Abstract
Background
The occurrence of hypovolemia in the setting of cerebral vasospasm reportedly increases the risk for delayed ischemic neurologic deficits. Few studies have objectively assessed blood volume (BV) in response to fluid administration targeting normovolemia (NV) or hypervolemia (HV) and none have done so with crystalloids alone. The primary purpose was to evaluate the BV of patients with SAH receiving crystalloid fluid administration targeting NV or HV.
Methods
The University of Washington IRB approved the study. Prospectively collected data was obtained from patients enrolled in a clinical trial and a concurrent group of patients who received IV fluids during the ICU stay. We defined a normovolemia (NV) and hypervolemia (HV) group based on the cumulative amount of IV fluid administered in mL/kg from ICU admission to day 5; ≥30–60 mL/kg/day (NV) and ≥60 mL/kg/day (HV), respectively. In a subgroup of patients, BV was measured on day 5 post ictus using iodinated 131I-labeled albumin injection and the BVA-100 (Daxor Corp, New York, NY). Differences between the NV and HV groups were compared using Student’s t-test with assumption for unequal variance.
Results
Twenty patients in the NV and 19 in the HV groups were included. The HV group received more fluid and had a higher fluid balance than the NV group. The subgroup of patients in whom BV was measured on day 5 (n = 19) was not different from the remainder of the cohort with respect to the total amount of administered fluid and net cumulative fluid balance by day 5. BV was not different between the two groups and varied widely.
Conclusions
Routinely targeting prophylactic HV using crystalloids does not result in a higher circulating BV compared to targeting NV, but the possibility of clinically unrecognized hypovolemia remains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Delayed ischemic neurologic deficits (DIND) after subarachnoid hemorrhage (SAH) remain an important cause of impaired functional neurologic recovery [1]. During the early brain injury period after aneurysmal rupture, a number of physiological derangements occur, which may initiate secondary injuries such as blood–brain barrier dysfunction, inflammation, and oxidative injuries, all culminating in neuronal cell death [2]. As a result, it is now increasingly recognized that cerebral vasospasm (CVS) may not necessarily be the major or only cause of DNID and poor outcomes. Nonetheless, the occurrence of hypovolemia, particularly in the setting of cerebral vasospasm (CVS), is a risk factor for DNID [3]. When manipulating the hemodynamic profile, the ultimate goal of volume expansion is optimization of the venous return and augmentation of stroke volume [4]. When hypovolemia is suspected, bolus administration of intravenous (IV) fluid to provide adequate circulating volume has been shown to increase regional cerebral blood flow [5] and may be attempted prior to other hemodynamic interventions. However, prophylactic therapy targeting hypervolemia (HV), compared to normovolemia (NV), does not result augmentation of circulating blood volume (CBV), regional cerebral blood flow, or the occurrence of CVS [6]. Thus, maintenance of euvolemia is currently recommended over attempts to induce hypervolemia [7].
Unfortunately, readily available bedside information such as patient history, vital signs, body weight and fluid balance, serum sodium concentration, and physical exam findings do not provide accurate information regarding the state of the CBV [8–13]. In two studies involving patients with aneurysmal SAH specifically, no correlation was noted between cumulative fluid balances and directly measured CBV [12, 13]. It has, therefore, been suggested that a hypervolemic fluid management strategy might be warranted to prevent low CBV states rather than to augment CBV [14]. On the other hand, any clinical benefits of hypervolemic therapy may be offset by an increase in cardiopulmonary or renal complications [15, 16]. Hypervolemia is also associated with prolonged hospital length of stay, and increased relative risk of death [17, 18].
Few studies have objectively assessed the CBV in response to fluid administration targeting NV compared to HV and those that have universally allowed for administration of a colloid solution in addition to a baseline crystalloid infusion. Thus, the primary purpose of the present study was to evaluate the CBV of patients with SAH receiving IV crystalloid fluid administration with the goal of achieving NV versus a goal of achieving HV. Secondarily, we sought to determine the degree of CBV augmentation that could be achieved relative to the amount of fluid administered.
Methods
Setting
The University of Washington (Seattle, WA) Human Subjects Division approved this study, which was conducted in the Neuroscience Intensive Care Unit (NICU) at Harborview Medical Center (HMC), University of Washington. HMC is the only level I trauma and comprehensive stroke center in a five state area including Washington, Wyoming, Alaska, Montana, and Idaho. Two neurocritical care teams provide medical coverage for 30 NICU beds. Each team consists of an attending physician, fellow, senior and junior residents, and acute care nurse practitioners. Dedicated intensivist coverage is provided 24 h a day, 7 days a week.
General Patient Care
All patients received treatment in the NICU according to published recommendations that included early securing of the aneurysm via open surgical or endovascular techniques, maintenance of normal serum Na+ (≥135 mEq/L) and glucose concentrations, euvolemia, and normothermia (temperature <37.5 °C), administration of anti-seizure prophylaxis with levetiracetam or phenytoin for 7 days (or longer in the event of documented seizure,) and oral nimodipine (Nimotop®, Bayer Healthcare Pharmaceuticals, Pittsburgh, PA) for 21 days. Maintenance fluids were provided as 0.9 % sodium chloride (NaCl) solution. Hyponatremia was defined as a serum sodium <135 mEq/L, and was treated with oral NaCl tablets or intravenous 2 % NaCl solution (684 mOsm/L).
The presence of CVS was assessed using neurological examination, daily transcranial Doppler (TCD) ultrasonography, and computed tomographic angiography (CTA). Moderate TCD vasospasm was defined by published criteria according to the vessel insonated; internal carotid artery (ICA) and middle cerebral artery (MCA) velocities >150 cm/s with Lindegaard ratios >3.0, and basilar velocities >80 to 85 cm/s with a ratio greater than 2.5. The anterior cerebral artery (ACA), vertebral arteries, and posterior cerebral arteries (PCA) were not graded due to their variability, with CVS defined as velocities >130, 80, and 100 cm/s, respectively. Severe CVS by TCD criteria was defined as an ICA or MCA velocity >200 cm/s with a Lindegaard ratio >6.0, or a basilar artery velocity >85 cm/s with a ratio greater than 3 [19].
In patients suffering neurologic deterioration despite clinically assessed adequate intravascular volume, hypertension was induced with either phenylephrine or norepinephrine infusions. If such therapies were ineffective or could not be maintained due to cardiopulmonary complications, digital subtraction angiography was performed. CVS present on angiography imaging was treated with angioplasty or intra-arterial vasodilator therapy. Central venous lines were not routinely placed and were only utilized when patients required vasoactive medications. Central venous pressures, therefore, were not routinely measured. The state of the extracellular fluid volume was clinically assessed at the bedside using patient history, vital signs, and changes in body weight and cumulative fluid balances.
Patient Population
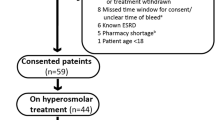
This is a cohort study of prospectively collected data in patients enrolled in the Intensive Management of Pressure and Volume Expansion in Patients With Subarachnoid Hemorrhage (IMPROVES) trial [20] and a concurrent group of patients who were not included in the clinical trial [13]. Briefly, in the IMPROVES trial, the NV group received maintenance IV fluids at a rate of 30 mL/kg/day, a target central venous pressure (CVP) when available of 4–8 mm Hg, and a target net fluid balance of even to positive 0.5 L/24 h; those in the HV group received maintenance IV fluids at a rate of 60 mL/kg/day, a target CVP when available of >8 mm Hg, and a net positive fluid balance of 1–2 L/24 h. In both groups, the hemodynamic goals were achieved with changes in the rate of the maintenance IV fluids or addition of IV fluid boluses with adjustments made based on 4-h interval fluid status assessments that included fluid balance, CVP (when available), and blood pressure. If moderate or severe CVS was detected by TCD without clinical symptoms, fluid management stayed the same in the NV group, but in the HV group IV maintenance fluids were increased to 70 mL/kg/day and CVP targets increased to >10 mm Hg. No colloids were allowed as part of the protocolized fluid management. The remainder of general patient care was the same as outlined above.
The concurrent cohort patients were enrolled in the present study during the same period of time the IMPROVES cohort was being enrolled, but the concurrent cohort was not part of the IMPROVES trial. Concurrent cohort patients were divided into NV and HV groups based on the cumulative amount of IV fluid administered in mL/kg from ICU admission to day 5 and combined with patients included in the clinical trial. Patients who received ≥30 mL/kg/day but <60 mL/kg/day were included in the NV group and patients who received ≥60 mL/kg/day of IV fluids were included in the HV group of the final pooled study cohort. Similar to the trial participants, none of the concurrent cohort received any colloid solutions.
Measurement of Weight, Height, and Fluid Balance
On admission to the ICU all patients were weighed in kilograms (kg) using the electronic bed weight. Beds were zeroed with only the linens prior to the patient being transferred from the stretcher as recommended by the manufacturer. Height in centimeters (cm) was recorded with the patient in supine position and the bed at zero degrees using a single-use paper measuring tape. Subsequent weighing was performed in the early morning hours prior to morning shift change. All tubes, lines, drains, and linens in excess of what was present when the bed was originally zeroed were held above the bed during the weighing process. Intake and output in mL was measured and recorded into the electronic medical record hourly. The fluid balance for any single day reflects the total balance from 0600 of one day to 0559 of the next. The day 1 fluid balance totals were computed from the time of ICU admit until 0559 the next morning.
Blood Volume Determination
In a subset of the study cohort CBV was measured. The following procedure was followed for each measurement CBV. After obtaining a baseline sample of 5 mL of blood as a control for background radiation, 1 mL of I131-labeled albumin (Volumex, Daxor Corporation, New York, NY) was injected intravenously over 1 min. Serial blood samples of 5 mL each were obtained at 12, 18, 24, 30, and 36 min post-injection from a large-bore peripheral catheter or indwelling arterial or central venous catheter when available. Sample radioactivity was measured in duplicate using a semi-automated counter (BVA-100®, Daxor Corporation, New York, NY), and a minimum of three samples with a standard deviation <4 % were used to determine plasma volume extrapolating to time 0. The red cell volume was calculated using a simultaneously measured hematocrit (gm %), with the total blood volume being the plasma and red cell volumes. The value was compared to the predicted normal blood volume, which is based upon the height, weight, and sex of the patient [18]. Values within ±8 % of predicted were considered normal. Mild, moderate, and severe hypovolemia were defined as 9–16, 17–24, and >24 % below predicted blood volume, respectively, whereas mild, moderate, and severe hypervolemia were defined by values of 9–16, 17–24, and >24 % above predicted blood volume, respectively.
Data Collection and Analysis
Data were abstracted from the electronic medical record and entered into an electronic database (Microsoft Excel for Mac 2011, v14.3.6, Redmond, WA). Age, sex, height in cm, weight in kg, severity of the bleed (Hunt and Hess, Fischer grade), total administered fluid, and the cumulative fluid balances in mL were collected for all groups. Body surface area (BSA, m2) was calculated using the Mosteller formula [21]: ([height in cm × weight in kg]/3600)1/2. Directly measured BV was recorded in mL/kg and as the percent deviation from expected when available. In order to compare the literature reports using weight-based estimates of expected BV, normal values were considered to be a CBV of 60–80 mL/kg [22]. Values <60 and >80 mL/kg were considered hypo- and hypervolemia, respectively. In order to gauge the “efficiency” of administered IV fluid in producing changes in CBV, the CBV (mL/kg) was divided by the total IV fluid administered (mL/kg/day) and multiplied by 100. The higher the number, the more effective the volume at producing changes in circulating CBV. Differences between the NV and HV groups were compared using Student’s t-test with assumption for unequal variance. Significance was defined as a two-tailed p value <0.05. The odds of being normovolemic (CBV of 60–80 mL/kg) at day 5 relative to a condition of hypo- and hypervolemia, among patients who received fluid with the goal of NV versus HV was estimated and is reported as OR (95 % CI). Analysis was performed using GraphPad Prism 5.0a (GraphPad Inc., La Jolla, CA). Data are expressed as mean ± SD or median (IQR) for measured characteristics or in frequency distributions for categorical variables.
Results
A total of 39 patients were included in the final analysis: 20 in the NV and 19 in the HV groups. A comparison of the groups is provided in Table 1. Overall, the HV group received significantly more fluid and had a higher fluid balance than the NV group. The subgroup of patients in whom CBV was measured on day 5 (n = 19, 10 NV group and 9 HV group) was not different from the remainder of the cohort with respect to the total amount of administered fluid and net cumulative fluid balance by day 5. However, CBV, whether expressed as a function of weight or as percent deviation from expected values based on gender, height, and weight was not different between the two groups, indicating that for any additional amount of fluid volume administered above the normovolemic fluid goal, there was not a corresponding effect on the increase in CBV.
Blood volumes varied widely. Among the 10 patients in the NV group, 5 (50 %) of them had a BV between 60 and 80 mL/kg. The remainder of patients was in the hypovolemic range from 40 to 57 mL/kg. Among the nine patients in the HV group with BV measurements, 4 (44 %) patients had a BV of 60–80 mL/kg, whereas the remainder of patients was in the hypovolemic range from 50 to 55 mL/kg. When normalized for height, weight, and gender as recommended by the manufacturer of the blood volume analyzer, 5 (50 %) patients in the NV subgroup were normovolemic (±8 % from expected), 4 (40 %) were mildly hypovolemic (>8, <16 % less than expected), and 1 (10 %) was severely hypervolemic (>24 % above expected). In the HV subgroup, 5 (56 %) patients were normovolemic (±8 % from expected), 1 (11 %) moderately hypovolemic (>16, <24 % less than expected), 2 (22 %) severely hypovolemic (>24 % less than expected), and 1 (11 %) hypervolemic (>24 % above expected). The relative odds of being normovolemic at day 5 with HV therapy was not higher than in the NV group whether BV was expressed by weight (OR = 0.8, 95 % CI 0.13–4.9; p = 0.8) or as % deviation from expected (OR = 1.25, 95 % CI 0.2–7.6; p = 0.8).
Discussion
Our findings suggest that protocolized IV fluid administration with crystalloid alone targeting HV, while able to achieve a significantly higher cumulative fluid balance than when NV was targeted, neither results in a higher CBV nor guarantees avoidance of hypovolemia. In addition, the efficiency of IV fluid in affecting changes in CBV is significantly diminished with increasingly high rates of IV fluid administration. Consistent with other reports, our findings also suggest that attempts to maintain NV in SAH by relying primarily on cumulative fluid balances are ineffective. Hoff and colleagues reported that despite a net positive fluid balance of 700 ± 900 mL by day 5–6 after SAH, only 52 % of all direct BV measurements made during the first 2 weeks after SAH were in the normal range [12]. In the only prospective trial of SAH in which patients were randomized to a protocolized NV or HV fluid therapy regimen within 24 h of securing the aneurysm, Lennihan and colleagues reported no differences in BV measured approximately 4 days from randomization between groups [6].
Differences in the absolute volumes administered and the fluid balances achieved between these studies and ours may be explained by the fact that their fluid management protocols utilized varying amounts of colloid solutions; 4 % succinylated gelatin in the first and 5 % human albumin solution in the second. Further limiting direct comparisons is the fact that these studies did not differentiate between the absolute volume of crystalloid and colloid solutions administered to their patients. Nevertheless, our assertion, that targeted volume strategies do not guarantee normal CBV is supported by these reports.
One explanation for the difficulty in assuring normal blood volumes by administration of IV fluid is the manner in which the effectiveness of the intervention is assessed. A lack of association between the cumulative fluid balance and directly measured blood volumes among patients with SAH has been reported [12, 13]. Changes in body weight also fail to accurately predict BV [13]. This may be particularly important when bedside clinical assessments are incorporated as more error may be introduced [9, 10, 23]. The potential for hypovolemia even when adequate fluid balance has been recorded may explain the findings of improved cerebral blood flow in response to a bolus administration of saline or albumin in this setting [5, 24]. Given the inability to accurately assess blood volume based on clinical information alone and the reported association between hypovolemia and DNID, it seems reasonable to hypothesize that fluid administration guided by direct CBV measurements may improve outcomes. In a prospective controlled study, daily BV-guided fluid management was compared to standard care in 102 patients with acute SAH [25]. In the BV-guided group, fluid administration was changed according to a standardized protocol based on whether or not the daily BV was outside of the normal range. Both groups received approximately 25 L of fluid over the first 5 days of the study, similar to what we report in our HV group. While the BV-guided management protocol decreased the number of measurements in the severe hypovolemia range during the 10-day study period (6.7 % of the BV measurements showed severe hypovolemia as compared with 17.1 % in controls), there were no differences in occurrence of DNID or neurologic outcome as assessed by modified Rankin score (mRS) at 3 months between groups. It is, however, possible that this study was not adequately powered to detect such differences. Nonetheless, these findings taken together do suggest that little is gained with a fluid strategy targeting HV and that specific knowledge of the state of the CBV in general may not translate into improved outcomes. Therefore, it remains unclear if volume expansion or CBV measurements are worthwhile.
To this point, a recently published post hoc analysis of the Clazosentan to overcome neurologic ischemia and infarction occurring after SAH (CONSCIOUS-1) trial, reported that the administration of colloids and induction of a positive fluid balance during the cerebral vasospasm risk period may be associated with worsened functional outcomes [26]. Using multivariate regression modeling, the authors reported that a positive fluid balance during days 3–14 was associated with an increased ICU length of stay and worsened mRS at 12 months post ictus. Only in the setting of severe angiographic cerebral vasospasm during this at risk period, a finding reported in only 14 % of patients enrolled in CONSCIOUS-1, was a negative fluid balance associated with delayed cerebral infarcts [27]. Other investigators have also reported an association between a composite of moderate or severe angiographic CVS, occurring in about 1/3 of patients, and delayed infarcts [28]. These findings are not conclusive, but suggest that the avoidance of hypovolemia may be warranted, but do not support HV as a treatment goal in the majority of SAH patients. Based on existing data and our present findings regarding the inefficiency and general ineffectiveness of HV therapy over NV, a more judicious IV fluid management strategy tailored to individual patients for SAH patients during the high-risk period for DIND seems to be prudent [29].
Limitations
We acknowledge that our study has limitations. Our sample size is small, since CBV was only measured in a subset of the study cohort. Due to the limited sample and variable effect size, we cannot exclude the existence of type 1 error. Additionally, all included patients were cared for within a single university-affiliated specialty ICU, which limits the external validity of our results. Furthermore, our patients were pooled from two cohorts, which may introduce additional sources of variability in the data. However, since our results were obtained from patients who were administered crystalloids exclusively, we believe they add to our current knowledge regarding CBV in SAH patients. This is particularly germane given published data regarding possible detrimental effects of colloids in patients with SAH [26, 30].
It should be noted that studies reporting directly measured CBV have utilized different techniques of measurement. All studies utilize some type of indicator dilution, which involves injection of a known quantity of a tracer, allowance for adequate time for complete mixing in the blood, and lastly, some type of sampling to provide a tracer count. This count is obtained by direct blood sampling, as is the case with case of nuclear tracers, or by a measure of the tracers density, as is the case with injected dyes, usually, but not exclusively by a peripherally placed device that measures light wavelength similar to a pulse-oximeter. The traditional gold standard is the use of a dual-labeled blood. Red cell volume (RCV) is obtained by dilution of 51Cr-labeled red cells and plasma volume (PV) obtained by dilution of 125I-labeled albumin [31]. The CBV is the sum of the RCV added to the PV. While extremely accurate, the test is cumbersome and time consuming, requiring 4–6 h to complete. As a result, it has limited clinical utility and is now infrequently performed. Our results were obtained using a single-tracer technique (131I-labeled albumin), which provides a simpler directly measured PV. A simultaneously obtained micro-hematocrit is used to calculate RCV. Total CBV is simply the addition of the two components. The two tests have been directly compared and reported to be equivalent [32]. In addition, controversy surrounds normative values for CBV, which are most often reported using a fixed weight ratio in mL/kg. However, as might be expected, average CBV to weight ratios vary considerably among people with different anthropomorphic body types due to the normal vascularity of various body tissues [33]. This makes the use of fixed weight ratios problematic. Body surface area has been reported to be a better predictor of CBV than body mass [22]. This is not a result of improved physiologic correlation, however. Two people with precisely the same body tissue composition who are different in size (height, width, and depth) will have identical normal CBV per unit mass. But, because the ratio of BSA to volume differs, their BSA per unit mass will be quite different and, thus, predictions of normal CBV based on a linear relationship to BSA in these individuals will be error-prone. Errors will be most marked at the extremes of height and weight. These limitations were recognized by the International Committee on Standardization in Hematology (ICSH) in 1995, which developed an improved method for predicting normal CBV based on BSA using ideal rather than actual body mass [34]. Unfortunately, the broad normal range produced by their final equation, ±25 %, has no clinical relevance for the acutely ill. An improved prediction model, which describes normal CBV per unit mass in relation to the percentage of deviation from ideal body weight, has been reported to have improved accuracy and precision compared to calculations based on BSA [35]. Additionally, the normal range, ±8 %, provides a high level of predictive discrimination for classifying blood volume status.
Lastly, the accuracy of nurse-recorded fluid intake and outputs can have errors. However, this was standard for all patients in our study, thus minimizing the differences.
Conclusion
Regardless of IV fluid administered, we observed similar CBVs in patients treated with a fluid management strategy targeting NV compared to a strategy targeting NV. Hypovolemia was equally common in both study groups. While routinely targeting a hypervolemic state does not appear efficacious, the possibility of clinically unrecognized hypovolemia exists and appears common. This finding, combined with the association between hypovolemia and DINDs support the practice of empiric fluid challenges in patients with changes in neurological status where vasospasm is suspected. Basing fluid management upon frequent serial BV determinations has not yet been shown to positively effect patient-centered outcomes in SAH. However, the utility of directly assessing BV in specific clinical circumstances (clinically relevant CVS, differentiating various causes of hyponatremia) in addition to targeting euvolemia based on cumulative fluid balance with crystalloids deserves further study.
References
Rinkel GJ. Medical management of patients with aneurysmal subarachnoid haemorrhage. Int J Stroke. 2008;3:193–204.
Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013;4:432–46.
Solomon RA, Post KD, McMurtry JG III. Depression of circulating blood volume in patients after subarachnoid hemorrhage: implications for the management of symptomatic vasospasm. Neurosurgery. 1984;15:354–61.
Lazaridis C. Advanced hemodynamic monitoring: principles and practice in neurocritical care. Neurocrit Care. 2012;16:163–9.
Dhar R, Scalfani MT, Zazulia AR, Videen TO, Derdeyn CP, Diringer MN. Comparison of induced hypertension, fluid bolus, and blood transfusion to augment cerebral oxygen delivery after subarachnoid hemorrhage. J Neurosurg. 2012;116:648–56.
Lennihan L, Mayer SA, Fink ME, et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke. 2000;31:383–91.
Diringer MN, Bleck TP, Claude Hemphill J III, et al. Neurocritical Care Society. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care 2011;15:211–40.
McGee S, Abernethy WB III, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281:1022–9.
Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83:905–8.
Stéphan F, Flahault A, Dieudonné N, Hollande J, Paillard F, Bonnet F. Clinical evaluation of circulating blood volume in critically ill patients—contribution of a clinical scoring system. Br J Anaesth. 2001;86:754–62.
Rothe CF, Murray RH, Bennett TD. Actively circulating blood volume in endotoxin shock measured by indicator dilution. Am J Physiol. 1979;236:H291–300.
Hoff RG, van Dijk GW, Algra A, Kalkman CJ, Rinkel GJ. Fluid balance and blood volume measurement after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2008;8:391–7.
Joffe AM, Healey L, Khandelwal N, Hallman MR, Van Cleve W, Treggiari MM. Validity of exchangeable solute balance as a measure of blood volume in neurologically injured adults. Neurocrit Care 2013 Sep 21. [Epub ahead of print].
Diringer MN, Wu KC, Verbalis JG, Hanley DF. Hypervolemic therapy prevents volume contraction but not hyponatremia following subarachnoid hemorrhage. Ann Neurol. 1992;31:543–50.
Solenski NJ, Haley EC Jr, Kassell NF, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Participants of the Multicenter Cooperative Aneurysm Study. Crit Care Med. 1995;23:1007–17.
Peled H. Volume status and fluid responsiveness. Advanced hemodynamic monitoring: neurocrit care. Neurocrit Care. 2012;16:485.
Treggiari MM, Walder B, Suter PM, Romand JA. Systematic review of the prevention of delayed ischemic neurological deficits with hypertension, hypervolemia, and hemodilution therapy following subarachnoid hemorrhage. J Neurosurg. 2003;98:978–84.
Martini RP, Deem S, Brown M, et al. The association between fluid balance and outcomes after subarachnoid hemorrhage. Neurocrit Care. 2012;17:191–8.
Kincaid MS, Souter MJ, Treggiari MM, Yanez ND, Moore A, Lam AM. Accuracy of transcranial Doppler ultrasonography and single-photon emission computed tomography in the diagnosis of angiographically demonstrated cerebral vasospasm. J Neurosurg. 2009;110:67–72.
Intensive Management of Pressure and Volume Expansion in Patients With Subarachnoid Hemorrhage (IMPROVES). http://clinicaltrials.gov/show/NCT01414894. Accessed 2/21/2014.
Lam TK, Leung DT. More on simplified calculation of body-surface area. N Engl J Med. 1988;318:1130.
Feldschuh J, Katz S. The importance of correct norms in blood volume measurement. Am J Med Sci. 2007;334:41–6.
Hoff RG, Rinkel GJ, Verweij BH, Algra A, Kalkman CJ. Nurses’ prediction of volume status after aneurysmal subarachnoid haemorrhage: a prospective cohort study. Crit Care. 2008;12:R153.
Jost SC, Diringer MN, Zazulia AR, et al. Effect of normal saline bolus on cerebral blood flow in regions with low baseline flow in patients with vasospasm following subarachnoid hemorrhage. J Neurosurg. 2005;103:25–30.
Hoff R, Rinkel G, Verweij B, Algra A, Kalkman C. Blood volume measurement to guide fluid therapy after aneurysmal subarachnoid hemorrhage: a prospective controlled study. Stroke. 2009;40:2575–7.
Ibrahim GM, Macdonald RL. The effects of fluid balance and colloid administration on outcomes in patients with aneurysmal subarachnoid hemorrhage: a propensity score-matched analysis. Neurocrit Care. 2013;19:140–9.
Macdonald RL, Kassell NF, Mayer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–21.
Brown RJ, Kumar A, Dhar R, Sampson TR, Diringer MN. The relationship between delayed infarcts and angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2013;72:702–7.
Orfanakis A, Brambrink AM. Long-term outcome call into question the benefit of positive fluid balance and colloid treatment after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2013;19:137–9.
Tseng MY, Hutchinson PJ, Kirkpatrick PJ. Effects of fluid therapy following aneurysmal subarachnoid haemorrhage: a prospective clinical study. Br J Neurosurg. 2008;22:257–68.
Manzone TA, Dam HQ, Soltis D, Sagar VV. Blood volume analysis: a new technique and new clinical interest reinvigorate a classic study. J Nucl Med Technol. 2007;35:55–63.
Dworkin HJ, Premo M, Dees S. Comparison of red cell and whole blood volume as performed using both chromium-51-tagged red cells and iodine-125-tagged albumin and using I-131-tagged albumin and extrapolated red cell volume. Am J Med Sci. 2007;334:37–40.
Gregersen MI, Nickerson JL. Relation of blood volume and cardiac output to body type. J Appl Physiol. 1950;3:329–41.
Pearson TC, Guthrie DL, Simpson J, et al. Interpretation of measured red cell mass and plasma volume in adults: Expert Panel on Radionuclides of the International Council for Standardization in Haematology. Br J Haematol. 1995;89:748–56.
Feldschuh J, Enson Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation. 1977;56:605–12.
Acknowledgments
This article was supported in part by an unrestricted grant from the Joseph F. Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was performed in the Neurosciences Intensive Care Unit, Harborview Medical Center, Seattle, WA.
Rights and permissions
About this article
Cite this article
Joffe, A.M., Khandelwal, N., Hallman, M.R. et al. Assessment of Circulating Blood Volume with Fluid Administration Targeting Euvolemia or Hypervolemia. Neurocrit Care 22, 82–88 (2015). https://doi.org/10.1007/s12028-014-9993-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-014-9993-8