Abstract
Background
Cerebral autoregulation and, consequently, cerebrovascular pressure reactivity, can be disturbed after traumatic brain injury (TBI). Continuous monitoring of autoregulation has shown its clinical importance as an independent predictor of neurological outcome. The cerebral pressure reactivity index (PRx) reflects that changes in seconds of cerebrovascular reactivity have prognostic significance. Using an alternative algorithm similar to PRx, we investigate whether the utilization of lower-frequency changes of the order of minutes of mean arterial blood pressure (MAP) and intracranial pressure (ICP) could have a prognostic value in TBI patients.
Materials and methods
Head-injured patients requiring continued advanced multimodal monitoring, including hemodynamic, ICP and microdialysis (MD) monitoring, were analyzed retrospectively. A low-frequency sample pressure reactivity index (L-PRx) was calculated, using 20-min averages of MAP and ICP data as a linear Pearson's correlation. The mean values per patient were correlated to outcome at 6 months after injury. Differences of monitoring parameters between non-survivors and survivors were compared.
Results
A total of 29 patients (mean age 37.2 years, 26 males) suffering from TBI were monitored for a mean of 109.6 h (16–236 h, SD ± 60.4). Mean L-PRx was found to be of 0.1 (−0.2 to 0.6, SD ± 0.20), six patients presented impaired (>0.2) values. The averaged L-PRx correlated significantly with ICP (r = 0.467, p = 0.011) and 6-month outcome (r = −0.556, p = 0.002). Significant statistical differences were found in L-PRx, cerebral perfusion pressure (CPP), lactate, and lactate-pyruvate ratio when comparing patients who died (n = 5) and patients who survived.
Conclusions
L-PRx correlates with the 6-month outcome in TBI patients. Very slow changes of MAP and ICP may contain important autoregulation information. L-PRx may be an alternative algorithm for the estimation of cerebral autoregulation and clinical prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autoregulation of cerebral blood flow (CBF) can be defined as the ability of brain arteries to vasodilate or constrict in response to blood pressure changes in order to maintain stable CBF [10]. It is regulated by the interplay of myogenic, metabolic, neurogenic, and endothelial factors. Nevertheless, the mechanisms underlying this phenomenon remain controversial [4, 12]. It is known that changes in arterial blood pressure usually produce a response in intracranial pressure (ICP), thus describing cerebrovascular pressure reactivity, defined as the capacity of vascular smooth muscle in the wall of cerebral arteries and arterioles to respond to changes in transmural pressure. Observations show that when the cerebral pressure-reactivity is intact, increases in the mean arterial blood pressure (MAP) will induce a vasoconstriction with reduction of cerebral blood volume and decrease of ICP, within a period of 5 to 30 s. When reactivity is impaired, the increase of MAP will increase the cerebral blood volume and ICP without compensation [18]. Therefore, cerebrovascular pressure reactivity represents a key mechanism responsible for cerebral autoregulation [4, 5, 18].
Autoregulation and, consequently, cerebrovascular pressure reactivity can be lost in a variety of insults such as traumatic brain injury (TBI) [12, 23], representing a significant risk factor for secondary brain damage [13]. The clinical importance of the continuous monitoring of these changes has been addressed by different indices, which can be used as independent predictors of the worse neurological outcome [5, 6]. The pressure reactivity index (PRx) is a monitoring index that quantifies cerebrovascular pressure reactivity and provides information of CBF fluctuations. Its prognostic importance applied in TBI patients has been recognized by various studies [5, 6, 9]. The PRx algorithm is based on the knowledge that the autoregulatory response is initiated within seconds after changes in pressure, as a consequence of myogenic regulation [19]. It calculates the correlation between 20-s and 2-min spontaneous slow waves of MAP and ICP. However, against this theoretical background, recently, a similar index of low-frequency pressure reactivity (L-PRx), which uses longer, 1-min average values and analyses much slower changes between MAP and ICP, correlates as well as PRx with the outcome in intracerebral hemorrhage (ICH) patients [15]. We tested the hypothesis that longer changes in MAP and ICP, between 1 and 20 min, are useful to evaluate cerebrovascular pressure reactivity.
Therefore, the aim of this study was to analyze low-frequency changes in MAP and ICP using an alternative algorithm derived from PRx, which uses minute-by-minute values to correlate it with patients’ follow-up outcome at 6 months and with the other monitoring variables. We also compared the difference in monitoring parameters between non-survivors and survivors.
Materials and methods
Patients
We conducted a retrospective study with TBI patients admitted to the neurosurgical intensive care unit of Heidelberg University Hospital. Demographic data, such as age, gender, mechanism of injury, neurological examination, CT findings and Glasgow Outcome Scale (GOS) after 6 months of follow-up were collected. All patients followed a standard protocol. They required sedation, intubation, artificial ventilation, and cardiovascular and neurological monitoring. Propofol and/or midazolam were used for sedation; analgesia was provided with fentanyl. Artificial ventilation aimed at keeping the PaO2 at 100 to 120 mmHg and the PaCO2 at 35 to 45 mmHg was performed. Arterial blood gas samples were obtained every 6 h, or as clinically indicated, to adjust ventilation parameters. Treatment targeted ICP pressure <20 mmHg and CPP between 60 and 70 mmHg. Mannitol was used for control of raised ICP at doses of 0.25 g/kg to 1 g/kg body weight. If needed, CPP was elevated with volume expansion and/or vasopressors, as clinically indicated. Space-occupying intracranial hematomas were surgically evacuated as deemed necessary by the neurosurgeon-on-call. The study protocol was approved by the local Ethics Committee for Human Research. Informed consent was obtained from the closest relatives of all patients.
Monitoring
As part of the routine, all patients underwent continued monitoring of MAP, ICP, and resulting cerebral perfusion pressure (CPP). Invasive MAP recordings were obtained via radial or femoral artery catheters. ICP measures were obtained from flexible intraparenchymal probes (Camino, CA, USA; Codman, Johnson & Johnson, New Brunswick, NJ, USA) inserted into the frontal white matter of the injured hemisphere. CPP was calculated as the difference between MAP and ICP (CPP = MAP - ICP). Analogue data of MAP and ICP were sampled at 1 min, from bed side monitors in the intensive care unit monitoring system via TCP/ICP, the Infinity Gateway Software Suite (Dräger Medical Deutschland GmbH, Lübeck, Germany) and recorded to a portable computer at the patient's bed side, using ICU Pilot™ (CMA Microdialysis AB, Solna, Sweden) software. Artefacts caused by movement, disconnection, or nursing interventions were manually eliminated from raw data sets.
Microdialysis
As part of the multimodal monitoring, all patients were monitored for cerebral extracellular chemistry using microdialysis (MD) probes with a molecular weight cut-off limit of 20 kDa and a length of 10 mm (CMA 70, CMA Microdialysis, and Stockholm, Sweden). MD probes were perfused with artificial CNS fluid at a rate of 0.3 μl/min and samples were taken every hour. The first-day samples were discarded from later examinations. Consecutive 1-h samples were continuously collected for on-line analysis and concentrations of glucose, glutamate, lactate, and pyruvate were analyzed using a mobile photometric, enzyme-kinetic analyzer (CMA600, CMA, Sweden).
L-PRx calculation
L-PRx was calculated as described elsewhere [15], as a moving linear (Pearson’s) correlation coefficient using a minute value in a time window of 20 min of 20 consecutive values of MAP and ICP. The window was repeated every minute to generate an overlapping index, expressing the correlation for 10 min before and after the desired time point. The value was expressed within ranges of −1 to 1. We assume that a negative value reflects preserved vascular reactivity, while a positive L-PRx implies that increases in MAP are associated with increases in ICP, thus reflecting nonreactive vessels in the same way that PRx does. We consider a value over 0.2 as a signal of impaired pressure-reactivity.
Statistical data analysis
The artefact-free episodes of MAP and ICP and the resulting CPP and L-PRx, as well as the MD values, were averaged over time, so every patient was represented by one set of data. Nonparametric statistical methods were generally used, due to the fact that the majority of our variables did not follow a normal distribution. The relationship between average L-PRx, GOS at 6 months post-injury (5 good; 4 with moderate disability; 3 with severe disability; 2 with persistent vegetative state; 1 death) and the studied variables was calculated using Spearman's rank correlation coefficient. The patients were divided into two groups for a better statistical comparison: patients with fatal outcome (GOS = 1) and survivors (GOS = 2–5). The Mann–Whitney U test was used to perform assessments when comparing such groups. Only variables with p < 0.05 entered into the model. Values of p < 0.05 were considered statistically significant in all tests. Statistical analyses were performed using the SPSS v18.0 (SPSS, Chicago, IL, USA) statistical package.
Results
A total of 29 patients suffering from TBI were retrospectively included in the study; all with a GCS score of ≤ 8 on presentation, or within 48 h of trauma. Twenty-six (89.6 %) of the patients were male and 3 (10.3 %) were female. The mean age was 37.2 (5–75) years. The patients were monitored, after artefact extraction, for a mean of 109.6 h (16–236 h, SD ± 60.4) with data containing MAP, ICP, CPP, and MD metabolites. The mean L-PRx value for all patients was 0.1 (−0.2 to 0.6, SD ± 0.20). It was found intact in 23 (79.3 %) patients with an L-PRx <0.16 and impaired in 6 (20.6 %) with an L-PRx >0.23.
Spearman's correlation coefficients and p values between averaged (for each patient) values of L-PRx, MAP, ICP, CPP, MD, and GOS at 6 months are presented in Table 1. Disturbed low-frequency pressure-reactivity (positive L-PRx) correlated significantly with ICP (r = 0.467, p = 0.011) and 6 months outcome (r = −0.556, p = 0.002), indicating that disturbances in MAP and ICP within periods of 1 to 20 min are associated with unfavorable prognosis. However, averaged L-PRx values did not show significant correlation with MAP and CPP, albeit the latter bordered on significance (p = 0.065). Moreover, correlation between averaged L-PRx and the various metabolites was not observed. However, a correlation between outcome, glutamate, and lactate was present.
From the analysis of L-PRx versus outcome, we confirm that the critical value for averaged L-PRx seems to be >0.2, above which the fatal outcome was 83.3 % (n = 6) (Fig. 1). The mortality rate at 6 months of follow-up was 17.2 %; these five patients had a mean age of 56 (SD ± 13.4) years and four of them were male. The remaining 82.8 % corresponded to the survivors, who had a mean age of 33 (SD ± 22.4) years. Among those who survived, 62.5 % of the patients had a good functional outcome (GOS 4 or 5). Patients with fatal outcome presented an averaged L-PRx of 0.4 while survivors presented an averaged L-PRx of 0.03 (Fig. 2).
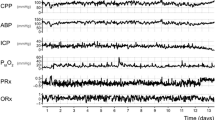
The relationship between L-PRx values over days in patients who survived and non-survivors showed that survivors presented a lower constant L-PRx during the entire monitoring period (Fig. 3). When comparing survivors and non-survivors, statistical significance was found in L-PRx, CPP, lactate, and lactate-pyruvate ratio (Mann–Whitney U test). Table 2 shows the parameter variables between the groups.
Discussion
The understanding of cerebral autoregulation has gained an important role in the evolution of critical care management in TBI patients. At present, a gold standard for measuring cerebral autoregulation does not exist, and the literature shows discrepancy in the several methods available. Nevertheless, the assessment of autoregulation can help to individualize treatment, in order to avoid secondary ischemic damage and to improve the outcome [13]. PRx allows a continuous estimation of cerebral autoregulation, reflecting the capacity of cerebral vessels to modify diameter in response to changes in blood pressure. It has also been reported as a useful secondary index of vascular deterioration, leading to fatal outcome and a significant increase in mortality has been observed to occur with increasing PRx [17].
In this work, we studied a variation of the PRx algorithm using a 20-min window of minute values that does not consider the changes between 5 to 30 s that MAP induces in ICP, but rather slower changes. In our study, we found an independent significant correlation between L-PRx and GOS at 6 months (r = −0.556, p = 0.002) and ICP (r = 0.467, p = 0.011), as well as a significant difference in L-PRx values between survivors and non-survivors (p = 0.001). Therefore, the results of this study help to support our hypothesis that changes in MAP and ICP in the range of minutes could be used in terms of autoregulation assessment and prognosis, suggesting that L-PRx can become a valid prognostic index in head injury patients.
The results found in our study are similar to those reported by other studies analyzing PRx, where PRx has shown a strong and independent correlation with outcome. In this regard, Czosnyka et al. [5], having examined the relationship between PRx and poor 6-month outcome in a group of 82 patients with brain injury, reported a significant correlation (r = 0.484, p < 0.00001) between these variables, proposing a persisting PRx value above 0.2 as critical for unfavorable outcome. However, others had found a value of 0.3 as determinant [9, 17, 22]. In another study with 114 TBI patients, Steiner et al. [18] reported a negative correlation between PRx and GOS at 6 months (r = −0.274, p = 0.003) and, recently, Jaeger et al. [8] found a significant negative correlation between these variables in a group of 27 head-injury patients (r = −0.52, p = 0.005). The mortality rate found in our group of patients is also similar to the 15–26 % reported by others [5, 9, 18], showing the similarity with other TBI populations. However, prognosis after severe TBI depends on a number of factors, not all of them determined in clinical trials. Such factors may account for differences in prognosis, clinical course and final outcome. Up to now, there is only one study that compares L-PRx and PRx with outcome in ICH patients. In this study, L-PRx and PRx correlated similarly with outcome (r = 0.667, p = 0.002; r = 0.563, p = 0.015, respectively) at time of discharge, using the National Institutes of Health Stroke Scale Score (NIHSS) [15]. In this study, we propose that cerebrovascular pressure reactivity may be assessed by using minute-by-minute data, as shown by the positive correlation between L-PRx and ICP value that was found similar to that reported between PRx and ICP (r = 0.366, p < 0.001) by Czosnyka et al. [5].
In analogy to PRx, the L-PRx algorithm provides a moving correlative index of slow spontaneous 20-min fluctuations in MAP and ICP [5, 6, 18]. This is true despite the common assumption that the autoregulatory response is a fast-acting mechanism that responds within seconds after changes in pressure and therefore requires high-frequency sampling for good time resolution [24]. L-PRx takes into account the consistency of slow changes in vasoreactivity and level of disturbance in physiological vascular responses exploring values by-the-minute, instead of by-the-second, as required by PRx. Thus, it records frequencies between 0.016 to 0.0008 Hz, instead of 0.05-0.008 Hz as analyzed by PRx. Indeed this strengthens support for the clinical significance of slow MAP waves lasting from 20 s to 10 min [1, 24], as well as ICP waves lasting from 2 or 3 min up to as long as 20 to 30 min [14]. Therefore, we speculate that minutely averaging values increases sensitivity through the denoising of MAP and ICP signals, where normally plenty of artefacts are abundant in the ICU environment. It is also possible that MAP and ICP waves with periods of less than 1–2 min may not be essential for autoregulation assessment and prognostic information as is believed [15]. The similarity found between other research and our study, shows that L-PRx can be used for outcome prediction. This suggests that autoregulation information available in the range of minutes and hours, important for vascular reactivity and the fate of injured brain tissue, may exist.
In our study, we did not find a correlation between average values of L-PRx and extracellular concentrations of brain tissue metabolites. This fact could be attributable to the limited number of patients. However, glutamate and lactate showed a correlation with outcome, demonstrating that secondary insults in TBI, which can be measured by MD at the bedside, have an impact on the long-term outcome [20, 21]. Nevertheless, the correlation between L-PRx and outcome was higher than that found between outcome and metabolites. Other studies have reported elevated extracellular glutamate concentrations as a strong predictor of poor outcome [2, 3]. Moreover, in TBI patients, MD variables in combination with MAP, ICP, and CPP have been shown to be valuable in outcome determination, with a significant improvement in prediction accuracy [11]. Thus, MD parameters are surrogate parameters of brain tissue health and every deterioration can be viewed as an indicator of tissue response to injury, with a potential role to influence the overall prognosis. A statistically significant difference in extracellular lactate concentrations and lactate-pyruvate ratio was found between survivors and non-survivors. In this regard, a recent study found that in the perilesional tissue of TBI patients, the loss of autoregulation led to increases in the lactate-pyruvate ratio concomitant with reductions in CPP and elevated ICP [21]. Significant correlations have also been reported between high ICP and low CPP values with elevated lactate-pyruvate ratio and high extracellular concentrations of glycerol in the acute phase after TBI, showing them to be important predictors for fatal outcome [7]. Interestingly, in a porcine model of spontaneous hemorrhage, values of PRx >0.3 were associated with an increasing lactate-pyruvate ratio and elevated extracellular glutamate [16]. Therefore, impaired autoregulation may have an important impact on tissue chemistry.
From this preliminary experience, L-PRx appears particularly attractive from the clinical perspective of routine medical purpose. Due to the fact that L-PRx is based on the standard parameters of MAP and ICP, which are easily recorded in a minute-by-minute fashion, it can be implemented on commercial monitoring devices. This prognostic information could be used for direct analysis, not requiring any additional device. In contrast, the traditional PRx methodology requires high-resolution data acquisition reserved for subspecialized units with special interfacing techniques and customized IT solutions, which may be not possible to obtain in all neurointensive care units. However, more experience with L-PRx from adequately powered prospective studies is needed to prove its advantage in the clinical field.
Conclusions
Slow wave changes in MAP and ICP may contain important autoregulation information and may contribute to the outcome prognostication of L-PRx. This new algorithm can be easily applied, due to the steadily increasing number of neurocritical care units with minute-by-minute sampling.
References
Aaslid R, Lindegaard KF, Sorteberg W, Nornes H (1989) Cerebral autoregulation dynamics in humans. Stroke 20:45–52
Chamoun R, Suki D, Gopinath SP, Goodman JC, Robertson C (2010) Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J Neurosurg 113:564–570
Chan TV, Ng SC, Lam JM, Poon WS, Gin T (2005) Monitoring of autoregulation using intracerebral microdialysis in patients with severe head injury. Acta Neurochir Suppl 95:113–116
Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA (2009) Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care 10:373–386
Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD (1997) Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 41:11–19
Czosnyka M, Smielewski P, Kirkpatrick P, Piechnik S, Laing R, Pickard JD (1998) Continuous monitoring of cerebrovascular pressure-reactivity in head injury. Acta Neurochir Suppl 71:74–77
Hejčl A, Bolcha M, Procházka J, Hušková E, Sameš M (2011) Elevated intracranial pressure, low cerebral perfusion pressure, and impaired brain metabolism correlate with fatal outcome after severe brain injury. Cen Eur Neurosurg. doi:10.1055/s-0031-1275745
Jaeger M, Schuhmann MU, Soehle M, Meixensberger J (2006) Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity. Crit Care Med 34:1783–1788
Lang EW, Lagopoulos J, Griffith J, Yip K, Yam A, Mudaliar Y, Mehdorn HM, Dorsch NW (2003) Cerebral vasomotor reactivity testing in head injury: the link between pressure and flow. J Neurol Neurosurg Psychiatry 74:1053–1059
Len TK, Neary JP (2011) Cerebrovascular pathophysiology following mild traumatic brain injury. Clin Physiol Funct Imaging 31:85–93
Low D, Kuralmani V, Ng SK, Lee KK, Ng I, Ang BT (2009) Prediction of outcome utilizing both physiological and biochemical parameters in severe head injury. J Neurotrauma 26:1177–1182
Paulson OB, Strandgaard S, Edvinsson L (1990) Cerebral autoregulation. Cerebrovasc Brain Metab Rev 2:161–192
Rangel-Castilla L, Gasco J, Nauta HJ, Okonkwo DO, Robertson CS (2008) Cerebral pressure autoregulation in traumatic brain injury. Neurosurg Focus 25:E7
Rosner MJ, Becker DP (1984) Origin and evolution of plateau waves. Experimental observations and a theoretical model. J Neurosurg 60:312–324
Santos E, Diedler J, Sykora M, Orakcioglu B, Kentar M, Czosnyka M, Unterberg A, Sakowitz OW (2011) Low-frequency sampling for PRx calculation does not reduce prognostication and produces similar CPPopt in intracerebral haemorrhage patients. Acta Neurochir 153:2189–2195
Santos E, Orakcioglu B, Kentar M, Diedler J, Uozumi Y, Schöll M Unterberg A, Sakowitz OW (2012) Pressure-related index of cerebrovascular reactivity correlates with metabolic dysfunction in a porcine model of intracerebral hemorrhage. In: Schuhmann M, Czosnyka M (eds) Intracranial pressure and brain monitoring XIV. Acta Neurochirurgica Supplementum, Springer-Verlag/Wien, pp 363–367
Sorrentino E, Diedler J, Kasprowicz M, Budohoski KP, Haubrich C, Smielewski P, Outtrim JG, Manktelow A, Hutchinson PJ, Pickard JD, Menon DK, Czosnyka M (2012) Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care 16:258–266
Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, Pickard JD (2002) Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med 30:733–738
Strandgaard S, Paulson OB (1984) Cerebral autoregulation. Stroke 15:413–416
Timofeev I, Carpenter KL, Nortje J, Al-Rawi PG, O'Connell MT, Czosnyka M, Smielewski P, Pickard JD, Menon DK, Kirkpatrick PJ, Gupta AK, Hutchinson PJ (2011) Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain 134:484–494
Timofeev I, Czosnyka M, Carpenter KL, Nortje J, Kirkpatrick PJ, Al-Rawi PG, Menon DK, Pickard JD, Gupta AK, Hutchinson PJ (2011) Interaction between brain chemistry and physiology after traumatic brain injury: impact of autoregulation and microdialysis catheter location. J Neurotrauma 28:849–860
Wang EC, Ang BT, Wong J, Lim J, Ng I (2006) Characterization of cerebrovascular reactivity after craniectomy for acute brain injury. Br J Neurosurg 20:24–30
White H, Venkatesh B (2008) Cerebral perfusion pressure in neurotrauma: a review. Anesth Analg 107:979–988
Zweifel C, Lavinio A, Steiner LA, Radolovich D, Smielewski P, Timofeev I, Hiler M, Balestreri M, Kirkpatrick PJ, Pickard JD, Hutchinson P, Czosnyka M (2008) Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurg Focus 25:E2
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Renán Sánchez-Porras and Edgar Santos contributed equally.
Rights and permissions
About this article
Cite this article
Sánchez-Porras, R., Santos, E., Czosnyka, M. et al. ‘Long’ pressure reactivity index (L-PRx) as a measure of autoregulation correlates with outcome in traumatic brain injury patients. Acta Neurochir 154, 1575–1581 (2012). https://doi.org/10.1007/s00701-012-1423-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1423-0