Abstract
Background
Cerebral blood flow (CBF) measurements are helpful in managing patients with traumatic brain injury (TBI), and testing the cerebrovascular reactivity to CO2 provides information about injury severity and outcome. The complexity and potential hazard of performing CBF measurements limits routine clinical use. An alternative approach is to measure the CBF velocity using bedside, non-invasive, and transcranial Doppler (TCD) sonography. This study was performed to investigate if TCD is a useful alternative to CBF in patients with severe TBI.
Method
CBF and TCD flow velocity measurements and cerebrovascular reactivity to hypocapnia were simultaneously evaluated in 27 patients with acute TBI. Measurements were performed preoperatively during controlled normocapnia and hypocapnia in patients scheduled for hematoma evacuation under general anesthesia.
Main Finding and Conclusion
Although the lack of statistical correlation between the calculated reactivity indices, there was a significant decrease in TCD-mean flow velocity and a decrease in CBF with hypocapnia. CBF and TCD do not seem to be directly interchangeable in determining CO2-reactivity in TBI, despite both methods demonstrating deviation in the same direction during hypocapnia. TCD and CBF measurements both provide useful information on cerebrovascular events which, although not interchangeable, may complement each other in clinical scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cerebral circulation can be compared to a finely tuned instrument, with the primary function of dynamically meeting the ever-changing metabolic demand of the brain. This delicate mechanism is thought to be based on small changes in the milieu surrounding the arteries. The increased metabolism of the brain cells increases the CO2 concentration and thereby reduces the peri-vascular pH resulting in the relaxation of the cerebral vessels [1, 2]. This is the most potent physiological property modulating the tone of the brain vessels and can be measured as the CO2 reactivity by evaluating the cerebral blood flow (CBF) at different arterial pCO2 levels. Changing the pCO2 in the arterial system creates uniform changes in the flow throughout the brain [3]. Normally, the change in CBF due to changes in pCO2 is 1–2 ml/100 g brain/mmHg change in mm Hg pCO2, depending on the CBF technique used [3]. In traumatic brain injured (TBI) patients, the global CBF varies with time and a 3D–CBF often shows a scattered perfusion. There are many vasoactive substances that could be responsible for this reaction. One theory behind this scattered perfusion refers to differences in lactate production in different areas of the brain with high lactate production, and thereby a lower pH, in damaged areas [4]. In addition, the arterial reactivity in brain damaged areas will be reduced, and a reduced CO2 reactivity therefore correlates to the extent of damaged tissue and thereby to the severity of the brain injury and outcome [5–7]. Over the years, a number of techniques have been utilized to measure CBF. The measurement of cerebral venous outflow can include heat clearance techniques in blood vessels and in brain tissue, hydrogen clearance, angiography, ultrasonography, diffusible and nondiffusible tracer-based measurements of cerebral flow, laser Doppler, positron emission tomography (PET), single photon computerized tomography (SPECT), magnetic resonance imaging (MRI), and computerized tomography (CT).
All CBF techniques are more or less invasive, involving advanced equipment and techniques in addition to the fact that these techniques are relatively inconvenient in acute TBI. It would therefore ease the evaluation of CO2 reactivity if measurement of the CBF velocity by transcranial Doppler (TCD) in large cerebral arteries could provide the same information [8]. Previous reports of the TCD technique has demonstrated that, in healthy subjects, blood flow velocity in the large intracranial arteries is directly related to the CO2-concentrations in the blood, reflecting global cerebral vasoconstriction and relaxation. [9]. However, this correlation is not found in patients with brain pathology, such as after subarachnoid hemorrhage [10], making it important to investigate the CBF–TCD correlation after traumatic brain injury. The present study was performed in acute severe TBI patients to evaluate if TCD measurements reflect cerebral vascular reactivity, measured by conventional CBF, in controlled hyperventilation.
Materials and Methods
27 Patients (36 ± 17 (mean ± SD) years, range 18–68 years,) suffering acute severe TBI (GCS 5.4 ± 1.4, range 3–8 on admission) were studied. Fifteen of the 27 patients had mass lesions and twelve were without. The study was approved by the local ethical committee.
Protocol
All the patients were admitted to surgery for evacuation of intracranial hemorrhage. Prior to the study, a ventricular catheter was placed in the ventricular system for intracranial pressure (ICP) monitoring. The CBF and TCD response to controlled hyperventilation was determined after induction of anesthesia, but prior to craniotomy. The measurements were performed bilaterally when possible. Anesthesia was induced with fentanyl (3–4 μg/kg of body weight), pancuronium (0.03 mg/kg of body weight) followed by a sleeping dose of thiopental (4–5 mg/kg of body weight), and suxamethonium (0.8–1.0 mg/kg of body weight) to facilitate the tracheal intubation. All patients were intubated and mechanically ventilated with a ventilator (Servo 900, Siemens Elema, Stockholm, Sweden) delivering a gas mixture containing 65 % nitrous oxide in oxygen. Ventilation was adjusted to maintain pCO2 at normocapnic levels. Simultaneous measurements of CBF and TCD were performed during controlled normoventilation, 20 min after induction of anesthesia, when the anesthetic depth had reached a steady state. Following a 25 % increase of the minute volume, aiming at a ≈1.0 kPa (7.6 mmHg) reduction in pCO2, the measurements were repeated after 20 min of steady state. Arterial blood pressure, electrocardiogram, end-tidal CO2-concentrations, and rectal temperature were continuously monitored. Arterial blood sampled 2 min after the start of each measurement was analyzed for blood gases and corrected for deviations in body temperature.
CBF Measurements
Mean hemispheric CBF was determined after intravenous administration of approximately 0.5 Gbq of 133Xe. The tracer substance was dissolved in normal saline and injected into a cubital vein as a bolus followed by a rapid injection of normal saline. Clearance of the tracer was monitored bilaterally by one extracranial scintillation detector at the parieto-temporal region over the middle cerebral artery (MCA) territory. A Novo Cerebrograph 10a (Simonsen Medical A/S, Randers, Denmark) was used for data collection with a sampling time of 11 min and subsequent flow calculation. The CBF was calculated as the initial slope index (ISI) from the early segment of the clearance curves by conventional bicompartmental analysis and a delayed start fit time [11, 12]. The ISI was chosen as it represents the blood flow of all tissue recorded, but is highly dominated by the gray matter blood flow with very little influence from extracerebral components [13]. The calculated CBF values, corrected for background and remaining 133xenon activity, were presented on a computer printout after each measurement. The validity and reproducibility of the CBF measurements with the Novo Cerebrograph have been previously reported [14].
TCD Measurements
TCD was performed with a transcranial 2 MHz pulsed Doppler instrument (EME TC2-64, Eden Medical Electronics, Uberlingen, Germany). The TCD recordings were performed simultaneously with the early portion of the CBF measurement. Insonation through the temporal bone, above the zygomatic arch, was performed to examine the main trunk of the middle cerebral artery (MCA) at a depth of 50–60 mm. The flow velocities are given as a mean flow velocity (mFV) for each MCA. The normal range for mFV in MCA with this equipment is 62 ± 12 cm/s.
Calculation and Statistical Analysis
The response to hypocapnia of both CBF and TCD mFV is expressed as a reactivity index to relate changes in CBF and TCD to hypocapnia. Reactivity index for CBF is expressed as % change in CBF (ISI)/unit change pCO2 (kPa) and for TCD as % change in mFV (cm/s)/unit change pCO2 (kPa). The correlation between % change in CBF and mFV was tested with repeated measurement ANOVA. The correlation between the CBF CO2 reactivity and the mFV CO2 reactivity is tested with Rank Kendall correlation test.
Results
Physiological values are presented in Table 1. Apart from the intended difference in PaCO2 (0.7 range 0.2–1.5 kPa) and pH between the normo and hypocapnic groups, there were no statistical significant disparity between hemoglobin (Hb), temperature (Temp), and mean arterial blood pressure (MAPB).
Mean CBF (ISI) changed nonsignificantly from 32 ± 11 (range 53–14) during normocapnia to 30 ± 10 (range 57–11) during hypocapnia (t = 1.23, p = 0.23) with a CO2 response of approximately 2.3 ISI/kPa (0.3 ISI/mmHg). Controlled hyperventilation was accompanied by significant decreases in the TCD readings in the MCA on both sides, dx: 52 ± 17–45 ± 15 (t = 3.6; p < 0.002), sin: 49 ± 19–43 ± 17 (t = 2.1; p = 0.05).There was a significant correlation (R 2 = 0.1191) between the CBF and MCA mFV in the entire material (Fig. 1).
Changing the ventilation from normocapnia to hypocapnia changed the ICP significantly (t = 3.70; p = 0.001) from 20 ± 11 to 14 ± 8 and the MABP nonsignificantly from 88 ± 14 to 90 ± 14 (t = 1.47; p = 0.15) resulting in a significant change (t = −4.02; p < 0.0005) in cerebral perfusion pressure (CPP = MAP − ICP) from 68 ± 21 to 76 ± 18. This change in CPP correlated to the CBF (F = 7.1; p = 0.014) with a correlation coefficient R 2 = 0.24
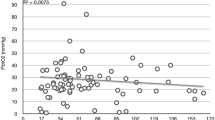
On the left side, a significant correlation between the absolute CBF and the mFV was found at normocapnia for the MCA (F = 4.97; p < 0.05), but not during hypocapnia. However, there was a significant mFV correlation (F = 4.82; p < 0.05; R 2 = 0.26) to the CBF change between normo and hypocapnia (Fig. 2). A near significant correlation to the CBF (F = 3.78; P = 0.08) was found for the ICA mFV at the same side, at rest.
No correlation was found between the % change in CBF/unit change pCO2 and %change in mFV/unit change pCO2 (F = 0.05; p = 0.82) (Fig. 3).
Discussion
The TCD technique is a noninvasive, nonradioactive, and technically simple method for determination of CBF velocities [8]. Flow velocity is measured in the large central arteries, mainly the medial cerebral artery (MCA), the anterior cerebral artery (ACA), or the posterior cerebral artery (PCA). If the blood flow is altered with an unchanged tone in these large arteries (i.e., by only changing the tone in the peripheral resistance arteries), there should be a direct positive correlation between the flow velocity in the central arteries and CBF. This direct correlation will be altered if the same changes are simultaneously taking place in both the central arteries as well as in the peripheral resistance arteries. If the change in arterial tone is only taking place in the central arteries, there should be a direct negative correlation between CBF and TCD FV readings. This relationship between the flow and flow velocity is described by Giller et al. [15] in the formula FV = CBF/vessel diameter in the insonated artery. Assumption about the change in one factor will therefore only hold if the other remains constant. Nevertheless, the simplicity and lack of invasiveness of the TCD technique warrants an evaluation to determine if it can replace the considerably more inconvenient CBF to evaluate the cerebral circulation and the CO2 response. In this study, the change from normo to hypocapnia significantly decreased the ICP and nonsignificantly decreased the CBF. The absolute change in mean CBF was small with a CO2 response of 2.3 ISI/kPa (0.3 ISI/mmHg). ISI is close to ml/100 g/min and can therefore be compared to the normal CO2 response of 10.6 ml/100 g/kPa (1.4 ml/100 g/mmHg) in the non-traumatized brain, measured by the same method [3]. The low CO2 response found in this study is in accordance with a severe brain injury in these patients [6, 7, 16].
Hypocapnia reduced the intracerebral volume, seen as a decrease in ICP. This effect reasonably reflects a reduction in the cerebral blood volume (CBV). A distinctive contractile effect was not found in the cerebral arteries as the CO2–CBF response was low, implying that the ICP lowering effect could be due to a contraction of the capacitance vessels. A diverse effect is normally found in the non-traumatized brain. The effect of hypercapnia on the arterial side (CBF) is more pronounced than on the venous side, seen in the fact that lowering the pCO2 reduces CBF more than it reduces the CBV [3, 17]. Hyperventilation has previously been described to profoundly decrease CBF, sometimes without a fall in ICP in brain trauma patients [18]. This discrepancy could be due to a different ICP between investigations. The volume change necessary to reduce ICP is much smaller when ICP is high, as in the current study patients with a mean ICP of 20 mmHg. In addition, the ICP-transducer was calibrated at the forehead level, thereby underestimating ICP by 5–7 mmHg. A minor and nonsignificant increase in MAP was found as an effect of the hyperventilation. This small increase in MAP together with the significant fall in ICP resulted in a significant increase in CPP. A direct and strong correlation between CPP and CBF was found, implying that cerebrovascular autoregulation was impaired in these patients. Such impairment in severe brain traumatized patients was in fact what could be expected and confirmed the severe magnitude of the brain injury in these patients [16, 19, 20]. In the present study, there was a correlation between the CBF and the mFV in MCA and ICA at normocapnia. This relationship, even though weak, is remarkable considering the large variance in CBF and mFV values. Considering the corresponding CBF–mFV values, the mFV cannot substitute the CBF in the single patient (Fig. 1); a fact that has previously been established for SAH patients [10], for brain trauma patients [21] and for different brain pathologies [22].
Compared to the slight attenuation in CBF due to hyperventilation, the fall in mFV was significant with a response of 10 cm/s/kPa (1.3 cm/s/mmHg). In general, responsiveness to CO2 is detected with TCD in brain traumatized patients [23]. The discrepancy, with a more pronounced effect on the flow velocity than on the CBF, has previously been reported in patients with SAH [10] but a weaker relationship was generally found between these two parameters in patients with different brain pathologies [22, 24]. In fact, a straightforward CO2 response relationship between CBF and mFV was not found even in normal brains, although the correlation was better compared with the patients with brain pathologies [24]. The CO2 response in patients with subarachnoid hemorrhage was 13 cm/s/kPa (1.9 cm/s/mmHg) compared to the normal 11 cm/s/kPa (1.4 cm/s/mmHg) [24]. In brain trauma patients, a change in mFV due to a change in CO2 is generally found [25, 26]. The CO2 response when comparing brain pathologies and normal brains is numerically diminutive and therefore not logical to use as a measure of an impaired CO2 response. No correlation was found between % change in mFv and CBF and % change in CO2, suggesting that CBF and mFV is not directly interchangeable. Thus, with the present application, CBF and mFV was not compatible in determining CO2-responsiveness in brain trauma patients. The CO2-response measured with CBF and the correlation to severity of the brain trauma and outcome has, as described earlier, been established [6, 16]. In this respect, impaired cerebral autoregulation measured with TCD did not correlate to outcome [27] even though the absolute mFv measured after admission could be promising [28].
In conclusion, hyperventilation was associated with a nonsignificant decrease of the CBF but a significant decrease of the mFV. Relationships of individual reactivity indices between the two parameters could not be established and CBF and mFV are therefore not interchangeable in patients with severe brain trauma.
References
Harder DR, Madden JA. Cellular mechanism of force development in cat middle cerebral artery by reduced CO2. Pflügers Archiv. 1985;403:402–4.
Reinstrup P, Uski T, Messeter K. Modulation by carbon dioxide and pH of the contractile response to potassium and prostagladin F2ά in isolated human pial arteries. Br J Anaesth. 1992;69:615–20.
Reinstrup P, Ryding E, Algotsson L, Berntman L, Uski T. Effects of nitrous oxide on human regional cerebral blood flow and isolated pial arteries. Anesthesiology. 1994;81:396–402.
Engstrom M, Polito A, Reinstrup P, et al. Intracerebral microdialysis in severe brain trauma: the importance of catheter location. J Neurosurg. 2005;102:460–9.
Cold GE, Jensen FT, Malmros R. The effects of PaCO2 reduction on regional cerebral blood flow in the acute phase after brain injury. Acta Anaesthesiol Scand. 1977;21:359–67.
Schalén W, Messeter K, Nordström CH. Cerebral vasoreactivity and the prediction of outcome in severe traumatic brain lesions. Acta Anaesthesiol Scand. 1991;35:113–22.
Poon WS, Ng SC, Chan MT, Lam JM, Lam WW. Cerebral blood flow (CBF)-directed management of ventilated head-injured patients. Acta Neurochir Suppl. 2005;95:9–11.
Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–74.
Markwalder TM, Grolimund P, Seiler RW, Roth F, Aaslid R. Dependency of blood flow velocity in the middle cerebral artery on end-tidal carbon dioxide partial pressure—a transcranial ultrasound Doppler study. J Cereb Blood Flow Metab. 1984;4:368–72.
Romner B, Brandt L, Berntman L, Algotsson L, Ljunggren B, Messeter K. Simultaneous transcranial Doppler sonography and cerebral blood flow measurements of cerebrovascular CO2-reactivity in patients with aneurysmal subarachnoid haemorrhage. Br J Neurosurg. 1991;5:31–7.
Obrist WD, Thompson HK Jr, Wang HS, Wilkinson WE. Regional cerebral blood flow estimated by 133-xenon inhalation. Stroke. 1975;6:245–56.
Risberg J, Ali Z, Wilson EM, Wills EL, Halsey JH. Regional cerebral blood flow by 133xenon inhalation. Stroke. 1975;6:142–8.
Risberg J, Uzzell BP, Obrist WD. Spectrum subtraction technique for minimizing extracranial influence on cerebral blood flow measurements by 133xenon inhalation. Stroke. 1977;8:380–2.
Messeter K, Nordström CH, Sundbärg G, Algotsson L, Ryding E. Cerebral hemodynamics in patients with acute severe head trauma. J Neurosurg. 1986;64:231–7.
Giller CA, Hatab MR, Giller AM. Estimation of vessel flow and diameter during cerebral vasospasm using transcranial Doppler indices. Neurosurgery. 1998;42:1076–84.
Cold GE, Jensen FT. Cerebral autoregulation in unconscious patients with brain injury. Acta Anaesthesiol Scand. 1978;22:270–80.
Reinstrup P, Ryding E, Ohlsson T, Dahm PL, Uski T. Cerebral blood volume (CBV) in humans during normo- and hypocapnia: influence of nitrous oxide. Anesthesiology. 2001;95:1079–82.
Marion DW, Firlik A, McLaughlin MR. Hyperventilation therapy for severe traumatic brain injury. New Horiz. 1995;3:439–47.
Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF. Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg. 1991;75:685–93.
Overgaard J. Cerebral circulation after head injury. 1. Cerebral blood flow and its regulation after closed head injury with emphasis on clinical correlations. J Neurosurg. 1974;41:531–41.
Romner B, Bellner J, Kongstad P, Sjöholm H. Elevated transcranial Doppler flow velocities after severe head injury: cerebral vasospasm or hyperemia? J Neurosurg. 1996;85:90–7.
Brauer P, Kochs E, Werner C, et al. Correlation of transcranial Doppler sonography mean flow velocity with cerebral blood flow in patients with intracranial pathology. J Neurosurg Anesthesiol. 1998;10(2):80–5.
Ng SC, Poon WS, Chan MT, Lam JM, Lam WW. Is transcranial Doppler ultrasonography (TCD) good enough in determining CO2 reactivity and pressure autoregulation in head injured patients? Acta Neurochir Suppl. 2002;81:25–7.
Hartmann A, Ries F, Tsuda Y, Lagrèze H, Seiler R, Grolimund P. Correlation of regional cerebral blood flow and blood flow velocity in normal volunteers and patients with cerebro-vascular disease. Neurochirurgia (Stutt). 1991;34:6–13.
Lee JH, Kelly DF, Oertel M, et al. Carbon dioxide reactivity, pressure autoregulation, and metabolic suppression reactivity after head injury: a transcranial Doppler study. J Neurosurg. 2001;95:22–32.
Rangel-Castilla L, Rivera Lara L, Gopinath SP, Swank PR, Valadka A, Robertson C. Cerebral hemodynamic effects of acute hyperoxia and hyperventilation after severe traumatic brain injury. J Neurotrauma. 2010;27:1853–63.
Newell DW, Aaslid R, Stooss R, Seller RW, Reulen HJ. Evaluation of hemodynamic response in head injured patients with transcranial Doppler monitoring. Acta Neurochir (Wien). 1997;139:804–17.
Moreno JA, Mesalles E, Gener J. Evaluating the outcome of severe head injury with transcranial Doppler ultrasonography. Neurosurg Focus. 2000;15:e8.
Acknowledgments
Supported by Grants from Regionalt Forskningsstöd.
Disclosure/Conflict of interest
None of the authors have any duality of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reinstrup, P., Ryding, E., Asgeirsson, B. et al. Cerebral Blood Flow and Transcranial Doppler Sonography Measurements of CO2-Reactivity in Acute Traumatic Brain Injured Patients. Neurocrit Care 20, 54–59 (2014). https://doi.org/10.1007/s12028-012-9727-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-012-9727-8