of presentation. Four of our patients (50%) who developed DCI presented with Hunt–Hess grades less than four. In these patients, supplemental CEEG was more accurate than clinical data alone in 27.2% of predictions. Although the improvement is modest, this technique might have clinical applicability in low clinical-grade patients who are deemed to be at high-risk of DCI using other criteria such as radiological features. However, this pilot study was not powered for a subgroup analysis of specific clinical grades.
Previous studies have concentrated on the utility of CEEG in the detection of DCI [4, 14, 15]. The second part of our study assessed the use of CEEG beyond this phase. Specifically, we wished to ascertain if qCEEG could trend the change in cerebral perfusion when treatment is instituted. Due to the stringent requirements of uninterrupted data for calculation of CAI, we used the daily mean alpha power instead for this purpose. Thus, all available artifact-free EEG data could be used. We observed that a reduction in the mean daily alpha power indicated a risk of recurrence. Alternatively a lack of increase in the alpha power following the institution of therapy suggested a poor response. This could prompt aggressive intervention at an earlier stage. These qCEEG patterns were observed in all five patients who were either poor responders or deteriorated following the down-titration of treatment. Patients who did well demonstrated improving daily alpha trends. Utilizing qCEEG beyond the stage of early detection of DCI is a novel application of CEEG in SAH. Supplemental qCEEG data might guide clinicians in deciding the appropriate time to commence, modulate, and wean treatment in patients with DCI.
CEEG did not delay the initiation of neuroimaging in the NICU. We used conductive plastic electrodes that were compatible with neuroimaging modalities such as CT, MRI, and angiography. Hence, the leads did not require removal and the patients were promptly transported. The recording quality of the EEG has been shown to be indistinguishable to that achieved with standard gold electrodes [29].
This study has several limitations. A small number of patients were studied due to the limited number of EEG machines available for this feasibility study. CEEG could not be performed on more than one patient at a time. Although clinical grade was not an inclusion criteria, patients who were felt to be at a higher risk of DCI based on radiological criteria were studied rather than a purely unselected cohort. Due to its design and our aim to simulate a clinical scenario as genuinely as possible, we did not control for the effects of sleep, sedation, and analgesia. However, information regarding the presence and manipulation of sedatives and analgesics was carefully recorded and provided to the reviewer so that interpretation of clinical and CEEG data could be made bearing these in mind. Sleep-related decreases in CAI occurred for only relatively brief periods of the night as patients in NICU are often woken for neurological assessments, medication, and nursing care [4]. We provided clinical and qCEEG data into the early hours of the following morning. The reviewer was, therefore, able to observe the resolution of the transient dips in the CAI during wakefulness as a favorable sign (see Fig. 5). Patients with DCI, however, had a sustained drop in the CAI.
In instances where dips in CAI preceded DCI by more than 24 h, predictions based on the CEEG differed from the ‘true’ clinical status as the decrease in CAI would have been asymptomatic in the following 24-h period. This would likely have had a negative impact on the accuracy of the CEEG as measured in this study.
The analysis of qCEEG did not include the parieto-occipital channels. We were concerned that physiological asymmetry of alpha power in patients of good clinical grade would be misinterpreted as abnormal. The inclusion of the temporal channels encompassed part of the territorial supply of the posterior cerebral artery. However, DCI restricted to the parietal and occipital regions would have been missed using our method.
Due to the post hoc nature of the analysis, we were unable to corroborate the pre-clinical episodes of significant CAI decrease with other diagnostic modalities to confirm the presence of DCI. Supportive radiological data in 7 of the 8 patients were available when DCI was clinically diagnosed. Based on previous physiologic studies correlating CEEG and alterations in cerebral flow and perfusion, there is little reason to suspect that the changes we observed were from other causes [11, 30]. Investigations were instituted at the time of clinical deterioration to rigorously exclude acute structural and metabolic disturbances. Similarly, the changes in daily alpha power in response to titration of DCI treatment were assumed to be secondary to changes in perfusion. While we correlated changes in daily mean alpha power with clinical evolution in response to treatment modulation, it should be emphasized that this study was not designed to assess the efficacy of milrinone which is a non-standard therapy for the treatment of DCI [6].
Although interpretations of the CAI trends could potentially be made by non-electroencephalographers, a significant limitation in our study is the need for an electroencephalographer to pre-process the data by manually removing artifacts. As our study assessed a novel qCEEG parameter in patients of various clinical grades, we felt that using artifact-free signals would be crucial as an initial proof-of-concept. This resulted in some loss of data on the graphical displays of CAI trends which may have had an effect on the ability of the reviewer to make an accurate prediction in some instances. In particular, this may have accounted for some of the poorer statistical results of CEEG such as in the specificity of predicting improvement and sensitivity in predicting stability. With advanced methods to automatically recognize a wide array of artifacts, a system may be developed to provide accurate and instantaneous quantification of EEG in the near future [31]. However, the quality of EEG signal from lead failures remains problematic unless trained personnel are continuously available on-site to correct for these. Recording directly from the cortex may overcome this limitation and provide a more direct assessment of neuronal function for the early detection of secondary neurological complications such as ischemia and non-convulsive seizures [32, 33].
The derivation of the CAI in our study was based on previous research which identified changes in the alpha band during ischemia notably in the penumbric region [14, 15, 34]. However, the use of alternate power bands and their ratios raises the possibility of a wide array of quantitative parameters that may be optimal markers of DCI [4, 34–36].
Conclusion
Quantitative CEEG supplements clinical data in patients with SAH to detect the onset of DCI and predict the evolution of patients. It is technically feasible to perform CEEG in all clinical grades of SAH. We demonstrate the utility of using a composite index that unifies previously reported variables that were identified as markers of the onset of symptomatic vasospasm. In addition, it may assist the clinician in the modulation of treatment of DCI. Further studies are required to determine if pre-clinical intervention based on CEEG changes can improve outcomes from this complication of SAH which carries a significant morbidity and mortality.
References
Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354:387–96.
Rigamonti A, Ackery A, Baker AJ. Transcranial Doppler monitoring in subarachnoid hemorrhage: a critical tool in critical care. Can J Anaesth. 2008;55:112–23.
Dumont AS, Dumont RJ, Chow MM, et al. Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery. 2003;53:123–33.
Claassen J, Hirsch LJ, Kreiter KT, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004;115:2699–710.
Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–72.
Bederson JB, Connolly ES Jr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025.
Greene KA, Marciano FF, Dickman CA, et al. Anterior communicating artery aneurysm paraparesis syndrome: clinical manifestations and pathologic correlates. Neurology. 1995;45:45–50.
Vespa PM, Nenov V, Nuwer MR. Continuous EEG monitoring in the intensive care unit: early findings and clinical efficacy. J Clin Neurophysiol. 1999;16:1–13.
Jordan KG. Emergency EEG and continuous EEG monitoring in acute ischemic stroke. J Clin Neurophysiol. 2004;21:341–52.
Courville CB. Etiology and pathogenesis of laminar cortical necrosis; its significance in evaluation of uniform cortical atrophies of early life. Arch Neurol Psychiatr. 1958;79:7–30.
Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723–5.
Claassen J, Mayer SA, Hirsch LJ. Continuous EEG monitoring in patients with subarachnoid hemorrhage. J Clin Neurophysiol. 2005;22:92–8.
Scheuer ML. Continuous EEG monitoring in the intensive care unit. Epilepsia. 2002;43:114–27.
Labar DR, Fisch BJ, Pedley TA, Fink ME, Solomon RA. Quantitative EEG monitoring for patients with subarachnoid hemorrhage. Electroencephalogr Clin Neurophysiol. 1991;78:325–32.
Vespa PM, Nuwer MR, Juhász C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol. 1997;103:607–15.
Fraticelli AT, Cholley BP, Losser MR, Saint Maurice JP, Payen D. Milrinone for the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2008;39:893–8.
Romero CM, Morales D, Reccius A, et al. Milrinone as a rescue therapy for symptomatic refractory cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2009;11:165–71.
Lannes M, Angle M. Milrinone for the treatment of cerebral vasospasm after subarachnoid hemorrhage: the MNH protocol. Intensive Care Med. 2007;33:S40.
Arakawa Y, Kikuta K, Hojo M, Goto Y, Ishii A, Yamagata S. Milrinone for the treatment of cerebral vasospasm after subarachnoid hemorrhage: report of seven cases. Neurosurgery. 2001;48:723–8.
Kraaier V, Van Huffelen AC, Wieneke GH, Van der Worp HB, Bär PR. Quantitative EEG changes due to cerebral vasoconstriction. Indomethacin versus hyperventilation-induced reduction in cerebral blood flow in normal subjects. Electroencephalogr Clin Neurophysiol. 1992;82:208–12.
Grandin CB, Cosnard G, Hammer F, Duprez TP, Stroobandt G, Mathurin P. Vasospasm after subarachnoid hemorrhage: diagnosis with MR angiography. AJNR Am J Neuroradiol. 2000;21:1611–7.
Jabre A, Babikian V, Powsner RA, Spatz EL. Role of single photon emission computed tomography and transcranial Doppler ultrasonography in clinical vasospasm. J Clin Neurosci. 2002;9:400–3.
Dankbaar JW, de Rooij NK, Velthuis BK, Frijns CJ, Rinkel GJ, van der Schaaf IC. Diagnosing delayed cerebral ischemia with different CT modalities in patients with subarachnoid hemorrhage with clinical deterioration. Stroke. 2009;40:3493–8.
Suarez JI, Qureshi AI, Yahia AB, et al. Symptomatic vasospasm diagnosis after subarachnoid hemorrhage: evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med. 2002;30:1348–55.
Dankbaar JW, Rijsdijk M, van der Schaaf IC, Velthuis BK, Wermer MJ, Rinkel GJ. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology. 2009;51:813–9.
Macdonald RL, Rosengart A, Huo D, Karrison T. Factors associated with the development of vasospasm after planned surgical treatment of aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;99:644–52.
Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59:21–7.
Carrera E, Schmidt JM, Oddo M, et al. Transcranial Doppler for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Neurosurgery. 2009;65:316–23.
Das RR, Lucey BP, Chou SH, et al. The utility of conductive plastic electrodes in prolonged ICU EEG monitoring. Neurocrit Care. 2009;10:368–72.
Sundt TM Jr, Sharbrough FW, Piepgras DG, Kearns TP, Messick JM Jr, O’Fallon WM. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc. 1981;56:533–43.
Mammone N, Morabito FC. Enhanced automatic artifact detection based on independent component analysis and Renyi’s entropy. Neural Netw. 2008;21:1029–40.
Waziri A, Claassen J, Stuart RM, et al. Intracortical electroencephalography in acute brain injury. Ann Neurol. 2009;66:366–77.
Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–37.
Machado C, Cuspineda E, Valdés P, et al. Assessing acute middle cerebral artery ischemic stroke by quantitative electric tomography. Clin EEG Neurosci. 2004;35:116–24.
van Putten MJ. The revised brain symmetry index. Clin Neurophysiol. 2007;118:2362–7.
Sheorajpanday RV, Nagels G, Weeren AJ, De Surgeloose D, De Deyn PP. Additional value of quantitative EEG in acute anterior circulation syndrome of presumed ischemic origin. Clin Neurophysiol. 2010;121:1719–25.
Acknowledgments
The authors would like to thank the staff of the NICU and the EEG technicians at the Montreal Neurological Institute and Hospital for their assistance in this study. Rahul Rathakrishnan was supported by a grant awarded by the Ministry of Health, Singapore, and the National University Hospital, Singapore.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rathakrishnan, R., Gotman, J., Dubeau, F. et al. Using Continuous Electroencephalography in the Management of Delayed Cerebral Ischemia Following Subarachnoid Hemorrhage. Neurocrit Care 14, 152–161 (2011). https://doi.org/10.1007/s12028-010-9495-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-010-9495-2