Abstract
Background
Continuous EEG (cEEG) may allow monitoring of patients with aneurysmal subarachnoid hemorrhage (SAH) for delayed cerebral ischemia (DCI) and seizures, including non-convulsive seizures (NCSz), and non-convulsive status epilepticus (NCSE). We aimed to evaluate: (a) the diagnostic accuracy of cEEG as a confirmatory test, (b) the prognostic value of EEG patterns suggestive of seizures and DCI, and (c) the effectiveness of intensified neuromonitoring using cEEG in terms of improved clinical outcome following SAH.
Methods
A systematic review was performed with eligible studies selected from multiple indexing databases through June 2014. The methodological quality of these studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2.
Results
Eighteen studies were identified, including cEEG data from 481 patients with aneurysmal SAH. NCSz were diagnosed in 7–18 % of patients; NCSE in 3–13 %. NCSE was associated with increased age (mean age 68 years) and mortality (82–100 %) compared to the entire patient population (53.9 years; mortality 13 %; p values <0.05). DCI was diagnosed in 20–46 % of patients. Quantitative EEG patterns suggestive of DCI included decreased alpha/delta ratio, relative alpha variability, and total power. All studies were subject to a high risk of bias concerning patient selection and cEEG methodology.
Conclusions
cEEG monitoring following SAH detects an increased number of subclinical seizures and may predict DCI many hours in advance. NCSE is associated with high mortality and morbidity, whereas for DCI identified by cEEG this association is less clear. Prospective randomized controlled multicenter trials are needed to evaluate the benefits (or risks) of intensified treatment of seizures and DCI following SAH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Once a ruptured aneurysm has been secured, patients with subarachnoid hemorrhage (SAH) may develop secondary complications, of which delayed cerebral ischemia (DCI) is the most devastating [1]. DCI is defined as a new focal or global neurological deficit and/or a new cerebral infarction revealed by neuroimaging or autopsy after other causes such as rebleeding and hydrocephalus have been excluded [2]. It affects most frequently the cerebral cortex with or without subcortical white matter involvement [3]. Symptomatic DCI occurs in roughly 35 % of patients, typically between days 4 and 14 following SAH [1, 2]. Computed tomography (CT) reveals new cerebral infarcts in about a third of these patients [4]; however, approximately 25 % of infarcts on CT are clinically silent [5]. The pathophysiology of DCI is not fully understood. Arterial vasospasm probably plays a role, but additional mechanisms include microembolism, vasospasm of peripheral arteries and arterioles (as opposed to proximal large vessels), and cortical spreading ischemia [2, 6, 7, 9–14].
Epileptic seizures, including non-convulsive seizures (NSCz) and non-convulsive status epilepticus (NCSE), are also common complications of SAH [15, 16]. In one study involving 48 comatose SAH patients, intracortical seizures were seen in 38 % of patients (using electrocorticography) and 8 % had surface seizures (detected by EEG) [13]. Although it remains unknown whether NCSz may contribute to neuronal damage or merely indicate an underlying brain injury, NCSE following SAH is associated with high mortality and morbidity [16].
Recent technical advances have made continuous EEG (cEEG) monitoring feasible for an increasing number of surgical and non-surgical patients in both general and neuro-intensive care departments [17]. Quantitative EEG (qEEG) software programs allow the many hours of raw EEG data to be condensed into a few screen shots which can be assessed instantly. Thus, real-time detection of adverse events is possible, and cEEG is increasingly used to monitor SAH patients for seizures and DCI. However, rhythmical and periodic EEG patterns of uncertain significance are frequently encountered, and it is unknown if and how rigorously they should be treated [18]. Treating EEG changes on the ictal-interictal spectrum too aggressively may induce serious adverse effects such as arterial hypotension, organ toxicity, and prolonged ventilator support. In addition, whereas EEG patterns of ischemia are well-described—subtle loss of alpha and beta, followed by excessive theta and delta and finally, suppression of all frequencies—there is lack of consensus about which of the many qEEG parameters are most suitable for the detection of DCI. It therefore remains unclear whether intensified monitoring by cEEG, an expensive and labor-intensive diagnostic tool, translates into better clinical outcome or if it indeed may lead to overtreatment and potentially harm the patient.
We aimed to evaluate the diagnostic accuracy of cEEG as a confirmatory test to detect seizures and DCI following SAH. Using the PICO approach [19], we phrased the following primary research question: In patients with acute SAH admitted to an intensive care unit (Population), does neuromonitoring using cEEG (Intervention) as compared to conventional clinical monitoring (Comparison) lead to earlier and more frequent detection of episodes with DCI and/or seizures (Outcome)? For secondary objectives we assessed (a) whether EEG patterns suggestive of seizures and DCI predict clinical outcome in SAH and (b) whether intensified neuromonitoring using cEEG translates into better clinical outcome of patients with SAH.
Methods
We performed a systematic review using standardized methods. The review protocol can be accessed from the Online supplemental files.
Criteria for Considering Studies for this Review
Types of Studies
We included cross-sectional, longitudinal, retrospective or prospective observational studies as well as interventional trials, and, if available, meta-analyses and reviews, comparing cEEG with conventional neuromonitoring [clinical examination, intracranial pressure measurement, routine EEG, neuroimaging including CT/magnetic resonance (MR) angiography, and transcranial Doppler (TCD)] in patients with acute SAH. Single case reports were excluded. Studies were restricted to those reporting on adults (age above 16 years) with non-traumatic and aneurysmal SAH, confirmed by neuroimaging, admitted to neuro-critical care units, general intensive care units, or specialist units (e.g. stroke units) and examined by cEEG during the acute period (days 0–30), irrespective of the severity of the disease or co-morbidities. Studies including patients assessed with simultaneous Electrocorticography were excluded because of the considerable methodological differences.
Index Test and Reference Standards
The index test was cEEG which comprises both prolonged measurement of raw EEG data and qEEG. EEG monitoring can be performed using a standard EEG montage (21 electrodes) or a reduced amount of EEG channels. Many different qEEG methods are available and are typically used in combination; these include qEEG based on amplitude (amplitude-integrated EEG, envelope trends), frequency (spectral arrays, spectrograms), rhythmicity, and asymmetry of the raw EEG.
With respect to NCSz and NCSE, clinical exam (including video EEG) and routine EEG were considered as reference standards. For vasospasm and DCI, clinical exam and neuroimaging [TCD; CT and MRI, including CT/MR angiography and perfusion; and digital subtraction angiography (DSA)] were reference standards.
Search Methods for Identification of Studies
We searched the following databases for relevant English and non-English literature from January 1, 1980 to June 15, 2014: Cochrane Central Register of Controlled Trials (The Cochrane Library), Medline (PubMed), EMBASE, Scopus, and clinicaltrials.gov. The following search terms were used: “subarachnoid* hemorrhage”, “subarachnoid* bleeding”, “electroencephalography”, “EEG”, “continuous EEG”, “cEEG”, “quantitative EEG”, “qEEG”, “ICU EEG monitoring”, and “neurotelemetry”. See Online supplemental files for details. References from relevant manuscripts were manually searched to identify additional articles. Further, papers were cross-referenced using the ‘cited by’ function on Scopus and PubMed. The literature search was supervised by an information specialist from the Copenhagen University Library Service.
Data Collection, Analysis, and Reporting
Selection of Studies, Data Extraction, and Management
Titles were reviewed first, followed by evaluation of the abstracts with titles suggesting that a study was of relevance. Eligible studies were then identified on the basis of their full text. The initial selection was performed by one author (DK), and then confirmation of eligibility and quality assessment were performed by two authors (DK, CF). Following identification of relevant studies, one author (DK) extracted relevant information from each study which was validated by a second author (CF).
Assessment of Methodological Quality, Including Investigations of Heterogeneity
Using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2), a recently modified version of QUADAS [20], two authors (DK, CF) independently assessed the methodological quality of each included study. The QUADAS-2 comprises four domains: (1) participant selection, (2) index test, (3) reference standard, and (4) flow of participants through the study and timing of the index tests and reference standard (flow and timing). Each domain is assessed for risk of bias, and the first three domains are also assessed for concerns regarding applicability, using pre-specified signaling questions. These questions were adjusted according to the purpose of this review (see Online supplementary files). Risk of bias and concerns about applicability were judged as “low”, “high”, or “unclear”. Disagreement between the two reviewing authors was resolved by consensus or an independent referee (MF) if required.
Statistical Analysis, Data Synthesis, and Reporting
We aimed to perform a meta-analysis of the available numerical data reporting on 1) the diagnostic accuracy of cEEG in detecting NSCz and NCSE, as well as vasospasm and DCI; 2) the positive and negative predictive values of cEEG for clinical outcome after SAH in terms of mortality and morbidity; and 3) potential clinical benefits from adjustment of therapy in response to intensified neuromonitoring using cEEG. However, this aim was subject to the availability and quality of the studies, study design, and risk of bias, and we planned only to perform a meta-analysis of the data when judged clinically meaningful and feasible. Descriptive statistics were performed, if appropriate, including the Student’s test for continuous variables; p < 0.05 was considered statistically significant. The data were reported according to the PRISMA criteria (see Online supplementary files) [21].
Results
Systematic Literature Search and Quality Assessment
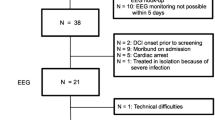
The initial literature research yielded 760 citations. Twenty original publications, reporting on 18 studies, were deemed eligible for inclusion and meta-analysis (Fig. 1) [14, 16, 22–39]. All studies were single-center case series (including one randomized clinical drug trial [29, 30] ), of which 8 (44 %) were retrospective. Using the QUADAS-2, we found that all studies were affected by a high risk of bias related to patient selection. In addition, nine studies (50 %) had a high or unclear risk of bias concerning cEEG methodology (index test), whereas risk of bias in terms of the reference standards, patient flow, and timing of investigations was in general low. In most studies there were no or only few concerns regarding applicability; thus, the conduct and interpretation of cEEG in the identified studies were in line with our review question (Table 1).
Patient Population
Adjusting for patients included in more than one study and excluding ambiguous data, we identified a total of 2,348 patients with acute aneurysmal SAH; unambiguous cEEG monitoring data were available for 481 patients (20.5 %; Table 2). Mean age of patients examined by cEEG was 55.8 years (an approximation based on data from 8 studies), 71 % were female (6 studies). Patients with NCSE had a mean age of 68 years (range 32–82; 2 studies) which was significantly older than that of the entire population (p < 0.05). In 12 studies the majority of patients had sustained severe SAH (Hunt and Hess grade 4–5, Fisher grade 3–4, and/or GCS ≤8; n = 305 patients). SAH was of moderate degree (Hunt and Hess grade 1–3, Fisher grade 1–2, and/or GCS ≥9) in most patients from 4 studies (n = 60). In 2 studies the severity of SAH was not specified. Mortality data were available from 5 studies; at the time of hospital discharge there were 26 deaths among 200 patients (13 %).
cEEG Methodology and Reference Standards
A full EEG montage was used in 9 studies (50 %), whereas the remaining publications did not specify the number of EEG electrodes or involved a reduced number (median 8 EEG channels, range 2–16). cEEG monitoring was performed on average for 5 days (range 1–60 days; based on data from 12 studies) (Table 2). Only 6 studies (33 %) provided qEEG data. A large variety of qEEG methods were employed. Claassen et al., for instance, assessed 12 different qEEG parameters, including parameters related to absolute and relative power, coherence and average frequency [25]. The indication for cEEG was monitoring for NCSz and NCSE in 15 studies (83 %), whereas in 8 studies (44 %) cEEG was performed for the detection of DCI and vasospasm. Only 5 studies (28 %) used cEEG for simultaneous assessment of ischemia and seizures.
The reference standards for DCI and vasospasm included CT, DSA, and TCD in most but not all studies, and thresholds for increased mean middle cerebral artery flow velocities varied from 120 to 140 cm/min. However, in 4 studies DCI was based on a clinical diagnosis, and the presence of radiological vasospasm or increased TCD flow velocities was considered supportive but not mandatory. Similarly, definitions of electrographic seizures varied significantly between studies: We counted 8 different definitions in 12 studies, excluding 3 studies without clearly specified seizure criteria (Table 3). In 4 papers, seizures were defined according to the American Clinical Neurophysiology Society's (ACNS) Standardized Critical Care EEG Terminology; 3 studies referred to the 2005 version, 1 to the 2012 version. Table 2 lists the available data on the incidence of DCI, NSCz, and NCSE, respectively. Due to the large heterogeneity of the present studies, we considered a more detailed meta-analysis inappropriate.
DCI and Seizures
Changing trends on qEEG correlated with DCI. In a study including 11 SAH patients, the most sensitive qEEG parameter was a change in total power (91 %) [22]. In another study, decreased relative alpha variability was found to precede TCD- or DSA-verified vasospasm by more than 2 days in 10 out of 19 patients, resulting in positive and negative predictive values of 76 % and 100 %, respectively [23]. In a third study, post-stimulation alpha/delta ratio (alpha power/delta power; ADR) had the strongest association with DCI [25]. Any single measurement with a >50 % ADR decrease had a sensitivity of 89 % and a specificity of 84 %. The finding of six consecutive recordings with a >10 % decrease in ADR from baseline had an even greater sensitivity (100 %), although specificity was lower (76 %) [25]. In yet another study, cEEG was predictive greater than 24 h prior to clinical change in 3 of 12 patients, and monitoring daily mean alpha power accurately identified recurrence of DCI, as well as poor responders to spasmolytic therapy [33]. Progressive electrographic deterioration (enhanced delta pattern) was associated with an increased risk of in-hospital death by almost 24 % compared to patients without worsening of EEG patterns [31]. However, DCI did not remain predictive of poor outcome in the largest of all studies involving 116 patients, perhaps because of the higher than expected DCI prevalence [25]. NCSE, periodic epileptic discharges, and lack of sleep architecture were associated with poor outcome, but the numbers were too small for meaningful statistic evaluation [14].
Seizures in SAH patients were common. In one study, 19 % of 108 patients with SAH had seizures recorded on cEEG. Most seizures were non-convulsive (95 %) and the majority of patients with NCSz had NCSE (70 %) [16]. Epileptiform discharges and NCSE were often associated with poor prognosis following SAH. In one study, 26 patients with SAH and decreased consciousness underwent cEEG monitoring and 8 were diagnosed as having NCSE (31 %). Despite successful termination of NCSE in 5, all 8 patients subsequently died [24]. In another study involving 11 SAH patients with NCSE, 9 died (82 %); the remaining 2 patients lived independently following rehabilitation [27]. However, one study reported a rather low incidence of NCSz (2/28 patients; 7 %) and NCSE (1/28 patients; 4 %) [34], and, in yet another study, rhythmical and periodic EEG patterns were very common but did not predict short-term outcome in critically ill patients with SAH [36]. The first NCSz was registered after a median of 8.5 days (median interquartile range 4.8–17) following SAH in one study [38], and, in another study, the first seizure detection occurred a median of 5.4 days (IQR 4.8–17) post-bleeding and 1.6 days (IQR 0.7–2.9) following the onset of cEEG monitoring [39].
Discussion
Continuous EEG allows uninterrupted, prolonged, and non-invasive real-time detection of adverse events in comatose patients, in whom neurological examination is typically unreliable. Patients with aneurysmal SAH are at risk for subclinical seizures and DCI, and thus, an obvious target group for cEEG monitoring [17].
As to the detection of DCI, physiological parameters (e.g. cerebral perfusion pressure), levels of sedation, medications, as well as artifacts, severely influence qEEG results [17, 22, 25]. In order to enhance sensitivity for changes related to DCI, investigators have used relative instead of absolute qEEG parameters [23, 25], analyzed exclusively artifact-free EEG [23], and restricted the evaluation to EEG clips following an alerting stimulus [25]. Quantitative EEG parameters should always be evaluated together with the raw EEG and in light of the clinical situation. Having implemented these principles, Claassen et al. diagnosed DCI in 46 % of patients with poor-grade SAH. They found post-stimulation ADR to be the most suitable qEEG parameter for predicting DCI for up to 24 h or even more prior to clinical deterioration [25]. Reduced values for total power, relative alpha variability, and the composite alpha index (CAI) were similarly predictive of ischemia hours to days in advance [22, 23, 33]. It must be noticed, though, that in the latter studies, the severity of SAH was moderate in most patients as compared to the poor-grade SAH population from Claassen et al.
A most significant confounding factor is the large variety of DCI definitions in clinical trials and observational studies on SAH, including the controversial equation of angiographic vasospasm with DCI, which makes comparison between studies challenging [2]. In most studies, a clear definition of DCI was lacking or DCI was diagnosed by combining radiographic or ultrasound evidence of vasospasm with clinical symptoms of cerebral ischemia [22, 23, 27, 31, 32]. This neither takes into account that delayed neurological deficits are subject to many other factors in addition to DCI and that clinical examination in the intensive care unit may be unreliable, nor the increasing evidence that a simple cause-effect relationship between angiographic vasospasm and DCI does not exist (Fig. 2) [8, 9]. Therefore, an international expert panel recently suggested a more reliable definition of DCI excluding any assumptions about pathogenesis [2]. The panel concluded that in observational studies and clinical trials aiming to investigate strategies to prevent DCI, the two main outcome measures should consist of (1) cerebral infarction, identified by CT or MRI or confirmed at autopsy (not including infarctions due to complications following surgery or endovascular treatment), and (2) functional outcome. Delayed neurological deficits or vasospasms detected by angiography or TCD may be used as secondary outcome measures, but must be assessed together with serial neuroimaging studies and functional outcome [2].
Schematic overview of the pathophysiology of DCI following SAH. Erythrocytes metabolites in the subarachnoid space lead to increased efficacy of vasoconstrictory agents while inhibiting vasodilators, and are the likely cause of DCI (inset A). They induce (1) delayed, prolonged vasospasm of large proximal cerebral vessels (which may be detected using CT- or MRI-based angiography, DSA or TCD) and (2) microvascular delayed chronic vasospasm (typically undetected by these techniques). In addition, (3) erythrocytes products promote the occurrence of spreading depolarizations (SDs) and (4) reverse the neurovascular coupling between SDs and cerebral blood flow [12]. SDs represent a near-complete breakdown of the cortical ion homeostasis. In the presence of erythrocytes metabolites, SDs typically lead to severe and acute microarterial spasm and spreading ischemia by inverse neurovascular coupling [12]. This microarterial vasospasm is superimposed on the prolonged vasospasm of larger vessels. Prolonged vasospasms and spreading ischemia prevent recovery from SDs, and neurons may eventually die. Using direct current (DC)/alternating current (AC) subdural Electrocorticography recordings (inset B), SDs are observed as slow potential changes (a) which are accompanied by depression of spontaneous neuronal activity (b). This depression can be quantified using the integral of power (c) [11]. Similarly, correlates of SDs in the scalp EEG are slow potential changes (a) and depressions of spontaneous activity (b and c), serving as potential biomarkers for DCI in cEEG recordings [13]. Pathomorphologically, SDs are associated with cytotoxic edema of cortical neurons and astrocytes, as well as acute intense spasms of cortical resistance arteries and arterioles. Microarterial spasms propagate in the tissue together with the neuronal depolarization wave at a speed of approximately 3 mm per minute (spreading ischemia; inset C) [12]
In the future, investigators will need to use standardized DCI definitions as outlined above, and systematically evaluate the effects on qEEG parameters by external factors (including medication, artifacts, and sedation), physiological data (such as intracranial pressure and metabolic abnormalities), and disease-related complications (e.g., intraventricular hemorrhage, obstructive hydrocephalus, rebleeding). Correlating, spreading depolarizations identified by Electrocorticography with changes in the scalp qEEG is crucial in this regard (Fig. 2) [13]. Nonetheless, it remains to be proven that treatment based on DCI detection by cEEG indeed improves outcome and does not lead to increased morbidity due to potentially risky procedures such as catheter-based angiography.
As to the detection of seizures, NCSz were diagnosed in 7–18 % of patients with SAH; NCSE was seen in 3–13 %. [16, 26, 27, 34] Thus, it appears that most patients with subclinical seizures are indeed having NCSE. However, the available studies were very heterogeneous with respect to electrographic seizure criteria. Excluding 3 studies without any clearly stated definition, we identified 8 different seizure definitions (Table 3). Only one study referred to the latest version of the ACNS Standardized Critical Care EEG Terminology [37]. This reflects the controversy about certain EEG patterns on the ictal-interictal continuum, which affects the diagnosis of epileptic seizures, including NCSz and NCSE. The incidence of incorrect cEEG interpretation remains unknown [18]. Only a minority of papers stated explicitly that board-certified electroencephalographers experienced with neuro-critical care were involved in EEG reading [32]. Continuous EEG monitoring obviously allows for detection of a larger amount of seizures (and closer monitoring of antiepileptic drug effects) in a given patient than spot EEG. Thus, the proportion of SAH patients with seizures identified by cEEG in the present studies was higher than in previous reports (5–11 %) [40]. However, the significance of subclinical seizures diagnosed with cEEG remains unclear. NCSE was associated with high mortality, but it is still unknown whether subclinical seizures have a deleterious effect on the brain or if they are merely a marker of the underlying disease. No study evaluated whether treatment of NCSz detected by cEEG translates into better clinical outcome of SAH patients in terms of reduced mortality and morbidity.
Using a quality assessment tool designed specifically for diagnostic accuracy studies, the QUADAS-2, we found that the literature on cEEG monitoring of SAH patients is subject to a high risk of bias. This was mainly related to the selection of patients and cEEG methodology. All available studies were single-center case series, roughly half of these were retrospective, many excluding a significant proportion of patients (e.g. due to logistic reasons), and patient numbers were in general low. Nearly, every second study involved a reduced number of EEG channels, qEEG data were available from only six studies, and very few investigators used cEEG for simultaneous assessment of ischemic and epileptic events.
In conclusion, our primary research question can be answered in the affirmative: cEEG monitoring of patients with SAH leads to detection of an increased number of subclinical seizures and may predict clinically symptomatic episodes of DCI many hours in advance. NCSE is associated with high mortality and morbidity, whereas for DCI identified by cEEG, this association is less evident. However, it remains unknown whether more aggressive treatment as a consequence of intensified neuromonitoring indeed leads to improved clinical outcome. In addition, the literature on cEEG in SAH is subject to a high risk of bias related to the selection of patients and cEEG methodology. Considering the lack of randomized or case–control studies, as well as the heterogeneity of the available data, no meta-analysis of extracted data was performed. Large scale systematic studies, including prospective randomized controlled multicenter trials and comparative effectiveness research, are clearly needed in order to evaluate the benefits (or risks) of early and aggressive treatment of seizures and DCI following SAH. Such studies should employ cEEG nomenclature and seizure criteria according to the latest version of the ACNS Standardized Critical Care EEG Terminology, standardized DCI criteria based on cerebral infarctions and functional outcome, board-specified electroencephalographers experienced with neuro-critical care, and preferably, full EEG montage.
References
Budohoski KP, Guilfoyle M, Helmy A, et al. The pathophysiology and treatment of delayed cerebral ischemia following subarachnoid hemorrhage. J Neurol Neurosurg Psychiatry. 2014. doi:10.1136/jnnp-2014-307711.
Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trial and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5.
Dreier JP, Sakowitz OW, Harder A, et al. Focal laminar cortical MR signal abnormalities after subarachnoid hemorrhage. Ann Neurol. 2002;52:82.
Ibrahim GM, Weidauer S, Macdonald RL. Interobserver variability in the interpretation of computed tomography following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2011;115:1191–6.
Schmidt JM, Wartenberg KE, Fernandez A, et al. Frequency and clinical impact of asymptomatic cerebral infarction due to vasospasm after subarachnoid hemorrhage. J Neurosurg. 2008;109:1052–9.
Dreier JP, Major S, Manning A, et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132:1866–8.
Woitzik J, Dreier JP, Hecht N, et al. Delayed cerebral ischemia and spreading depolarization in absence of angiographic vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2012;32:203–12.
Vergouwen MD, et al. Vasospasm versus delayed cerebral ischemia as an outcome event in clinical trials and observational studies. J Cereb Blood Flow Metab. 2011;31:1545–53.
Etminan N, Vergouwen MD, Macdonald RL. Angiographic vasospasm versus cerebral infarction as outcome measures after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:33–40.
Stein SC, Browne KD, Chen XH, et al. Thromboembolism and delayed cerebral ischemia after subarachnoid hemorrhage: an autopsy study. Neurosurgery. 2006;59:78–781.
Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–7.
Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17:439–47.
Drenckhahn C, Winkler MKL, Major S, et al. Correlates of spreading depolarization in in human scalp electroencephalography. Brain. 2012;135:853–68.
Claassen J, Hirsch LJ, Frontera JA, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006;4:103–12.
Claassen J, Perotte A, Albers D, et al. Nonconvulsive seizures after subarachnoid hemorrhage: multimodal detection and outcomes. Ann Neurol. 2013;74:53–64.
Claassen J, Mayer SA, Kowalski RG, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–8.
Claassen J, Taccone FS, Horn P, et al. Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensive Care Med. 2013;39:1337–51.
Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22:79–91.
Schardt C, Adams MB, Owens T, et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16.
Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Labar DR, Fisch BJ, Pedley TA, et al. Quantitative EEG monitoring for patients with subarachnoid hemorrhage. Electroencephalogr Clin Neurophysiol. 1991;78:325–32.
Vespa PM, Nuwer MR, Juhász C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol. 1997;103:607–15.
Dennis LJ, Claassen J, Hirsch LJ, et al. Nonconvulsive status epilepticus after subarachnoid hemorrhage. Neurosurgery. 2002;51:1136–44.
Claassen J, Hirsch LJ, Kreiter KT, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004;115:2699–710.
Claassen J, Mayer SA, Hirsch LJ. Continuous EEG monitoring in patients with subarachnoid hemorrhage. J Clin Neurophysiol. 2005;22:92–8.
Little AS, Kerrigan JF, McDougall CG, et al. Nonconvulsive status epilepticus in patients suffering spontaneous subarachnoid hemorrhage. J Neurosurg. 2009;106:805–11.
Amantini A, Fossi S, Grippo A, et al. Continuous EEG-SEP monitoring in severe brain injury. Clin Neurophysiol. 2009;39:85–93.
Szaflarski JP, Sangha KS, Lindsell CJ, et al. Prospective, randomized, single-blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocrit Care. 2010;12:165–72.
Steinbaugh LA, Lindsell CJ, Shutter LA, et al. Initial EEG predicts outcomes in a trial of levetiracetam vs. fosphenytoin for seizure prevention. Epilepsy Behav. 2012;23:280–4.
Bosco E, Marton E, Feletti A, et al. Dynamic monitors of brain function: a new target in neurointensive care unit. Crit Care. 2011;15:R170.
Park S, Roederer A, Mani R, et al. Limitations of threshold-based brain oxygen monitoring for seizure detection. Neurocrit Care. 2011;15:469–76.
Rathakrishnan R, Gotman J, Dubeau F, et al. Using continuous electroencephalography in the management of delayed cerebral ischemia following subarachnoid hemorrhage. Neurocrit Care. 2011;14:152–61.
Lindgren C, Nordh E, Naredi S, et al. Frequency of non-convulsive seizures and non-convulsive status epilepticus in subarachnoid hemorrhage patients in need of controlled ventilation and sedation. Neurocrit Care. 2012;17:367–73.
Ong C, Gilmore E, Claassen J, et al. Impact of prolonged periodic epileptiform discharges on coma prognosis. Neurocrit Care. 2012;17:39–44.
Crepeau AZ, Kerrigan JF, Gerber P, et al. Rhythmical and periodic EEG patterns do not predict short-term outcome in critically ill patients with subarachnoid hemorrhage. J Clin Neurophysiol. 2013;30:247–54.
Gaspard N, Manganas L, Rampal N, et al. Similarity of lateralized rhythmic delta activity to periodic lateralized epileptiform discharges in critically ill patients. JAMA Neurol. 2013;70:1288–95.
Claassen J, Albers D, Schmidt JM, et al. Nonconvulsive seizures in subarachnoid hemorrhage link inflammation and outcome. Ann Neurol. 2014;75:771–81.
O’Connor KL, Westover MB, Phillips MT, et al. High risk of seizure following subarachnoid hemorrhage regardless of referral bias. Neurocrit Care. 2014. doi:10.1007/s12028-014-9974-y.
Claassen J, Peery S, Kreiter KT, et al. Predictors and clinical impact of epilepsy after subarachnoid hemorrhage. Neurology. 2003;60:208–14.
Acknowledgments
This study was supported by the following Grants DFG DR 323/5-1, BMBF 01 EO 0801, BMBF 01GQ1001C B2, FP7 no 602150 CENTER-TBI.
Ethics statement
This paper is a systematic review of previously published literature, which is why the local ethics committee (Copenhagen University) waived the need for formal approval.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kondziella, D., Friberg, C.K., Wellwood, I. et al. Continuous EEG Monitoring in Aneurysmal Subarachnoid Hemorrhage: A Systematic Review. Neurocrit Care 22, 450–461 (2015). https://doi.org/10.1007/s12028-014-0068-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-014-0068-7