Abstract
Background
Conivaptan is an arginine-vasopressin-receptor antagonist approved for the treatment of hyponatremia. We hypothesized that administration of conivaptan to normonatremic patients with traumatic brain injury (TBI) is safe and could reduce intracranial pressure (ICP).
Methods
Open-label, randomized, controlled trial enrolling 10 subjects within 24 h of severe TBI to receive a single 20 mg dose of conivaptan (n = 5) or usual care (n = 5). The primary endpoint was the evaluation of the safety profile defined by serum sodium increases averaging >1 mEq/h when measured every 4 h and any adverse events. Secondary endpoints were 48-h serum sodium, sodium load, change in ICP, and urine output.
Results
Ten patients were included in the intention-to-treat analysis. Three patients (2 conivaptan, 1 usual care group) experienced brief sodium increases averaging >1 mEq/h, with no patients achieving Na >160 mEq/l. There were no drug-related serious adverse events. At 48 h, the mean sodium was 142 ± 6 mEq/l (conivaptan) and 144 ± 10 mEq/l (usual care, P = 0.71). 48-h sodium load was 819 ± 724 mEq in the conivaptan and 1,137 ± 1,165 mEq in the usual care group (P = 0.62). At 4 h, serum sodium was higher (P = 0.02) and ICP was lower (P = 0.046) in the conivaptan compared with usual care group. 24-h but not 48-h urine output was different between the two groups (P < 0.01 and P = 0.20, respectively).
Conclusions
These data suggest that a single dose conivaptan is safe in non-hyponatremic patients with severe TBI and may reduce ICP. Further studies are needed to establish the effect of conivaptan on clinically relevant endpoints, and its role in the management of intracranial hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditional modalities for the treatment of increased intracranial pressure (ICP) have included both mannitol and hypertonic saline solutions, which elevate the osmolarity within the cerebral vasculature and promote water movement across the blood–brain barrier and into the capillary system. This fluid shift allows the reduction of cerebral edema fluid and ICP [1]. Although hypertonic saline solutions are effective at reducing ICP, administration of high loads of exogenous sodium is often necessary to maintain hypernatremia due to natriuresis. This necessitates frequent measurement of serum sodium to avoid uncontrolled, rapid increase or decrease in sodium levels. In the experimental setting, when the blood–brain barrier is disrupted, rapid correction of hyponatremia has been shown to result in osmotic demyelination [1].
Conivaptan (Vaprisol®) is an antagonist of arginine vasopressin V1A and V2 receptors and promotes aqueresis. Conivaptan and other vasopressin antagonists have been demonstrated to increase serum sodium in a controlled fashion, and conivaptan is FDA approved for the treatment of euvolemic and hypervolemic hyponatremia. Numerous studies have attempted to address the management of hyponatremia with conivaptan in the neurocritical care setting [1–5]. Given the goal of maintaining a stable state of normal or high serum sodium in most patients with increased ICP, we hypothesized that conivaptan may have beneficial effects in this setting.
As an initial step in testing this hypothesis, we conducted an open-label, randomized, controlled trial of conivaptan compared with usual care in patients with severe traumatic brain injury (TBI). This study was designed to assess the safety and tolerability of administering a single dose of conivaptan to normonatremic critically ill patients with severe TBI. Our secondary goals included the assessment of sodium load and level, as well as changes in ICP between the groups.
Materials and Methods
The study was conducted between August 2009 and May 2010 at Harborview Medical Center, an urban level I trauma center that receives patients from a wide geographic area in the Pacific Northwest. The study was approved by the Human Subjects Division Review Board of the University of Washington. A signed informed consent form was obtained from all participants’ legally authorized representatives. The FDA determined that the study met all of the requirements for exemption from the IND regulations and, therefore, an IND was not required to conduct the trial.
Patient Population
Admission criteria included age ≥18 years, admission to the ICU, an admission diagnosis of isolated, severe TBI as defined by a Glasgow Coma Scale (GCS) of 8 or less upon initial evaluation, and requiring placement of an intraparenchymal fiberoptic device to monitor ICP. Additionally, patients were eligible if there was an order by the primary care team to initiate supplemental sodium with an intention to raise the serum sodium to a goal approximately 10 mEq/l higher than admission baseline in order to reduce cerebral edema and/or ICP.
Patients were excluded from the study based on the following criteria: 1. Unable to receive the medication based on contraindications indicated by the manufacturer; 2. Age <18 years; 3. Hemodynamic instability defined by hypotension (defined as systolic blood pressure <90 mmHg) at the time of evaluation or requirement for vasopressors; 4. Liver disease defined by jaundice and ascites, or elevated liver function tests (AST >35 units/l, ALT >35 units/l); 5. Renal disease including end stage renal disease on chronic dialysis, or elevated renal function tests (serum creatinine >1.5 mg/dl, BUN >20 mg/dl); 6. Serum sodium >145 mEq/l at baseline (admission labs or any time prior to randomization); 7. No ICP monitor placement; 8. Pregnant or lactating females; 9. Concomitant use of digoxin, ketoconazole, itraconazole, clarithromycin, ritonavir, and indinavir (as recommended by the manufacturer due to CYP3A inhibition); 10. Presentation to the tertiary care hospital >24 h post-injury; 11. Multi-system traumatic injuries; 12. Diabetes insipidus; 13. Not expected to survive within 48 h of admission, or a presumed diagnosis of brain death.
Study Design
After consent was obtained, patients were randomized to receive the study medication (conivaptan) in addition to usual care (n = 5) or usual care alone (n = 5). Given the open-label administration of the medication, clinicians, participants, and researchers knew if the patient had received the medication. The study medication, conivaptan, was administered in a single dose of 20 mg, mixed with 100 ml of 5% dextrose in water, and infused over 30 min. The usual care group did not receive any study medication.
For safety reasons, patients were not allowed to receive hypertonic saline solutions in concentrations greater than 3%. Usual care also included sedation and analgesia as needed, elevation of the head of the bed, mannitol and/or 3% saline as needed to reduce ICP, and temperature control with antipyretics such as acetaminophen. During the ICU stay, all study participants had serum sodium assessment every 4 h.
Study Endpoints
The primary study endpoint was the evaluation of the safety profile of conivaptan compared with standard care. The primary safety endpoint was the number of events of increase in serum sodium averaging greater than 1 mEq/h, when measured every 4 h. Additional endpoints used to evaluate safety included: 1. Any increase in serum sodium above the target goal range established by the primary care team, and 2. Any drug-related adverse events occurring during the study period.
Secondary endpoints were the mean serum Na and Na load (cumulative amount of sodium administered by enteral and parenteral routes) in the first 48 h after randomization, mean ICP values and change in ICP after randomization, and cerebral perfusion pressure (CPP). Urine volume in 4-h intervals was evaluated during the first 48 h after randomization.
Statistical Methods
As the trial was designed to investigate safety and tolerability, the study was not powered to detect differences in the efficacy endpoints. We determined that a sample of 10 patients was adequate to evaluate the safety of the administration of conivaptan in non-hyponatremic patients.
The analysis was done using an intention-to-treat approach. Descriptive statistics were used to describe the distribution of baseline characteristics using two-sample Student’s t-test or chi-square statistic, as appropriate. Linear mixed effects models were used to compare the longitudinal change of variables with repeated measures, specifically serum sodium and ICP, between the two groups. A two-sided level of significance of 0.05 was considered for significance in all analyses. All hypothesis testing was two-sided. The analyses were performed using the statistical software STATA vers 11 (Stata Corp, College Station, TX).
Results
Baseline Characteristics
A total of 216 patients were assessed for eligibility and 10 met inclusion criteria and were included in the intention-to-treat analysis. Figure 1 shows the population with severe TBI that was assessed for eligibility. The mean age was 50 ± 13 years in the conivaptan group and 48 ± 20 years in the usual care group (P = 0.83). There were no substantial differences in demographics, pre-admission, and patient baseline characteristics, except for mean arterial pressure and cerebral perfusion pressure that were higher at baseline in the usual care group compared with the conivaptan group (Table 1).
Drug Safety and Effect on Sodium Balance
Three patients experienced brief (less than 4 h) sodium increases >1 mEq/l/h, two in the treatment group, one in the usual care group. There were no instances of patients achieving sodium levels >160 mEq/l. There were no adverse events related to the study drug during the study period.
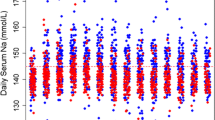
The peak effect on serum sodium was seen at 4 and 8 h post study drug administration and the change from baseline was significantly different between the two groups at 4 h (Table 2; Fig. 2a, P = 0.02). The effect on sodium change was accompanied by a significant decrease in ICP (change from baseline −8 ± 7.7 and +2.5 ± 4.4 mmHg in the conivaptan compared with the usual care group, respectively, P = 0.046, Fig. 2b). Concomitant measurements of arterial blood pressure remained stable and there was no change in cerebral perfusion pressure (Fig. 2c, d).
The average serum sodium at 24 h (142 ± 5 and 143 ± 7 mEq/l, P = 0.76) and 48 h (142 ± 6 and 144 ± 10 mEq/l at 48 h, P = 0.71) was not different between the conivaptan versus the usual care group, respectively. There were no differences in the peak sodium level achieved in 48 h, in the time to achievement of sodium >145 mEq/l or in the number of hours with serum sodium above 150 mEq/l during the first 48 h after randomization (Table 2). The amount of sodium administered in the first 48 h was 819 ± 724 mEq in the conivaptan and 1,137 ± 1,165 mEq in the usual care group (P = 0.62).
Urine output at 24 h was significantly different between the conivaptan and usual care group (3,959 ± 504 and 2,174 ± 717 mL, P < 0.01). In the subsequent 24 h there was no difference between the two groups (3,096 ± 1,203 and 2,199 ± 772 ml, respectively, P = 0.20). However, the cumulative 48 h urine volume was different between the two groups (7,055 ± 1,436 and 4,373 ± 1,413, respectively, P = 0.02). The peak difference in 4-h urine output was observed at 8 h after randomization (Fig. 3, P = 0.045).
Discussion
This study was designed to evaluate the safety of single dose conivaptan in normonatremic patients with severe TBI. We did not identify any significant excessive increases in serum sodium or any adverse events related to the administration of conivaptan. The study was not powered to effectively study efficacy, although we were able to demonstrate that serum sodium increased significantly 4 h after drug administration, which is consistent with the anticipated onset of action for conivaptan. Concomitantly, ICP was noted to be significantly lower in the conivaptan compared with the usual care group, supporting possible therapeutic utility of conivaptan in controlling intracranial hypertension.
The observation that conivaptan has significant effects on ICP associated with a steep change in sodium level is important. Moreover, our data suggest that while mean arterial pressure remained comparable between the two groups, the ICP dropped in the intervention group only. However, it should be noted that conivaptan induced dieresis could potentially be associated with intravascular volume contraction. Consistent with our findings, conivaptan has been reported to result in a fall in ICP 3–5 h after the medication administration in a patient with severe TBI and elevated ICP [3]. This time frame is consistent with the kinetics we observed for maximum drop in ICP and increased serum sodium. The authors of this previous report concluded that vasopressin-receptor antagonism and aquaresis reduced brain water, which reduced ICP and dramatically increased fluid output.
Our data suggest that the effect on ICP may be more pronounced than the effect on aquaresis, possibly indicating that the effect on ICP was mediated by mechanisms other than merely V2 receptor antagonism. Emerging data in experimental stroke suggest that V1 receptor antagonism attenuates injury volume and cerebral edema [6], and that inhibition of V1a receptors reduces ICP and brain water content in a murine model of trauma-induced brain edema [7], and via modulation of aquaporin-4 expression by selective V1a receptor antagonism in rats [8]. Because there is an increase in the expression of vasopressin receptors in the brain following TBI, it is conceivable that V1a receptor antagonism has a role in the treatment of cerebral edema following trauma [9–11]. On the other hand, stimulation of V1 receptors has cerebro-vascular effects that appear to have variable effects on ICP [12–16]. Further investigation is necessary to provide an explanation for our findings and to better delineate the mechanisms of action of conivaptan in the injured brain.
One safety issue of major concern was the rate of rise in serum sodium after conivaptan administration due to the potential risk of central pontine myelinolysis [17]. Our data indicated only isolated increases >1 mEq/l/h, neither of which lasted more than a single 4-h time interval. Naidech et al. [5] also noted that conivaptan resulted in significant increases in serum sodium at a rate that was not concerning for the development of sequelae. These two studies differed in the baseline serum sodium levels; however, the rate of rise of serum sodium was comparable.
Another safety issue is that conivaptan should be avoided in the presence of hypovolemia given the observation that conivaptan administration is associated with diuresis. Hyponatremia associated with cerebral salt wasting is a good example of a scenario where conivapatan may be harmful, and illustrates the need for careful assessment of intravascular volume prior to administration of this agent.
The dosing of conivaptan (a single 20 mg IV bolus) in our study was conservative given the safety-oriented primary endpoint. For treatment of hyponatremia, the manufacturer recommends a 20 mg loading bolus followed by 20 mg/day continuous infusion for up to a maximum of 4 days. This dose can be titrated up to a maximum daily infusion of 40 mg. Future studies of conivaptan in patients with elevated ICP are necessary to evaluate if a more sustained effect on ICP can be achieved with higher doses or continuous infusion of the drug.
Our report has some important limitations. As mentioned above, our study was designed to address the safety and tolerability of conivaptan and did not have adequate power for establishing efficacy. As well, it was not targeted specifically at intracranial hypertension and some patients had ICP’s <20 mmHg at the time of drug administration. Nonetheless, the significant changes in ICP observed after drug administration would represent a clinically relevant effect size. Future studies will need to be adequately powered to assess efficacy in terms of effect on ICP and patient outcomes, with less restrictive inclusion criteria and allowing the administration of higher drug doses.
Conclusions
These data suggest that a single dose of conivaptan is safe in non-hyponatremic patients with severe TBI for whom increases in serum sodium is desirable for the purpose of ICP control. Conivaptan caused an increase in serum sodium within 4 h of administration with a concomitant significant reduction in ICP without adverse effects. These data suggest a potentially important role of conivaptan among the treatment options to achieve ICP control, especially in patients already receiving maximal medical therapy. Further studies are needed to establish the effect of higher doses and longer administration on clinically relevant endpoints.
References
Rangel-Castilla L, Gopinath S, Robertson CS. Management of intracranial hypertension. Neurol Clin. 2008;26:521–41.
Wright WL, Asbury WH, Gilmore JL, Samuels OB. Conivaptan for hyponatremia in the neurocritical care unit. Neurocrit Care. 2009;11:6–13.
Dhar R, Murphy-Human T. A bolus of conivaptan lowers intracranial pressure in a patient with hyponatremia after traumatic brain injury. Neurocrit Care. 2011;14(1):97–102.
Murphy T, Dhar R, Diringer M. Conivaptan bolus dosing for the correction of hyponatremia in the neurointensive care unit. Neurocrit Care. 2009;11:14–9.
Naidech AM, Paparello J, Leibling SM, et al. Use of conivaptan (Vaprisol) for hyponatremic neuro-ICU patients. Neurocrit Care. 2010;13:57–61.
Liu X, Nakayama S, Amiry-Moghaddam M, Ottersen OP, Bhardwaj A. Arginine-vasopressin V1 but not V2 receptor antagonism modulates infarct volume, brain water content, and aquaporin-4 expression following experimental stroke. Neurocrit Care. 2010;12:124–31.
Trabold R, Krieg S, Scholler K, Plesnila N. Role of vasopressin V(1a) and V2 receptors for the development of secondary brain damage after traumatic brain injury in mice. J Neurotrauma. 2008;25:1459–65.
Taya K, Gulsen S, Okuno K, Prieto R, Marmarou CR, Marmarou A. Modulation of AQP4 expression by the selective V1a receptor antagonist, SR49059, decreases trauma-induced brain edema. Acta Neurochir. 2008;102:425–9.
Kozniewska E, Romaniuk K. Vasopressin in vascular regulation and water homeostasis in the brain. J Physiol Pharmacol. 2008;59(Suppl 8):109–16.
Bemana I, Nagao S. Treatment of brain edema with a nonpeptide arginine vasopressin V1 receptor antagonist OPC-21268 in rats. Neurosurgery. 1999;44:148–54. (discussion 54–5).
Kagawa M, Nagao S, Bemana I. Arginine vasopressin receptor antagonists for treatment of vasogenic brain edema: an experimental study. J Neurotrauma. 1996;13:273–9.
Fernandez N, Martinez MA, Garcia-Villalon AL, Monge L, Dieguez G. Cerebral vasoconstriction produced by vasopressin in conscious goats: role of vasopressin V(1) and V(2) receptors and nitric oxide. Br J Pharmacol. 2001;132:1837–44.
Suzuki Y, Satoh S, Kimura M, et al. Effects of vasopressin and oxytocin on canine cerebral circulation in vivo. J Neurosurg. 1992;77:424–31.
Suzuki Y, Satoh S, Oyama H, Takayasu M, Shibuya M, Sugita K. Vasopressin mediated vasodilation of cerebral arteries. J Auton Nerv Syst. 1994;49(Suppl):S129–32.
Tsugane S, Suzuki Y, Kano T, Takayasu M, Shibuya M, Sugita K. Differing effects of vasopressin on regional cerebral blood flow of dogs following intracisternal vs. intra-arterial administration. Life Sci. 1994;54:PL241–6.
Lluch S, Conde MV, Dieguez G, et al. Evidence for the direct effect of vasopressin on human and goat cerebral arteries. J Pharmacol Exp Ther. 1984;228:749–55.
Adler S, Verbalis JG, Williams D. Effect of rapid correction of hyponatremia on the blood-brain barrier of rats. Brain Res. 1995;679:135–43.
Acknowledgment
Funding: Financial support for this investigator initiated study was provided by Astellas. The sponsor had no role in the design, interpretation, and reporting of the study findings.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galton, C., Deem, S., Yanez, N.D. et al. Open-Label Randomized Trial of the Safety and Efficacy of a Single Dose Conivaptan to Raise Serum Sodium in Patients with Traumatic Brain Injury. Neurocrit Care 14, 354–360 (2011). https://doi.org/10.1007/s12028-011-9525-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-011-9525-8