Abstract
Background
Bowel ischemia is a rare life threatening complication seen in patients with refractory status epilepticus (RSE). The few reported cases of bowel ischemia in this setting have been associated with the use continuous barbiturate infusions. We report two patients with RSE in the absence of barbiturate infusion and without clear structural, infectious, anatomic, vascular, or autoimmune etiology. We review the clinical details of the cases and potential factors involved in the development of non-occlusive bowel ischemia in patients with RSE.
Methods
The following is a retrospective review of two cases of non-occlusive mesenteric ischemia that occurred during the management of RSE. The clinical data and the details of pathological examination of the infarcted segments of bowel are presented in both cases.
Results
In both cases, the bowel ischemia occurred in the absence of barbiturate infusion or evidence of clear thrombosis, infection, or autoimmune etiology. Case 1 had extensive ischemic necrosis of the small bowel with secondary pseudomembrane formation, and case 2 had full thickness infarction of both the large and small bowel.
Conclusions
The mechanism of bowel infarction in these cases is likely multifactorial and was not associated with barbiturate use. Likely contributors to ischemia include RSE itself, systemic hypotension, vasopressor use, general anesthesia, and abnormal cardiac function. During the management of RSE, every effort must be made to avoid the secondary complications such as bowel ischemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Refractory status epilepticus (RSE) requires management of ongoing seizures using a combination of antiepileptic medications (AEDs) and induction of coma using continuous AEDs (cAEDs). Bowel ischemia is a rare life threatening complication seen in RSE [1]. There are two reported cases of bowel ischemia during RSE in association with continuous infusions of barbiturates namely thiopental [2], and one of bowel ischemia associated with barbiturate overdose [3]. A reported case exists of bowel ischemia associated with RSE not attributable to barbiturate infusion [4]. We report two patients with RSE (2012) and bowel ischemia in the absence of barbiturate infusion.
Case Reports
Patient 1
A 21-year-old previously healthy man presented with one week of frontal headache and fever followed by RSE. Infectious, vasculitic, and autoimmune workup was unremarkable. On day 7, he was transferred to our tertiary care hospital for continuous EEG monitoring (cEEG). He required propofol, midazolam, phenytoin, valproic acid, levetiracetam, phenobarbital (100 mg IV q12 h, serum level 109–136 μmol/L), and lacosamide to achieve burst suppression (Status Epilepticus Severity Score (STESS) = 4) [5]. Isoflurane anesthesia was required to maintain burst suppression. Intravenous immunoglobulin and IV methylprednisolone were trialed without success.
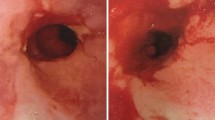
On day 8, he developed an ileus, and all feeds and medications were provided parenterally. On day 10, there was abdominal distention and bowel ischemia on CT abdomen (Fig. 1a). There was systemic inflammatory response syndrome (SIRS) (leukocytosis, fever (38.5 °C), pulse >100), elevated serum lactate 3.7 mmol/L, and cultures were negative. He was hypotensive in the setting of deep anesthesia and received vasopressors. Surgery revealed small bowel ischemia, requiring a 130-cm small bowel resection. Histopathology confirmed ischemic necrosis of the small intestinal mucosa and wall (Fig. 2a) with extensive mucosal ulceration and pseudomembrane formation. There was no large vessel thrombosis or embolism. The findings were related to small vessel ischemia with subsequent superimposed infection (Clostridium difficile) [6].
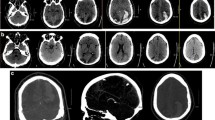
a Patient 1’s abdominal CT, on day 10 after onset of SE, confirms the presence of pneumatosis intestinalis involving a long segment of the small bowel (arrow). There was no evidence of either thrombus on arterial or venous phases (not shown) or of gas in the portal venous system of the liver. b Patient 2’s abdominal X-ray, on day 81 after admission, showed pneumatosis intestinalis of the right colon (arrows); dilated loops and air involving the small and large bowel
He required continuous vasopressors throughout and echocardiography was unremarkable. On day 20, he developed central diabetes insipidus and a CT angiogram demonstrated cerebral edema with absent cerebral blood flow.
Patient 2
A 58-year-old woman presented with decreased oral intake, nausea, vomiting, abdominal pain and weight loss. She had a prior history of seizures (old left temporal lobe contusion, controlled for years on phenytoin, and no history of right-sided epileptiform activity). She was diagnosed with missed appendicitis and treated with piperacillin-tazobactam. She subsequently developed confusion and self-limited seizures without clear focality. A Proteus urinary tract infection was diagnosed and treated. On day 5, she became obtunded and hypotensive with non-convulsive seizures.
Neuroimgaging, infectious, toxic, vasculitic, and autoimmune workups were unremarkable.
On day 22, she developed RSE with focal EEG discharges and seizures arising from the right hemisphere as well as intermittent generalized periodic discharges. MRI showed new right temporal pole and hippocampal T2 signal change, attributable to recent seizures. Phenytoin, valproic acid, levetiracetam, phenobarbital (150 mg NG q12 h, serum level 94–191 μmol/L), propofol, midazolam, isoflurane, and ketamine were required to maintain burst suppression (STESS = 3). A pelvic ultrasound revealed an enlarged right ovary, CA-125 was elevated (124 U/mL; Reference range <35), and RSE secondary to a paraneoplastic/autoimmune etiology was suspected. Methylprednisolone 4 g IV total was trialed without success. She underwent a bilateral salpingo-oophorectomy on day 76, but no abnormality was found. Post-operatively, she had bowel movements and her abdomen remained non-distended.
On day 81 post-admission, after 59 days in SE, she developed vomiting with abdominal distension, and intra-abdominal pressure measuring 26 mmHg. She had SIRS, was treated for hypotension with vasopressors, cultures remained negative, and serum lactate was 2.7 mmol/L. There was pneumatosis of the right colon and multiple segments of dilated small and large bowel (Fig. 1b). Laparotomy revealed ischemic necrosis of the small bowel and colon.
Pathology showed extensive small and large bowel ischemic necrosis (Fig. 2b) with perforation. No thromboembolus was found. There was no volvulus or herniation, and no microscopic evidence of vasculitis. She was found to have cardiomegaly with left ventricular hypertrophy, despite normal echocardiography on day 10. The appendix was hidden by adhesions and the presence of a pericolonic abscess was suggestive of missed appendicitis. Histopathology of the large and small intestine confirmed full thickness infarction of the cecum, ascending, transverse, descending colon (Fig. 2b), and the small intestine with the exception of the most proximal 50 cm. There was perforation of the colon. There was no evidence of large vessel thrombosis or embolism and the remainder of the gastrointestinal tract appeared uninvolved.
Discussion
Our patients developed non-occlusive bowel ischemia after prolonged cryptogenic RSE despite multiple AEDs and cAEDs, in the absence of barbiturate infusion and without clear structural, infectious, anatomic, vascular, or autoimmune etiology. Non-occlusive mesenteric ischemia (NOMI) is relatively common in intensive care medicine. The mechanism is thought to be multifactorial, including large vessel vasoconstriction secondary to sympathomimetics or vasoactive agents and hypoperfusion of mesenteric vessels from anemia, hypoxia, cardiovascular shock, and sepsis [7]. Cereda et al. proposed that patients with RSE and NOMI, the mechanism may be thiopental-associated paralytic ileus, complicated by therapeutic hypothermia [2]. Neither factor was present in these cases.
In our patients, there was no known underlying renal or cardiovascular disease. They received vasopressors and cAEDs, which may have caused transient relative hypotension [4]. Patient 1 received vasopressors around the time of his ileus. Patient 2 had no associated episode of hypotension; there was no evidence of bowel pathology noted during surgery for salpingo-oophorectomy, and no clinical evidence of bowel disease post-operatively until day 81. In a series of seven patients with RSE treated with inhalational anesthetics, all developed hypotension. One patient, in the absence of barbiturate infusion, died of bowel infarction [4].
Our patients developed signs of SIRS prior to bowel ischemia, despite a lack of infectious etiology. Intestinal blood flow decreases as cardiac output is redistributed to essential organs. The combination of vascular sludging, vasospasm, and local hypoxemia leads to necrosis of the villous tips. Experimental studies have shown that reduction of intestinal blood flow by 50–70 % can lead to infarction within 2 h [8]. Intestinal hypoperfusion sufficient to cause infarction may even occur in normotensive patients [3, 8].
Excessive stress on the nervous system influences the function of other viscera, causing neurovisceral injury [9]. Patient 2, despite a normal echocardiogram early on, developed cardiomyopathy in the setting of RSE, which may have resulted in hypoperfusion of the bowel and NOMI. Similarly, a brain-gut axis exists for autonomic control of the gastrointestinal system [10]. Its impairment, secondary to RSE, may contribute to ileus and bowel ischemia. In patient 2, the acquired cardiomyopathy likely contributed more to bowel hypoperfusion than did direct impairment of the brain-gut axis, given significant delay between the onset of RSE and development of bowel ischemia. Increased abdominal pressures may also have contributed to the ischemic bowel in patient 2 [11].
In summary, the bowel ischemia occurred in the absence of barbiturate infusion. We propose that the bowel ischemia was multifactorial including the presence of RSE, systemic hypotension, vasopressor use, general anesthesia, abnormal cardiac function and, in case 2, recent abdominal surgery. The pathological findings were similar in both cases, showing small and large bowel ischemic necrosis without thromboembolism or vasculitis. While aggressive management of RSE is required, every effort must be made to improve outcomes both by improving our understanding of the underlying disease process and by avoiding secondary complications of RSE and its management.
References
Rossetti, et al. A randomized trial for the treatment of refractory status epilepticus. Neurocrit Care. 2011;14:4–10.
Cereda C, et al. Bowel ischemia: a rare complication of thiopental treatment for status epilepticus. Neurocrit Care. 2009;10:355–8.
Olson KR, et al. Intestinal infarction complicating phenobarbital overdose. Arch Int Med. 1984;144:407–8.
Mirsattari SM, Sharpe MD, Young GB. Treatment of refractory status epilepticus with inhalational anesthetic agents isoflurane and desflurane. Arch Neurol. 2004;61:1254–9.
Rossetti AO, Logroscino G, Milligan TA, Michaelides C, Ruffieux C, Bromfield EB. Status Epilepticus Severity Score (STESS): a tool to orient early treatment strategy. J Neurol. 2008;255:1561–6.
Tsutaoka B, Hansen J, Johnson D, Holodniy M. Antibiotic-associated pseudomembranous enteritis due to Clostridium difficile. Clin Infect Dis. 1994;18:982–4.
Kolkman JJ, Mensink PBF. Non-occlusive mesenteric ischemia: a common disorder in gastroenterology and intensive care. Best Pract Res Clin Gastroenterol. 2003;17:457–73.
Haglund U, Lundgren O. Non-occlusive acute intestinal vascular failure. Br J Surg. 1979;66:155–8.
Samuels MA. ‘Voodoo’ death revisited: the modern lessons of neurocardiology. Cleve Clin J Med. 2007;74(Suppl. 1):S8–16.
Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–66.
Ogawa K, Kanemoto K, Shirasaka Y, Kawasaki J, Yamasaki S. Acute pancreatic damage associated with convulsive status epilepticus: a report of three cases. Psychiatry Clin Neurosci. 2001;55:619–22.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rizek, P., Ikeda, K.M., Mele, T. et al. Bowel Ischemia in Refractory Status Epilepticus: Report of Two Cases and Review of the Literature. Neurocrit Care 24, 128–131 (2016). https://doi.org/10.1007/s12028-015-0181-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-015-0181-2