Abstract
Introduction
Conivaptan is the first arginine vasopressin antagonist to be FDA-approved for the treatment of euvolemic hyponatremia, a common complication in neurointensive care patients. Due to risks for cerebral edema and seizures, sodium levels are generally aggressively maintained within normal levels (135–145 meq/l) in this patient population.
Objective
To assess the safety and efficacy of conivaptan for the treatment of euvolemic hyponatremia in the neurocritical care unit.
Methods
Data were obtained retrospectively on 22 patients treated with conivaptan for euvolemic hyponatremia. End points evaluated included time to [Na] increase of ≥6 meq/l; incidences of rapid overcorrection of [Na] (defined as an increase of >12 meq/l in a 24-h period while on conivaptan), infusion site reactions, or other adverse events; and whether sodium levels decreased after discontinuation of conivaptan.
Results
A [Na] increase of ≥6 meq/l was reached in 19/22 (86%) patients, with an average time to goal of 13.1 h. No patients experienced a rapid overcorrection of [Na]. Five patients had an infusion site reaction necessitating an IV change. One patient experienced hypotension and another complained of thirst during infusion. Conivaptan was initiated in 11/22 patients (50%) who were hyponatremic despite already being on conventional therapies.
Conclusion
Conivaptan was safe and effective in this small series of neurointensive care patients, including many patients who were hyponatremic despite traditional treatments to maintain normal sodium levels. Further studies are needed to clarify the role of conivaptan as an adjunctive and/or alternative therapy for hyponatremia in this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyponatremia, defined as sodium concentration ([Na]) below 136 meq/l [1], is common in the neurocritical care unit. Unfortunately for critically ill neurologic or neurosurgical patients, hyponatremia is risky beyond the usual symptoms associated with low [Na], including headache, nausea, vomiting, confusion, and muscle weakness. Resulting seizures can cause an abrupt increase in intracranial pressure and destabilization of an already tenuous clinical status. Most concerning, though, is the risk for cerebral edema, a leading cause of secondary neuronal injury. Many patients are in the neurocritical care unit because of diseases that put them at risk for cerebral edema [2]. To minimize the risks of cerebral edema, normal serum sodium levels are typically maintained in critically ill neurologic patients [3]. These same diseases that can cause cerebral edema may also lead to hyponatremia due to the syndrome of inappropriate antidiuretic hormone secretion (SIADH) [4, 5].
Conivaptan is the first arginine vasopressin (AVP) receptor antagonist approved for treatment of euvolemic and hypervolemic hyponatremia [6, 7]. It is a mixed V1A/V2 receptor antagonist. In blocking AVP V2 receptors, it produces an effect known as “aquaresis,” which is defined as the excretion of free water without the loss of electrolytes [7, 8]. This is in contrast to the natriuresis caused by furosemide, which will cause the excretion of urine with tonicity approximately equal to that of one-half isotonic saline solution [1]. Adverse reactions include phlebitis, hypotension, headache, hypokalemia, and atrial fibrillation. Patients with hypovolemic hyponatremia, such as with cerebral salt wasting (CSW), should not be treated with conivaptan [7].
There are several potential advantages to using conivaptan for the treatment of euvolemic hyponatremia in the neurointensive care unit. If the cause is SIADH, it may be favorable to block the effect of excessive AVP (also known as antidiuretic hormone) at the receptor site as a more direct method of treatment. If SIADH is treated by adding back fluid and salt, the potential exists that salt could be excreted and fluid retained as more AVP is released, thereby worsening hyponatremia [1, 9]. The use of conivaptan may allow the ongoing administration of medications and hyperalimentation in the intensive care unit (ICU) without exceeding traditional fluid restriction goals [10]. Additionally, it may offer a more controllable pharmacologic effect over hypertonic saline [11]. Potential disadvantages include the possibility of volume depletion to the point of compromising cerebral perfusion pressure and the concern for “rapid overcorrection” of [Na]. The threshold for rapidity of sodium rise with conivaptan is likely patient dependent, but in general, exceeding a rise in serum [Na] of greater than 12 meq/l within 24 h is not recommended. The feared complication of rapid overcorrection of [Na] is osmotic demyelination syndrome (ODS) [7].
Clinical experience with conivaptan is fairly limited. Since AVP dysregulation is commonly implicated as the cause of euvolemic hyponatremia in critically ill neurologic and neurosurgical patients [12], conivaptan is probably a reasonable treatment, provided that intravascular volume is appropriately maintained. To further understand the role of conivaptan in the neurocritical care unit, patients who had been treated for euvolemic hyponatremia were studied to evaluate this drug’s safety and efficacy in this patient population.
Methods
With IRB approval, patients who had been treated with conivaptan in the neurocritical care unit from September 2006 to September 2007 were reviewed. Twenty-three patients were identified by a combination of a log kept for quality assurance purposes and reviewing pharmacy charges, but one patient could not be included in the data analysis due to lab error impacting sodium values, therefore data will be reported on 22 patients.
Based on our pharmacy’s initial guideline implemented while conivaptan was being evaluated for addition to the hospital’s formulary, patients were only eligible to receive conivaptan if their Na level was below 135 meq/l and if they were euvolemic. Specifically, patients were excluded from receiving conivaptan if they had a negative fluid balance for the previous 24 h, but clinical factors such as hypotension, the need for vasopressors, or low central venous pressure (CVP) would also indicate that a patient was hypovolemic and therefore conivaptan therapy would not be appropriate. The recommended dosing strategy of a 20 mg bolus followed by a 20 mg/day infusion or 40 mg/day infusion were the only dosages released from the pharmacy during this time period. Furthermore, patients were typically not treated beyond the FDA-approved course of 4 days. These conservative dosing strategies were to ensure that usage was within that approved by the FDA, and no experimental design was employed in the treatment of these patients. We did not administer conivaptan if there was any clinical suspicion for the presence of CSW, which, at our discretion, included hyponatremic subarachnoid hemorrhage patients at high risk for vasospasm. Additionally, per existing hospital guidelines, conivaptan could only be administered in an ICU, so if a patient was otherwise eligible for transfer out of the ICU, conivaptan was discontinued upon transfer.
The primary [Na] goal was an increase by ≥6 meq/l from the triggering [Na]. Primary safety measures were incidences of rapid overcorrection of [Na] (defined as an increase of [Na] by >12 meq/l in a 24-h period while on conivaptan) and rate of infusion site reaction, which is the most common side effect [7]. Other data points collected included time to reach the [Na] goal, potassium levels, creatinine levels, any other adverse effects of the conivaptan (including hypotension defined as SBP <90 mmHg or requiring vasopressor use), I/O balance, other therapies used for hyponatremia, and if the patients maintained their sodium levels after the conivaptan infusion was discontinued. “Maintained” sodium level was defined as [Na] above 135 meq/l for all but one lab draw for the 24-h time period after the conivaptan was discontinued. Sodium trends were graphed for each patient to try to gain a better understanding of the patterns of response to conivaptan.
Results
The 22 patients (13 male and 9 female) ranged in age from 18 to 82. Diagnoses included various infections (n = 7), subarachnoid hemorrhage (n = 6), other vascular lesions (n = 4), and one patient each with metastatic brain tumor, hydrocephalus, subdural hygroma, subdural hematoma, and Guillian–Barre disease. Most patients (n = 16) were treated with a 20 mg bolus followed by a 40 mg/day infusion. One patient received a 20 mg bolus with a 20 mg/day infusion. Five patients had dose adjustments according to [Na] response. The length of infusion varied depending on how long the patient was in the ICU or if other treating physicians discontinued the medication.
Nineteen of 22 (86%) patients reached the goal rise in serum [Na] of ≥6 meq/l from the triggering [Na]. Of those who reached this endpoint, the average time to achieve it was 13.1 h from starting conivaptan. Five patients reached their peak [Na] levels one draw after the infusion was discontinued, which may still be consistent with medication effect as the elimination half-life is 6–9 h [7]. There were no incidences of “rapid overcorrection” of [Na] while on conivaptan. No one had any decline in neurologic function while receiving conivaptan, therefore ODS was not of concern. However, no patients required an MRI of the brain for other reasons after conivatpan therapy to document the absence of myelinolytic changes. Similarly, lack of exam changes with decline in [Na] caused no concern for symptomatic cerebral edema.
Five patients had infusion site reactions, none of which required a discontinuation of the medication, but all required a change in peripheral IV. An additional 11 patients receiving the medication through peripheral IVs had no infusion site reactions. Six patients had central lines only, therefore an infusion site reaction would not be expected. The rate of infusion site reactions for this series was therefore 31.25% (5/16), which is lower than previously reported [7]. One patient had hypotension related to volume depletion which resolved with fluid boluses, and one patient reported thirst. One patient had a potassium value of 2.9 meq/l, which responded to potassium supplementation, and none of the patients had cardiac arrythymias while on conivaptan. No patients had an increase in plasma creatinine levels over 1.5 mg/dl, or a notable change from baseline creatinine. No other adverse events were reported.
Of the 22 patients evaluated, 8 (36.4%) patients were more than 1 liter negative for the entire time that they were on conivaptan, 8 (36.4%) patients were roughly euvolemic (that is plus or minus one liter) and 6 patients (27.2%) were more than 1 liter positive overall. The three “non-responders” that did not meet the [Na] goal of an increase ≥6 meq/l had one representative in each of these three categories. BUN/Cr ratios did not correlate well with I/O balances. Looking at the maximal BUN/Cr ratio for the days on conivaptan, 15 patients had a BUN/Cr ratio ≤20 and seven patients had a BUN/Cr ratio >20.
Eleven of the 22 patients were already receiving more traditional therapies for hyponatremia when they were started on conivaptan, including: hypertonic (3%) saline infusion (five patients, with infusion rates varying from 40 to 60 cc/h; two patients, #2 and #9, had 3% saline held when conivaptan was initiated, and three patients, #14, #15 and #17, were continued on 3% saline with conivaptan); salt tablets (three patients, doses ranging from 1 g enterally q8 hours to 2 g q6 h, all continued when conivaptan was initiated); and fludrocortisone (one patient, 0.1 mg enterally q12 h which was continued when conivaptan was initiated). Patient #1 was on both 3% saline and salt tabs prior to conivaptan infusion; 3% saline was held when conivaptan was started. Patient #22 was on 3% saline, salt tablets and fludricortisone when conivaptan was initiated, and her 3% saline was continued with conivaptan infusion. With the addition of conivaptan, all but 2 of the 11 patients met the primary Na goal of an increase of serum [Na] ≥ 6 meq/l. One patient was bolused with 250 cc of 3% saline for euvolemic hyponatremia prior to conivaptan, but [Na] did not increase above 135 meq/l; he was subsequently started on salt tablets on the second day of conivaptan therapy. Two patients were fluid restricted after the start of conivaptan, and one patient received an intravenous dose of furosemide as part of the treatment for SIADH while on conivaptan. Three patients had salt tablets initiated with the infusion of conivaptan, and in two patients, conivaptan was the only treatment used for hyponatremia.
Of the 19 patients who reached the primary [Na] endpoint, six patients maintained [Na] above 135 meq/l for 24 h, with three additional patients only having one draw below 135 meq/l before eventually settling out above 135 meq/l for the 24-h period. Therefore, by the definition proposed, 9/19 (47%) responders maintained [Na] above our desired range for 24 h after discontinuation of conivaptan. Six patients (32%) had [Na] below 135 meq/l prior to discontinuation of conivaptan, indicating that while on therapy hyponatremia recurred after initially meeting the primary [Na] goal of an increase of ≥6 meq/l. Four patients (21%) were above 135 meq/l when conivaptan was discontinued, but returned to hyponatremic sodium levels within 24 h of ending the course of therapy.
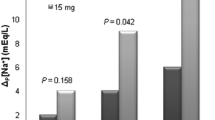
When the sodium results were graphed, some patients had a desirable improvement in [Na] and maintained this increase after the medication was discontinued (see Fig. 1). Some patients responded more dramatically to the bolus, and then the sodium levels would normalize, or even drift back down to hyponatremic levels (see Fig. 2). Other patients had a favorable therapeutic response to conivaptan, but their [Na] would drop after the medication was discontinued (see Fig. 3, and Table 1).
“Start” means start of conivaptan infusion. “Stop” means conivaptan infusion was stopped. “Trigger” indicates the sodium value that initiated the conivaptan treatment. “Na ≥ 6” indicates the sodium value that marks the primary endpoint achieved. The case number corresponds to the patient number in Table 1
Discussion
To our knowledge, this case series is the first to address the use of AVP antagonists in neurocritical care patients. The obvious concerns prior to using this medication were rapid overcorrection of [Na] with the potential of ODS and inducing intravascular volume depletion to the point of compromising cerebral perfusion pressure. No patients had any clinical changes to suggest ODS, but MRI data were not available on any of the patients.
As conivaptan effectively induces diabetes insipidus, it would be anticipated that patients treated with it would have a negative fluid balance. Many of the patients in our series did not have an overall negative fluid balance for the length of their conivaptan infusion. Perhaps this was due to the need for intravenous medications, the continuous infusion of hypertonic saline in several patients, and/or an overall treatment plan to maintain euvolemic or positive fluid balances as is common in the neurointensive care unit, although no specific fluid replacement protocol was in place. The patients with positive or euvolemic fluid balances were as likely to meet the primary sodium goal as the patients with negative fluid balances. Only one patient in this series developed hypotension, but this responded quickly to fluid boluses. Judicious monitoring of urine output, blood pressure, and other parameters such as CVP could alert the clinician to the presence of intravascular volume depletion, allowing for volume replenishment before a clinically relevant decline in cerebral perfusion pressure occurs.
Six patients had subarachnoid hemorrhages, a disease in which volume depletion is typically avoided. Fluid restriction after SAH to treat hyponatremia leads to an increased rate of cerebral ischemia [13]. Furthermore, it is difficult to distinguish SIADH from CSW in these patients [5]. We did not use conivaptan in patients with suspected CSW, or with hypovolemia based on a negative I/O balance. All of these patients were thought to be at low risk of vasospasm either because they were far from the peak vasospasm risk period, they were angiogram negative for aneurysm, they had favorable Fisher grades or because of advanced age. These six patients were on “conventional” treatments to prevent hyponatremia yet had sodium levels below 135 meq/l. Of these six patients, three had an overall negative fluid balance during conivaptan infusion (including the non-responder), one patient was within the range of euvolemia, and two patients had positive fluid balances. None of these patients developed vasospasm by clinical or transcranial Doppler criteria.
The endpoint of an increase in serum [Na] ≥6 meq/l was chosen based on information found in the package insert. In retrospect, it may have been more clinically relevant to choose a different endpoint, such as the percentage of time that the patient was in a goal [Na] range (for example, 135–145 meq/l). Regardless, the majority (86%) of our patients met our predefined end point. Interesting information about patterns of sodium response was gained when sodium values were graphed over time. Although it was unknown to us at the time data collection began, the robust response to the initial bolus in some patients has recently been reported in the literature [6]. It is unclear if bolusing conivaptan will offer a more controllable pharmacologic effect over hypertonic saline. For example, in Fig. 2, the patient had a significant aquaresis and an increase in [Na] following a 20 mg conivaptan bolus but then this patient’s [Na] decreased 9 meq/l in 6 h as her aquaresis tapered off. The patient had no hemodynamic instability to indicate intravascular volume depletion, so she may have tolerated either a repeat bolus, a higher dose of conivaptan, or volume repletion with hypertonic saline, depending on what this patient’s individual fluid balance goals would have been. Alternatively, perhaps patients like this would respond in a more controlled manner to a lower bolus, such as 10 mg. At the time the patients in this series were treated, no novel dosing strategies (such as repeated boluses or doses higher than 40 mg/day infusion) had been attempted. Like the other patients in this series, this patient had no clinical changes to suggest ODS, despite such fluctuations in serum sodium levels.
Although protocols for preventing hyponatremia were not standardized ahead of time, half of the patients in this series were on one or more therapies commonly used in neurocritical care units to prevent hyponatremia. Despite this, their [Na] drifted below 135 meq/l and therefore they became eligible to receive conivaptan per our hospital’s guidelines. Nine of these 11 patients met the primary [Na] goal with the addition of conivaptan to their existing treatment regimen, or by substituting it for hypertonic saline. We feel that this is one of the more interesting insights gained from this review, and indicates that conivaptan may have a role as an adjunctive therapy or as an alternative therapy in patients with refractory hyponatremia. Four patients received both 3% saline and conivaptan without rapid overcorrection of [Na], or any clinical changes indicative of ODS. One strategy could be to aquarese off free water with conivaptan, then add back volume with hypertonic saline [11], depending on the patient’s sodium and fluid balance goals, and individual response to conivaptan.
Patients in the neurocritical care unit with SIADH and other causes of euvolemic hyponatremia have a variety of disease processes, some of which may resolve over the 4-day treatment course of conivaptan, but some diseases may outlast the recommended 96 h infusion. No one in this series had a repeat course of conivaptan for recurrent hyponatremia after discontinuation of the first round of therapy. We were not able to adequately address the issue of “maintenance of sodium levels” in a retrospective fashion. However, it is important because as illustrated in Fig. 3, some patients had a sharp decline in Na levels after medication discontinuation, albeit without neurologic sequelae.
This study is limited by the retrospective nature of the data collection in a wide variety of patient types. We did not have intracranial pressure measurements in these patients and therefore the effect on cerebral perfusion pressure is ultimately unknown. Side effects such as headache and thirst would be more accurately tracked prospectively. There are many additional parameters that could be effectively incorporated into a prospective study, including a more accurate assessment of volume status, and more clinically relevant end points for [Na] goals both during and after conivaptan infusion. We are currently seeking a more thorough, effective and objective way of evaluating sodium response patterns.
Conclusion
Many clinical questions remain unanswered, but this series represents initial steps to clarify the risks and benefits of conivaptan in the neurocritical care unit. Further studies could help elucidate more relevant sodium goals and a better understanding of how sodium levels respond to treatment in this diverse population. No head-to-head studies with hypertonic saline have been performed, but this study shows that both treatments can be used together [11]. Novel dosing strategies such as repeated boluses [14], increasing the infusion dose to 80 mg/day as reported previously [8], or courses of therapy longer than 4 days may improve sodium levels in certain patients. Frequent monitoring of [Na] and intravascular volume status should help minimize the risks of both rapid overcorrection of sodium and decline in cerebral perfusion pressure. Prospective studies are needed to further assess the role of conivaptan in critically ill neurologic and neurosurgical patients.
References
Androgué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–9. doi:10.1056/NEJM200005253422107.
Sterns RH, Silver SM. Brain volume regulation in response to hypo-osmolality and its correction. Am J Med. 2006;119:S12–6. doi:10.1016/j.amjmed.2006.05.003.
Bhardwaj A, Ulatowski JA. Hypertonic solutions and brain injury. Curr Opin Crit Care. 2004;10:126–31. doi:10.1097/00075198-200404000-00009.
Diringer MN, Zazulia AR. Hyponatremia in neurologic patients: consequences and approaches to treatment. Neurologist. 2006;12(3):117–26. doi:10.1097/01.nrl.0000215741.01699.77.
Nathan BR. Cerebral correlates of hyponatremia. Neurocrit Care. 2007;6:72–8. doi:10.1385/NCC:6:1:72.
Zeltser D, Rosansky S, van Rensburg H, Verbalis JG, Smith N, for the Conivaptan Study Group. Assessment of the efficacy of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am J Nephrol. 2007;27:447–57. doi:10.1159/000106456.
Vaprisol package insert. Deerfield: Astellas Pharma, U.S. Inc.; 2007
Verbalis JG. AVP receptor antagonists as aquaretics: review and assessment of clinical data. Cleve Clin J Med. 2006;73(Suppl 3):S24–33.
Palmer BF. Hyponatremia in patients with central nervous system disease: SIADH versus CSW. Trends Endocrinol Metab. 2003;14(4):182–7. doi:10.1016/S1043-2760(03)00048-1.
Gross P, Reimann D, Henschkowski J, Damian M. Treatment of severe hyponatremia: conventional and novel aspects. J Am Soc Nephrol. 2001;12:S10–4.
Verbalis JG. Vasopressin V2 receptor antagonists. J Mol Endocrinol. 2002;29:1–9. doi:10.1677/jme.0.0290001.
Bhardwaj A. Neurological impact of vasopressin dysregulation and hyponatremia. Ann Neurol. 2006;59:229–36. doi:10.1002/ana.20788.
Hasan D, Vermeulen M, Wijdicks EF, Hijdra A, van Gijn J. Effect of fluid intake and antihypertensive treatment on cerebral ischemia after subarachnoid hemorrhage. Stroke. 1989;20(11):1511–5.
Murphy T, Dhar R, Axelrod A, Corry J, Russel E, Diringer M. Conivaptan for the correction of hyponatremia in the neurocritical care unit. Neurocrit Care. 2008;8:196.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wright, W.L., Asbury, W.H., Gilmore, J.L. et al. Conivaptan for Hyponatremia in the Neurocritical Care Unit. Neurocrit Care 11, 6–13 (2009). https://doi.org/10.1007/s12028-008-9152-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-008-9152-1