Abstract
Background
Prevention and detection of secondary brain insults via multimodality neuromonitoring is a major goal in patients with severe traumatic brain injury (TBI).
Objective
Explore the underlying pathophysiology and clinical outcome correlates as it pertains to combined monitoring of ≥2 from the following variables: partial brain tissue oxygen tension (PbtO2), pressure reactivity index (PRx), and lactate pyruvate ratio (LPR).
Methods
Data sources included Medline, EMBASE, and evidence-based databases (Cochrane DSR, ACP Journal Club, DARE, and the Cochrane Controlled Trials Register). The PRISMA recommendations were followed. Two authors independently selected articles meeting inclusion criteria. Studies enrolled adults who required critical care and monitoring in the setting of TBI. Included studies reported on correlations between the monitored variables and/or reported on correlations of the variables with clinical outcomes.
Results
Thirty-four reports were included (32 observational studies and 2 randomized controlled trials) with a mean sample size of 34 patients (range 6–223), and a total of 1,161 patient-observations. Overall methodological quality was moderate. Due to inter-study heterogeneity in outcomes of interest, study design, and in both number and type of covariates included in multivariable analyses, quantitative synthesis of study results was not undertaken.
Conclusion
Several literature limitations were identified including small number of subjects, lack of clinical outcome correlations, inconsistent probe location, and overall moderate quality among the included studies. These limitations preclude any firm conclusions; nevertheless we suggest that the status of cerebrovascular reactivity is not only important for cerebral perfusion pressure optimization but should also inform interpretation and interventions targeted on PbtO2 and LPR. Assessment of reactivity can be the first step in approaching the relations among cerebral blood flow, oxygen delivery, demand, and cellular metabolism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term multimodality neuromonitoring encompasses a number of monitoring techniques and tools that measure and attempt to continually “interrogate” different aspects of pathophysiology in the traumatized brain. These are aspects of pressure-flow-oxygenation dynamics, biochemistry-metabolic tissue profiles, and electrophysiological states. Subjecting monitoring techniques to the challenges of evidence-based medicine has been particularly difficult, especially since outcomes are primarily dependent on interventions and not on the monitoring data that inform them. However, use of monitoring data without rigorous underlying physiologic models not only misguides clinical trial design and result but also hampers clinical decision-making and potentially endangers patient outcomes. Several excellent narrative reviews in neuromonitoring have been recently published [1–6]. Here, we attempt a systematic review focused on studies combining monitoring of brain tissue oxygen tension (PbtO2), cerebrovascular pressure reactivity index (PRx), and lactate-pyruvate ratio (LPR). We chose these three variables for the following reasons: 1. These are the three factors recommended in the most recent version of the brain trauma foundation (BTF) guidelines, as ancillary measurements in an effort to identify patient-specific optimal cerebral perfusion pressure (CPP) thresholds [7]. 2. They are all obtainable via the same source, a common intracranial access device or “multilumen bolt” [an oxygen sensor for PbtO2, a microdialysis (MD) catheter for LPR, and an intracranial pressure (ICP) parenchymal probe, used for derivation of PRx] [8] 3. Contemporary literature in severe traumatic brain injury (TBI) over these three indices overwhelms other commonly used modalities including electroencephalography, direct cerebral blood flow (CBF) monitoring, and near-infrared spectroscopy among others. Finally, we did chose to investigate PbtO2 over jugular venous bulb oxygenation (JvO2), because we intended to correlate local metabolic/redox states with local tissue oxygenation and because JvO2 has been suggested to provide a potentially lower percentage of high quality, reliable data [9, 10].
Our specific goal is to identify all clinical studies in patients with TBI combining ≥2 of PbtO2, PRx, and LPR in view of the following questions: Are changes in local brain tissue metabolism and flow reflected by a consistent pattern as it pertains to changes in PbtO2 and LPR? What is the role of the state of pressure autoregulation (as represented by PRx) in these patterns? Can we use combined information from all three variables to describe a patient’s clinicophysiologic state and thus tailor our interventions in optimizing oxygen delivery? These research questions were further reviewed in the context of the methodological quality of the included studies.
Methods
This systematic review was conducted according to the recommendations of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement [11]. The PRISMA checklist, with the related PICOS (participants, interventions, comparisons, outcomes, and study design) appendix, is shown in the Supplemental Digital Content-Appendix A.
Study Selection
We selected articles meeting the following inclusion criteria. First, studies enrolled adults (>18 years old) who required critical care and monitoring in the setting of TBI. Second, patients were monitored with ≥2 of PbtO2, PRx, and LPR. We included studies that either reported on correlations between the monitored variables and/or reported on correlations of the variables with clinical outcomes. Studies involving mixed neurocritical care cohorts (including populations other than TBI) and experimental animal studies were excluded. Also, studies that did not specifically combine PbtO2, PRx, and/or LPR were excluded; we did not include studies that performed MD but did not report on LPR. Despite the fact that MD provides several potentially useful measures of cerebral energy metabolism, we decided to focus on the LPR since it is the marker that has been highlighted by expert consensus as the most representative of cellular redox state and mitochondrial respiratory chain integrity [12, 13].
Data Sources and Searches
Relevant publications were identified by searching Medline (1966 to present), EMBASE (1974 to present), and evidence-based medicine databases (Cochrane DSR, ACP Journal Club, DARE, and the Cochrane Controlled Trials Register from 1990 to present). The following terms and/or MeSH headings were used in different combinations: TBI, neuromonitoring, multimodality monitoring, brain tissue oxygen, brain oxygenation, brain tissue hypoxia, brain ischemia, Licox, Neurotrend, MD, lactate pyruvate ratio, brain tissue metabolism, brain tissue acidosis, redox state, mitochondria, pressure autoregulation, pressure reactivity, PRx. Citations were not excluded on the basis of language. In addition to the electronic search, references from selected reports and review articles, as well as personal files, were hand searched. Only full-length reports published in peer-reviewed journals were included. The search was independently performed by two investigators (C.L., C.M.A) and was completed on August 01 2013.
Data Extraction
Via a standardized data collection form, we extracted the following information from each study: year it was published, study design, objective, number of patients, modalities been monitored (PbtO2, PRx, LPR), other relevant neuromonitoring variables [e.g., tissue oxygen reactivity (TOR)], site of monitoring probes, and conclusions drawn.
Quality Assessment and Data Synthesis
A predefined standardized scoring system was adapted to include 8 items relevant to neuromonitoring observational studies, assessing study methods, analysis, and presentation (Table 1). This scoring system was adapted from a recent systematic review on prognostic factors, and modified to cover the topic of our review [14]. We predefined high quality as a score of >10 and low quality as <6 out of 15. For the two randomized controlled trials (RCTs) included in this review, methodological quality was assessed using the Cochrane Collaboration’s risk of bias assessment tool [15]. In light of inter-study heterogeneity in outcomes of interest (clinical outcome prediction vs. correlations among monitored variables), study design, sites of probes location, and in both number and type of covariates included in multivariable analyses, quantitative synthesis (i.e., meta-analysis) of study results was not undertaken.
Results
Study Characteristics and Quality
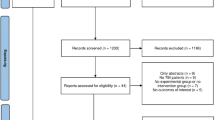
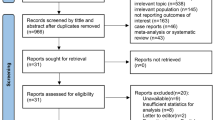
The initial search retrieved 5,572 citations. The electronic search strategy can be found in Supplemental Digital Content-Appendix B. Sequential review of titles, abstracts, and full length articles ultimately yielded 34 reports (Fig. 1) [16–49]. The included studies had a mean sample size of 34 (range 6–223), and totaled 1161 patient-observations. Of the 34 studies, 25 were prospective cohorts [16, 23–26, 29–37, 39–49], 5 were based on prospectively collected data with retrospective analyses [18, 21, 22, 28, 38], 1 was a retrospective cohort [20], 1 was a retrospective case series [19], and 2 were RCTs [17, 27]. Table 2 provides first author, year of study, study design, number of patients enrolled, monitored variables, site of monitoring probes, and conclusions drawn. The frequencies of monitoring of the three variables and the combinations thereof have as follows: PbtO2 (31), LPR (26), PRx (13), PbtO2 + LPR (21) [17, 18, 23, 26, 27, 29, 31, 33, 34, 37, 39–49], PbtO2+PRx (8) [16, 19, 25, 28, 32, 35, 36, 38], LPR+PRx (3) [20, 21, 24], and PbtO2+LPR+PRx (2) [22, 30]. The overall methodological quality of the observational studies was moderate (mean of 9 out of 15; Supplemental Digital Content, Online Appendix Table 1a). Seven studies were scored with >10 and thus classified as high quality [21, 22, 35, 36, 42–44]. Online Appendix Table 1b (Supplemental Digital Content) shows the Cochrane Collaboration’s tool for assessing risk of bias in the two RCTs included.
PbtO2+LPR
Three themes emerge. Effects of hyperoxia (seven studies), effects of common interventions in the management of TBI, namely CPP augmentation, extraventricular drain (EVD) insertion, mannitol, propofol administration, and packed red blood cell (PRBC) transfusion (six studies); the third theme is reporting on associations among PbtO2, LPR, ICP, and clinical outcome (eight studies).
Hyperoxia
Increasing availability of dissolved oxygen, both under normobaric and hyperbaric conditions lead to an increase of PbtO2, and a decrease in LPR in the majority of the included investigations. The studies by Rockswold et al. [17, 27] suggest that hyperbaric oxygen maybe more effective in achieving PbtO2 levels required for improvement of oxidative metabolism. Although none of these studies was primarily aimed in assessing clinical outcomes and thus not adequately powered, there is the suggestion of better clinical outcomes in patients treated with hyperoxia. To this end, Tolias et al. [42] used historical controls, and Rockswold et al. [17] performed a randomized prospective phase II clinical trial. Nortje et al. using PET found that normobaric hyperoxia resulted in increased PbtO2 and augmentation of cerebral metabolic rate for oxygen (CMRO2) in “tissue at risk” that was unaccompanied by a significant change in LPR [31]. Similarly, Magnoni et al. [45] found increased PbtO2 that did not translate to reduced LPR.
Interventions
Both studies using CPP augmentation showed a significant increase in PbtO2, with potentially preferential effects of Norepinephrine over Dopamine; neither study documented an effect on LPR despite decreasing arteriovenous oxygen gradient (AVDO2) and oxygen extraction fraction (OEF) [39, 41]. ICP reduction and improvement of craniospinal reserve, after EVD insertion, lead to both an increase in PbtO2 and a reduction in LPR [33]. Neither mannitol nor propofol had effects on PbtO2 and LPR despite reducing ICP and increasing burst-suppression levels respectively; both studies were limited by small number of patients [37, 46]. Increasing Hemoglobin levels via PRBC transfusion had a positive effect on PbtO2 but did not affect the LPR [29].
Observational Cohort Studies
The most recent prospective report by Timofeev et al. [18] on 56 patients explored the relationship between brain tissue oxygenation, brain tissue pH (pHbt), and metabolic profile. These authors found a significant, negative correlation between LPR-PbtO2 only at low pHbt, and increased mortality to be associated with brain tissue acidosis. The relationship between the two markers was not concordant for Merino et al. [26], Hlatky et al. [40, 43], and Sarrafzadeh et al. [48]. Finally, Hlatky et al., Sarrafzadeh et al., and Vespa et al. [43, 44, 47] found late LPR increases after either PbtO2 < 10 mm Hg or terminal herniation.
PbtO2+PRx
Jaeger et al. [38] first suggested that impaired cerebrovascular PRx increases the risk for secondary cerebral hypoxia. The authors found negative correlations between PRx, PbtO2, and outcome. In this work, they also calculated an index of oxygen reactivity, ORx, as the cross correlation coefficient between PbtO2 and CPP. They found a close correlation between PRx and ORx. More recently the same group showed that PbtO2 increases linearly as it approximates PRx-based, optimal CPP; furthermore, any pressure augmentation beyond CPPOPT yields no additional benefit in brain tissue oxygenation [25]. These relationships among PbtO2, ORx, and PRx did not hold in the investigation by Radolovich et al. [28] (it should be noted that a Neurotrend probe and a shorter monitoring period were used here versus a Licox probe in the investigation by Jaeger et al.) Thorat et al. reported concordant, favorable changes in ICP, PRx, and PbtO2 in survivors treated with barbiturate coma, and Ang et al. found that changes in PbtO2 and CPP correlated negatively with the change in PRx in non-survivors [32, 35]. Most recently, Jaeger et al. reported on TOR and PRx in a small cohort of 11 TBI patients with the premise that both indices assay cerebrovascular resistance vessels; they found a significant correlation between TOR and PRx [16].
PRx+LPR
Timofeev et al. [21] reported observational neuromonitoring data from 223 patients with TBI, prospectively collected during a 10-year period in a single tertiary centre. In a multivariate logistic regression model, which employed data averaged over the whole monitoring period, significant independent positive predictors of mortality were glucose (p = 0.024), LPR (p = 0.016), ICP (p = 0.029), PRx (p = 0.036), and age (p = 0.003); while pyruvate was a significant independent negative predictor of mortality (p = 0.004). According to the authors, the most consistent finding was the significant association of higher LPR with increased mortality and unfavorable outcome; an LPR of 25 was found as the best discriminator. A temporal relationship between changes in autoregulation and biochemical impairment was not conclusively shown. Sanchez-Porras et al. [20] calculated a low-frequency sample pressure reactivity index (L-PRx), using 20-min averages of MAP, and ICP data; significant statistical differences were found in L-PRx, CPP, lactate, and LPR when comparing patients who died and patients who survived. Regarding the temporal association between changes on PRx and LPR, Yokobori et al. [24] found early after injury disturbed biochemistry and pressure reactivity that improved over 4 days with decreasing LPR and negative PRx. An example of combined monitoring with concurrent deterioration of PRx and LPR is shown in Fig. 2.
PbtO2+PRx+LPR
Only two studies were identified reporting on “triple monitoring”. Ho et al., first published a prospective observational study of 16 patients with refractory ICP who underwent decompressive craniectomy (DC) [30]. Six months following TBI, 11 patients had a poor outcome, whereas the remaining 5 patients had a favorable outcome. DC resulted in a significant reduction (p < 0.001) in the mean ICP and PRx to autoregulatory values (PRx < 0.3) in both groups of patients. There was a significant improvement in PbtO2 in the favorable-group patients from 3 to 17 mm Hg; in addition, the durations of abnormal PbtO2 and biochemical indices were significantly reduced after DC, whereas there was no improvement in the biochemical indices in patients who did poorly. More recently, Timofeev et al. [21], in a group of 97 patients found that perilesional tissue chemistry exhibited a significant independent relationship with ICP, PbtO2, and CPP thresholds, with increasing LPR in response to decreases in PbtO2 and CPP, and increase in ICP. The relationship between CPP and chemistry depended upon the state of PRx. Significantly higher levels of MD lactate (p < 0.001), glycerol (p = 0.013), LPR (p < 0.001), and lactate/glucose ratio (p = 0.003) were found in perilesional tissue, compared to ‘‘normal’’ brain tissue. The authors concluded that decreases in perfusion and oxygenation were associated with deteriorating neurochemistry, and these effects were more pronounced in perilesional tissue and when cerebrovascular reactivity was impaired.
Discussion
The interplay between cerebrovascular pressure reactivity, tissue oxygenation, and metabolism, is multifaceted and not fully understood. We systematically investigated the existing clinical literature in an effort to understand physiologic relationships among these variables. Arguably, the main finding of this review relates to the limitations of the available clinical literature that impede reaching safe conclusions on the nature of the physiologic interactions and on outcome prediction. The inconsistent results, aside the limitations, seem to fit more the idea that both PbtO2 and LPR should not be simplistically viewed as markers of ischemic hypoxia but rather as complex measures resulting from the various mechanisms involved in the oxygen delivery-demand-consumption and utilization pathways [50, 51]. Gupta et al. [52] demonstrated that PbtO2 does not represent end-capillary oxygen tension. Subsequently, Menon et al. [53] highlighted the importance of diffusion barriers in the oxygen pathway from blood to the mitochondrial respiratory chain; this barrier is localized in the microvasculature with structural substrates of vascular collapse, endothelial swelling, and perivascular edema. Diringer et al. [54] found no improvement in CMRO2 after normobaric hyperoxia, “disconnecting” PbtO2, and CMRO2. Finally, Rosenthal et al. [55] reinforced the idea that PbtO2 is not closely related to total oxygen delivery nor to cerebral oxygen metabolism, instead identified a parabolic relationship between PbtO2 and the product of CBF and arteriovenous oxygen tension. The LPR is been thought as a sensitive marker of brain redox state and secondary ischemic injury [12]. Positron emission tomography studies have found variable relationships among CMRO2, OEF, and LPR based on the thresholds for ischemia used, timing of monitoring, and probe location [56, 57]. Type of tissue hypoxia is also expected to affect the OEF-LPR relationship, as OEF is expected to be increased in ischemic and decreased in shunt or diffusion-barrier hypoxia [50]. Recent works further demonstrate that an increased LPR may have a wide differential diagnosis [58]. Importantly, energy crisis has been demonstrated to occur in the absence of ischemia or defects in oxygen delivery [57] and on the basis of primary mitochondrial dysfunction [59]. A pattern of increased lactate with near normal pyruvate may indicate mitochondrial failure rather than ischemia [60]. Knowledge of the functional status of mitochondria and of the presence of oxygen diffusion barriers are critical in the interpretation of an increased LPR.
Cerebrovascular pressure reactivity is an intrinsic underlying mechanism of CBF regulation; Steiner et al. and more recently Aries et al. have demonstrated the value of using the PRx in identifying an optimal CPP, under and above which outcome worsens [61, 62]. In the studies we reviewed, the link between disturbed PRx and low PbtO2 could be interpreted as a failure to meet oxygen demands in the face of inadequate vascular reactivity. In this setting and under the consideration that PRx and TOR were shown to correlate [16], the practice of increasing PaO2, as a means of increasing PbtO2, becomes questionable. This is because of data showing that an increase of PbtO2 in the face of disturbed TOR may be actually associated with adverse outcomes, related to oxygen neurotoxicity [63–65]. Another inference to be made is that a close correlation between PRx and ORx suggests that PbtO2 behaves as a CBF surrogate and thus making the presence of a significant diffusion barrier unlikely. In reverse, when PRx and ORx do not correlate, diffusion barriers, and mitochondrial dysfunction have to be considered. In conjunction, monitoring of pressure and oxygen reactivity should inform the decision to increase PaO2 as a means to higher PbtO2. This is particularly relevant since the effects of hyperoxia are not fully understood with mixed clinical and experimental results; artificial augmentation of dissolved oxygen maybe better informed by assessment of vascular reactivity.
The largest to date series of patients with monitoring of PRx and LPR did not find a mechanistic, temporal relation between the two, although they both correlated with outcome [21]. A similar suggestion comes from Asgari et al. [66] who found no connection between high LPR and a measure of vascular reactivity. The link between vascular reactivity and cellular redox state seems not to be direct in these investigations. On the other hand, and when looking specifically at perilesional tissue, Timofeev et al. [22]. showed that the relationship between CPP and tissue biochemistry depended on PRx pointing again to the idea of an inability to meet tissue demands in the face of disturbed vascular reactivity and/or an uncoupled flow-metabolism state.
Limitations
Only 20 % of included studies reached high quality; the main limitations included small number of subjects, lack of clinical outcome correlations, and multivariable analyses looking at multiple predictors among limited numbers of patients. The heterogeneity of aims, variables, and outcomes precluded quantitative synthesis of the data. A similar conclusion was reached by a recent evaluation on the diagnostic accuracy of MD in the setting of brain surgery [67]. An additional source of heterogeneity relates to the rate of data recording (hourly vs. high frequency) and to the technology used for data recording and storage; often this information is not reported.
PbtO2 and LPR are local measures and may not reflect global oxygenation and metabolism, especially in patients suffering from focal injuries. On the contrary, the PRx is a global or composite measure of pressure reactivity thought to arise from cerebral blood volume fluctuations within pressure arterioles. Correlations between local and global measures are further fraught by probe location. There is an active debate on the most appropriate location of monitoring probes and this is apparent in the studies included here. Eleven studies used the right frontal lobe by default, 9 studies positioned probes in the less traumatized hemisphere, 6 studies monitored perilesional (“penumbral”) tissue, 4 studies had probes placed in the more injured hemisphere, and 3 studies had mixed locations. It has been recently shown that probe location may affect the relationship between PbtO2 and neurologic outcome [68]. Also, although most studies have used intraparenchymal probes for ICP monitoring, several studies have used EVDs.
Understanding the nature of monitored variables and the physiology of flow, oxygen, and metabolism pathways is greatly facilitated by PET imaging since it provides measurements of CBF, CMRO2, and OEF. We have referenced above PET studies that have significantly added to current knowledge. The fact is, that PET imaging is restricted to few select centers with the resources and the expertise. Similar restrictions apply to multimodality brain monitoring. Finally, this review has only looked at combinations among 3 specific variables, and it should not be viewed as an all-inclusive review on the plethora of neuromonitoring variables currently available.
Conclusions
We sought to compile, evaluate, and synthesize the literature on combination neuromonitoring of PbtO2, LPR, and PRx. Our ultimate goal is to generate further hypotheses on how to integrate information provided from these variables. We conclude with the following suggestions 1. Included studies are highly heterogeneous; larger number of patients, consistent probe location, and prospective outcome data are needed. 2. Knowledge of the status of cerebrovascular reactivity is not only important for CPP optimization but should also inform interpretation and interventions targeted on PbtO2 and LPR. Assessment of reactivity can be the first step in approaching the relations among CBF, oxygen delivery and demand, and cellular metabolism; concurrent and subsequent steps should consider issues of oxygen diffusion barriers, oxygen toxicity, and mitochondrial failure.
References
Stocchetti N, Roux PL, Vespa P, et al. Clinical review: neuromonitoring—an update. Crit Care. 2013;17(1):201.
Feyen BF, Sener S, Jorens PG, Menovsky T, Maas AI. Neuromonitoring in traumatic brain injury. Minerva Anestesiol. 2012;78(8):949–58.
Messerer M, Daniel RT, Oddo M. Neuromonitoring after major neurosurgical procedures. Minerva Anestesiol. 2012;78(7):810–22.
Kirkman MA, Smith M. Multimodal intracranial monitoring: implications for clinical practice. Anesthesiol Clin. 2012;30(2):269–87.
Oddo M, Villa F, Citerio G. Brain multimodality monitoring: an update. Curr Opin Crit Care. 2012;18(2):111–8.
Hemphill JC, Andrews P, De Georgia M. Multimodal monitoring and neurocritical care bioinformatics. Nat Rev Neurol. 2011;7(8):451–60.
Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury IX. Cerebral perfusion thresholds. J Neurotrauma. 2007;24(Suppl 1):S59–64.
Hutchinson PJ, Hutchinson DB, Barr RH, Burgess F, Kirkpatrick PJ, Pickard JD. A new cranial access device for cerebral monitoring. Br J Neurosurg. 2000;14(1):46–8.
Kiening KL, Unterberg AW, Bardt TF, Schneider GH, Lanksch WR. Monitoring of cerebral oxygenation in patients with severe head injuries: brain tissue PO2 versus jugular vein oxygen saturation. J Neurosurg. 1996;85:751–7.
Gopinath SP, Valadka AB, Uzura M, Robertson CS. Comparison of jugular venous oxygen saturation and brain tissue Po2 as monitors of cerebral ischemia after head injury. Crit Care Med. 1999;27(11):2337–45.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Bellander BM, Cantais E, Enblad P, et al. Consensus meeting on microdialysis in neurointensive care. Intensiv Care Med. 2004;30(12):2166–9.
Andrews PJ, Citerio G, Longhi L, et al. NICEM consensus on neurological monitoring in acute neurological disease. Intensiv Care Med. 2008;34(8):1362–70.
de Rooij NK, Rinkel GJ, Dankbaar JW, Frijns CJ. Delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review of clinical, laboratory, and radiological predictors. Stroke. 2013;44(1):43–54.
Higgins JP, Altman DG, Gøtzsche PC, Cochrane Bias Methods Group, Cochrane Statistical Methods Group, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Jaeger M, Lang EW. Cerebrovascular pressure reactivity and cerebral oxygen regulation after severe head injury. Neurocrit Care. 2013;19(1):69–73.
Rockswold SB, Rockswold GL, Zaun DA, Liu J. A prospective, randomized Phase II clinical trial to evaluate the effect of combined hyperbaric and normobaric hyperoxia on cerebral metabolism, intracranial pressure, oxygen toxicity, and clinical outcome in severe traumatic brain injury. J Neurosurg. 2013;118(6):1317–28.
Timofeev I, Nortje J, Al-Rawi PG, Hutchinson PJ, Gupta AK. Extracellular brain pH with or without hypoxia is a marker of profound metabolic derangement and increased mortality after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33(3):422–7.
Bowles AP, Pasierb L, Simunich T, Updyke M. Implications of neurophysiological parameters in persons with severe brain injury with respect to improved patient outcomes: a retrospective review. Brain Inj. 2012;26(12):1415–24.
Sánchez-Porras R, Santos E, Czosnyka M, Zheng Z, Unterberg AW, Sakowitz OW. ‘Long’ pressure reactivity index (L-PRx) as a measure of autoregulation correlates with outcome in traumatic brain injury patients. Acta Neurochir (Wien). 2012;154(9):1575–81.
Timofeev I, Carpenter KL, Nortje J, et al. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain. 2011;134(Pt 2):484–94.
Timofeev I, Czosnyka M, Carpenter KL, et al. Interaction between brain chemistry and physiology after traumatic brain injury: impact of autoregulation and microdialysis catheter location. J Neurotrauma. 2011;28(6):849–60.
Vilalta A, Sahuquillo J, Merino MA, et al. Normobaric hyperoxia in traumatic brain injury: does brain metabolic state influence the response to hyperoxic challenge? J Neurotrauma. 2011;28(7):1139–48.
Yokobori S, Watanabe A, Matsumoto G, et al. Time course of recovery from cerebral vulnerability after severe traumatic brain injury: a microdialysis study. J Trauma. 2011;71(5):1235–40.
Jaeger M, Dengl M, Meixensberger J, Schuhmann MU. Effects of cerebrovascular pressure reactivity-guided optimization of cerebral perfusion pressure on brain tissue oxygenation after traumatic brain injury. Crit Care Med. 2010;38(5):1343–7.
Merino MA, Sahuquillo J, Borrull A, Poca MA, Riveiro M, Expósito L. Is lactate a good indicator of brain tissue hypoxia in the acute phase of traumatic brain injury? Results of a pilot study in 21 patients. Neurocirugia (Astur). 2010;21(4):289–301.
Rockswold SB, Rockswold GL, Zaun DA, et al. A prospective, randomized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injury. J Neurosurg. 2010;112(5):1080–94.
Radolovich DK, Czosnyka M, Timofeev I, et al. Reactivity of brain tissue oxygen to change in cerebral perfusion pressure in head injured patients. Neurocrit Care. 2009;10(3):274–9.
Zygun DA, Nortje J, Hutchinson PJ, Timofeev I, Menon DK, Gupta AK. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Crit Care Med. 2009;37(3):1074–8.
Ho CL, Wang CM, Lee KK, Ng I, Ang BT. Cerebral oxygenation, vascular reactivity, and neurochemistry following decompressive craniectomy for severe traumatic brain injury. J Neurosurg. 2008;108(5):943–9.
Nortje J, Coles JP, Timofeev I, et al. Effect of hyperoxia on regional oxygenation and metabolism after severe traumatic brain injury: preliminary findings. Crit Care Med. 2008;36(1):273–81.
Thorat JD, Wang EC, Lee KK, Seow WT, Ng I. Barbiturate therapy for patients with refractory intracranial hypertension following severe traumatic brain injury: its effects on tissue oxygenation, brain temperature and autoregulation. J Clin Neurosci. 2008;15(2):143–8.
Timofeev I, Dahyot-Fizelier C, Keong N, et al. Ventriculostomy for control of raised ICP in acute traumatic brain injury. Acta Neurochir Suppl. 2008;102:99–104.
Tisdall MM, Tachtsidis I, Leung TS, Elwell CE, Smith M. Increase in cerebral aerobic metabolism by normobaric hyperoxia after traumatic brain injury. J Neurosurg. 2008;109(3):424–32.
Ang BT, Wong J, Lee KK, Wang E, Ng I. Temporal changes in cerebral tissue oxygenation with cerebrovascular pressure reactivity in severe traumatic brain injury. J Neurol Neurosurg Psychiatry. 2007;78(3):298–302.
Reinert M, Andres RH, Fuhrer M, Müller A, Schaller B, Widmer H. Online correlation of spontaneous arterial and intracranial pressure fluctuations in patients with diffuse severe head injury. Neurol Res. 2007;29(5):455–62.
Sakowitz OW, Stover JF, Sarrafzadeh AS, Unterberg AW, Kiening KL. Effects of mannitol bolus administration on intracranial pressure, cerebral extracellular metabolites, and tissue oxygenation in severely head-injured patients. J Trauma. 2007;62(2):292–8.
Jaeger M, Schuhmann MU, Soehle M, Meixensberger J. Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity. Crit Care Med. 2006;34(6):1783–8.
Johnston AJ, Steiner LA, Coles JP, et al. Effect of cerebral perfusion pressure augmentation on regional oxygenation and metabolism after head injury. Crit Care Med. 2005;33(1):189–95.
Hlatky R, Valadka AB, Goodman JC, Robertson CS. Evolution of brain tissue injury after evacuation of acute traumatic subdural hematomas. Neurosurgery. 2004;55(6):1318–23.
Johnston AJ, Steiner LA, Chatfield DA, et al. Effect of cerebral perfusion pressure augmentation with dopamine and norepinephrine on global and focal brain oxygenation after traumatic brain injury. Intensiv Care Med. 2004;30(5):791–7.
Tolias CM, Reinert M, Seiler R, Gilman C, Scharf A, Bullock MR. Normobaric hyperoxia—induced improvement in cerebral metabolism and reduction in intracranial pressure in patients with severe head injury: a prospective historical cohort-matched study. J Neurosurg. 2004;101(3):435–44.
Hlatky R, Valadka AB, Goodman JC, Contant CF, Robertson CS. Patterns of energy substrates during ischemia measured in the brain by microdialysis. J Neurotrauma. 2004;21(7):894–906.
Vespa PM, McArthur D, O’Phelan K, et al. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: a microdialysis study. J Cereb Blood Flow Metab. 2003;23(7):865–77.
Magnoni S, Ghisoni L, Locatelli M, et al. Lack of improvement in cerebral metabolism after hyperoxia in severe head injury: a microdialysis study. J Neurosurg. 2003;98(5):952–8.
Johnston AJ, Steiner LA, Chatfield DA, et al. Effects of propofol on cerebral oxygenation and metabolism after head injury. Br J Anaesth. 2003;91(6):781–6.
Sarrafzadeh AS, Sakowitz OW, Callsen TA, Lanksch WR, Unterberg AW. Detection of secondary insults by brain tissue Po2 and bedside microdialysis in severe head injury. Acta Neurochir Suppl. 2002;81:319–21.
Sarrafzadeh AS, Sakowitz OW, Callsen TA, Lanksch WR, Unterberg AW. Bedside microdialysis for early detection of cerebral hypoxia in traumatic brain injury. Neurosurg Focus. 2000;9(5):e2.
Hutchinson PJ, al-Rawi PG, O’Connell MT, et al. Head injury monitoring using cerebral microdialysis and Paratrend multiparameter sensors. Zentralbl Neurochir. 2000;61(2):88–94.
Haitsma IK, Maas AI. Advanced monitoring in the intensive care unit: brain tissue oxygen tension. Curr Opin Crit Care. 2002;8(2):115–20.
Nortje J, Gupta AK. The role of tissue oxygen monitoring in patients with acute brain injury. Br J Anaesth. 2006;97:95–106.
Gupta AK, Hutchinson PJ, Fryer T, et al. Measurement of brain tissue oxygenation performed using positron emission tomography scanning to validate a novel monitoring method. J Neurosurg. 2002;96(2):263–8.
Menon DK, Coles JP, Gupta AK, et al. Diffusion limited oxygen delivery following head injury. Crit Care Med. 2004;32:1384–90.
Diringer MN, Aiyagari V, Zazulia AR, Videen TO, Powers WJ. Effect of hyperoxia on cerebral metabolic rate for oxygen measured using positron emission tomography in patients with acute severe head injury. J Neurosurg. 2007;106(4):526–9.
Rosenthal G, Hemphill JC 3rd, Sorani M, et al. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit Care Med. 2008;36(6):1917–24.
Hutchinson PJ, Gupta AK, Fryer TF, et al. Correlation between cerebral blood flow, substrate delivery, and metabolism in head injury: a combined microdialysis and triple oxygen positron emission tomography study. J Cereb Blood Flow Metab. 2002;22(6):735–45.
Vespa P, Bergsneider M, Hattori N, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25(6):763–74.
Larach DB, Kofke WA, Le Roux P. Potential non-hypoxic/ischemic causes of increased cerebral interstitial fluid lactate/pyruvate ratio: a review of available literature. Neurocrit Care. 2011;15(3):609–22.
Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J Neurosurg. 2000;93(5):815–20.
Nielsen TH, Schalén W, Ståhl N, Toft P, Reinstrup P, Nordström CH. Bedside Diagnosis of Mitochondrial Dysfunction After Malignant Middle Cerebral Artery Infarction. Neurocrit Care. 2013. doi:10.1007/s12028-013-9875-5.
Steiner LA, Czosnyka M, Piechnik SK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30(4):733–8.
Aries MJ, Czosnyka M, Budohoski KP, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40(8):2456–63.
van Santbrink H, Maas AI, Avezaat CJ. Continuous monitoring of partial pressure of brain tissue oxygen in patients with severe head injury. Neurosurgery. 1996;38:21–31.
Menzel M, Doppenberg EM, Zauner A, et al. Cerebral oxygenation in patients after severe head injury: monitoring and effects of arterial hyperoxia on cerebral blood flow, metabolism and intracranial pressure. J Neurosurg Anesthesiol. 1999;11:240–51.
van Santbrink H, vd Brink WA, Steyerberg EW, Carmona Suazo JA, Avezaat CJ, Maas AI. Brain tissue oxygen response in severe traumatic brain injury. Acta Neurochir (Wien). 2003;145(6):429–38.
Asgari S, Vespa P, Hu X. Is there any association between cerebral vasoconstriction/vasodilatation and microdialysis lactate to pyruvate ratio increase? Neurocrit Care. 2013;19(1):56–64.
Bossers SM, de Boer RD, Boer C, Peerdeman SM. The diagnostic accuracy of brain microdialysis during surgery: a qualitative systematic review. Acta Neurochir (Wien). 2013;155(2):345–53.
Ponce LL, Pillai S, Cruz J, et al. Position of probe determines prognostic information of brain tissue PO2 in severe traumatic brain injury. Neurosurgery. 2012;70(6):1492–502.
Acknowledgments
We would like to thank Professor Marek Czosnyka, Academic Department of Neurosurgery, University of Cambridge, Cambridge, UK, for graciously providing Fig. 2 and Dr. Laith Altaweel, Department of Critical Care, Inova Fairfax Hospital, Fairfax, VA for his careful reading of our manuscript.
Conflict of interest
Christos Lazaridis and Charles Andrews declare that they have no conflict of interest.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lazaridis, C., Andrews, C.M. Brain Tissue Oxygenation, Lactate-Pyruvate Ratio, and Cerebrovascular Pressure Reactivity Monitoring in Severe Traumatic Brain Injury: Systematic Review and Viewpoint. Neurocrit Care 21, 345–355 (2014). https://doi.org/10.1007/s12028-014-0007-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-014-0007-7