Abstract
Continuous renal replacement therapy (CRRT) is a renal replacement modality that is often used in the ICU setting, including the neuro-ICU. This form of renal replacement therapy has been used classically for acute renal failure in patients with hemodynamic compromise, but is gaining acceptance as a method to control vascular and extra-vascular volume and mediate cytokines in non-renal diseases. Although these uses are briefly discussed, this review concentrates on the different forms of continuous renal replacement, mainly focusing on the technology of convective versus diffusive modalities and briefly on filter technology. There is also discussion on the various anticoagulation regimes used in CRRT including data on performing CRRT without anticoagulation. This review is not meant to be a discussion on the pros and cons of CRRT versus intermittent dialysis, but rather a primer on the technology of CRRT and how this therapy may affect general care of the ICU patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute renal failure (ARF) related to critical illness is a significant contributor to poor outcome regardless of the primary diagnoses or condition that warranted ICU care [1–4]. Intermittent or continuous renal replacement therapy (CRRT) remains the cornerstone in treating ARF and/or refractory volume overload in the ICU and has been for over 30 years.
There is ongoing debate as to the type of renal replacement therapy (RRT) that is superior in the intensive care setting. Debates range from who should manage the therapy, what the goals of the therapy should be to what type of therapy are best—intermittent or continuous. Even timing of the initiation of RRT is debated and remains an unsettled issue [5]. The CRRT or intermittent hemodialysis (IHD) debate generates strong biases and opinions an in spite of recent studies to suggest equipoise, the controversy remains [6, 7]. Many institutions offer both therapies and have different criteria in choosing which to use. This is a discussion of CRRT technology and not a position paper advocating one therapy over another.
CRRT is a form of RRT that is used continuously to treat not only ARF, but has also been utilized to treat a variety of ICU conditions without ARF such as shock and pancreatitis [8, 9]. CRRT incorporates several modes of RRT utilizing different mechanisms of clearance. The modes include slow continuous ultra-filtration (SCUF), continuous ateriovenous hemofiltration (CAVH), Continuous veno-venous hemofiltration (CVVH), continuous veno-venous hemodiafiltration (CVVHDF), and high volume hemofiltration (HVHF) [10]. Each mode has unique clearance mechanics with some using convective clearance (CVVH) while other modes use diffusive clearance or some combination of convective and diffuse clearance (CVVHDF). This complexity extends further to machine configuration, such as pre-filter or post-filter solution replacement in convective modes, to different filter technology. In spite of these numerous modalities, CRRT has been used as a catchall phrase with inference that all continuous modalities are the same. This gives little regard to the specifics and uniqueness of each mode or possible machine configuration. This is equivalent to reporting that a patient is on mechanical ventilation ignoring mode, rate, PEEP, tidal volume or FiO2.
CRRT at most institutions resides within the realm of the nephrologist being a natural extension or offshoot of intermittent dialytic therapy. However, the goals of an intensivist utilizing continuous therapy may differ from that of a consulting nephrologist [11]. In specialized units like a Neuroscience ICU, nephrologist may round daily, but are not in charge of adjusting vasopressors or monitoring triple-H therapy, adding new antibiotics, initiating and adjusting nutritional support, giving fluids or colloids for hemodynamic issues or continually monitoring hemodynamic parameters. That is the domain and responsibility of the intensivist. Therefore, understanding CRRT, and the particular modalities, is extremely important since CRRT affects, among other things, drug clearance and dosing [12–14], including antibiotics and vasopressors [15–17], and affects nutritional support [18, 19]. These affects are dependent on CRRT mode, type of clearance, mode configuration, and filter type [20]. In addition, the use of anticoagulation for CRRT opens another area that needs to be controlled and understood by the intensivist.
IHD vs CRRT
Intermittent dialysis provides renal replacement for brief intervals (3–4 h), usually daily or every 2–3 days. CRRT is a therapy that provides continuous 24-h per day therapy. Both IHD and continuous hemodialysis (HD) circuits utilize similar principles. Blood is removed from the patient, pumped through a dialysis filter and returned to the patient following removal of surplus water and waste. The major difference between intermittent and continuous therapies is the speed and mechanism by which water and wastes are removed. IHD removes large amounts of water and wastes in a short period of time using diffusion, whereas, continuous renal replacement therapies remove water and wastes at a slow and steady rate using diffusion, convection or combination thereof. While intermittent dialysis allows chronic renal failure patients to limit the amount of time that they receive dialysis, the rapid removal of water and wastes during intermittent treatments may be poorly tolerated in unstable patients.
Hemodynamically unstable, fluid overloaded, catabolic and septic patients better tolerate CRRT [21]. Because of these advantages, the popularity of CRRT for the treatment of critically ill patients with renal failure has increased considerably. Major advantages of CRRT use in the ICU setting includes: (1) slower rate of solute or fluid removal per unit of time leading to a steady state fluid equilibrium [22]; (2) improved control of body temperature, fluid, and metabolic balance; (3) continuous control of azotemia, electrolytes, and acid–base balance; (4) continuous removal of fluid in volume overload clinical situations; (5) aid in administration of parenteral nutrition and other fluids without concern for volume excess due to continuous ultrafiltration; and (6) can effectively lower intracranial pressure as opposed to routine IHD which can sometimes raise intracranial pressure [23, 24]. CRRT has also demonstrated removal of circulating inflammatory substances including cytokines, activated components of complement, and derivatives of the arachidonic acid [25–28].
Presently, most equipment that performs CRRT is used exclusively for providing CRRT. A few manufactures offered machines that can provide both intermittent and continuous modalities. However, these machines usually have configuration limitations, are more costly and require dedicated dialysis nursing presence. Because of this, most centers maintain separate equipment for both IHD and CRRT for both logistics and cost.
Indications for CRRT
The indications for CRRT can be separated into renal and non-renal indications. The traditional indication for CRRT in the ICU setting has been ARF complicated by hemodynamic instability [22] and ARF associated with heart failure, volume overload, hypercatabolism, acute or chronic liver failure, and/or brain swelling [29–31]. Non-renal indications include systemic inflammatory response (SIRS), sepsis, multiorgan failure (MOF), and adult respiratory distress syndrome to name a few [32–34]. Table 1 list several non-renal diseases processes that have utilized CRRT as part of the therapeutic regime. Although these therapies do not meet rigourous evidence based standards, this technology is broadening its scope and expanded uses are increasingly reported. It will take some time before enough data accumulates to sparse out efficiacy and to define which patient populations may benefit from non-renal uses.
Modes and Principles of CRRT
HD refers to the transport process by which a solute passively diffuses down its concentration gradient from one fluid compartment (either blood or dialysate) into the other. During HD, urea, creatinine, and potassium move from blood to dialysate, while other solutes, such as calcium and bicarbonate, move from dialysate to blood. The dialysate flows countercurrent to blood flow through the dialyzer to maximize the concentration gradient between the compartments and therefore to maximize the rate of solute removal. The net effect is the desired changes in the plasma concentrations of blood urea nitrogen and plasma creatinine concentration along with an elevation in the plasma calcium and bicarbonate concentrations.
Hemofiltration (HF) refers to the use of a hydrostatic pressure gradient to induce the filtration (or convection) of plasma water across the membrane of the hemofilter [38]. The frictional forces between water and solutes (called solvent drag) results in the convective transport of small and middle molecular weight solutes (<5000 Da) in the same direction as water [39]. To prevent hypovolemia, any water removed during HF is returned to the blood before it reaches the patient. This is called “replacement” fluid or “substitution” fluid. The process of HF itself removes smaller solutes (such as urea and electrolytes) in roughly the same concentration as the plasma. Therefore, little change in the plasma concentrations of these solutes is seen with HF contrasting those achieved by HD. However, the administration of substitution fluid will lower the plasma concentrations of those solutes by dilution since solutes such as urea and creatinine are not present in the substitution fluid.
Original continuous HD circuits required arterial to venous access sites, because they did not utilize a blood pump to pull blood through the filter. Consequently, they were referred to as continuous arterial–venous (CAV) circuits. Today’s technology uses a blood flow pump; therefore, most continuous circuits are continuous venous–venous (CVV).
The following are the different CVV therapies that can be provided:
SCUF. SCUF is the removal of water from the patient’s blood as it travels through the filter. Water removal is referred to as ultrafiltration. Ultrafiltration is the movement of water across a semi-permeable membrane because of a pressure gradient (hydrostatic, osmotic or oncotic). The increased blood pressure in the hollow fibers of the filter creates a favorable driving pressure to force water across the membrane.
Blood pressure within the hollow fibers is positive, while the pressure outside the hollow fibers is lower. Increased negativity can be generated outside the hollow fibers by an effluent pump thus increasing the fluid removal rate. The difference between the blood pressure in the hollow fibers and the surrounding pressure is the transmembrane pressure (TMP). The TMP determines the ultrafiltrate production.
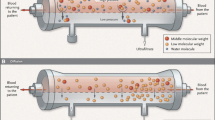
Different filter membrane properties can produce different ultrafiltration rates at a constant TMP. A filter that is more permeable to water will allow more water to travel across the membrane at a given TMP. A filter with a high permeability to water is called high-flux membranes (Fig. 1) [40].
CVVH. CVVH is the removal of large amounts of water across the filter membrane for the purpose of clearing wastes. When large volumes of water are washed across the membrane, solutes are dragged along with the water (convection). HF is the removal of water over and above the surplus water removed during ultrafiltration. To prevent hypovolemia, water removed during HF must be given back before the blood is returned to the patient. This is referred to as replacement. CVVH is the use of replacement fluid without dialysis fluid, plus or minus fluid removal (with ultrafiltration, the correct nomenclature would be CVVHF) (Fig. 2).
Continuous venous–venous hemodialysis (CVVHD). CVVHD is the countercurrent infusion of dialysis fluid into the filter canister. The dialysis fluid (dialysate) surrounds the blood filled filter segments. Solutes that are small enough to fit through the membrane of the dialysis filter will move from an area of high concentration to low concentration (diffusion). The dialysate determines the solutes that will be removed. To remove solutes, the concentration in the dialysate is lower than the blood concentration. To give something to the patient, the concentration in the dialysate is higher than the blood. CVVHD is the removal of wastes by diffusion only, without the use of HF (replacement fluid). It can be administered with or without fluid removal from the patient.
CVVHDF. CVVHDF is the use of dialysis and HF. Therapy will include the use of both dialysate and replacement fluids and can be administered with or without fluid removal from the patient (Fig. 3).
The configuration for CVVHD and CVVHDF is the addition of counter-current dialysate. This mode utilizes diffusive and convective methods of clearance. If the volume of filtration exceeds the substitution rate (a net negative fluid balance) then the configurations are considers a hemodiafiltration. No net fluid removal CVVHD, net fluid removal CVVHDF
Hybrid Therapies
Extended Daily Dialysis
Extended daily dialysis (EDD) is a modality that extends dialysis over a longer than normal period and can be an alternative to CRRT in the ICU. With EDD blood flow rates are around 200 ml/min with a dialysate flow rate of approximately 300 ml/min for treatment time of 6–8 h. EDD utilizes conventional IHD equipment. EDD in small studies has shown to be safe and effective alternative to CRRT that offers comparable hemodynamic stability and excellent small solute control [40]. EDD uses the principle of diffusion for solute removal. Anticoagulation during the treatment is similar to other modalities. A few reported advantages of EDD include less cumbersome technique, patient mobility, decreased requirements for anticoagulation while providing hemodynamic stability and volume control [41]. In spite of possible advantages, there is insufficient evidence that it offers any clear advantage to the critically ill patient, particularly those with significant hemodynamic compromise.
Slow Low Efficiency Daily Dialysis
Slow low efficiency daily dialysis (SLEDD) is a hybrid technique between CRRT and IHD. It is aimed to reduce rate of ultrafiltration for optimal hemodynamic stability while also providing low efficiency solute removal to minimize solute disequilibrium [42]. SLEDD reportedly provides a sustained treatment duration to maximize dialysis dose but also has periods off therapy that can give an opportunity for therapeutic procedures during scheduled down-time. The disadvantages of SLEDD include lower clearance rates of small and particularly large solutes in comparison to CRRT. SLEDD can be performed using traditional IHD machines or CRRT machines. The blood flow rate can be set between 50 and 200 ml/min and treatment can last anywhere from 6 to 12 h and can be done nocturnally. There is insufficient data to date to advocate SLEDD over another form of RRT in the ICU setting [43].
Vascular Access
Historically early circuits removed blood from arterial access sites and returned the purified blood via a venous catheter. This promoted blood flow through the filter by utilizing the patients own arterial to venous blood pressure gradient [44]. Advantage to this technique was that there was no pump required and the mean arterial pressure drove the blood into the filter. However, there was increased risk of atheroembolism, ischemia of limb, hematoma formation, hemorrhage, arterial wall injury and spasm of the artery cannulated.
Current CRRT machines have a pump and hence use a veno-venous access. Temporary double-lumen venous dialysis catheters are the most common form of access. They can be inserted quickly at the bedside and used immediately. This method gives rapid and constant blood flow rate, improved dialyzer performance and decreased circuit and dialyzer clotting. Disadvantages include air embolism due to inadvertent disconnection and vein thrombosis and stenosis risk.
Anticoagulation
Anticoagulation is often used to maintain circuit patency. The strategies include systemic anticoagulation and regional anticoagulation. Also, CRRT can be successfully administered without anticoagulation, however, the common strategy is systemic anticoagulation with growing use of regional anticoagulation [45].
Systemic Anticoagulation
Different systemic anticoagulation options include heparin, low-molecular weight heparin (LMWH), nafamostat, prostacyclin, hirudin, argatroban, activated protein C (aPC).
Heparin
Heparin is the most common anticoagulant used for CRRT. Advantages of heparin include low cost, ease of administration, ease of monitoring, short half life, and easily reversible with protamine. Disadvantages include risk of heparin-induced thrombocytopenia, hypoaldosteronism, dependency of antithrombin III, transaminitis, and hemorrhage [46]. With increased bleeding noted in patients with PTT > 45 s. Strategies include bolus of 2000–5000 units injected into the circuit followed by an infusion of 3–15 units/kg/h to maintain PTT 1.5–2 times upper limit of normal.
LMWH
Advantages of LMWH include lower incidence of HIT, lower affinity for antithrombin III, less platelet activation, constant bioavailability, and less metabolic side effects. Disadvantages include need to monitor anti-Xa levels, which are not readily available, and no reversal agent available for bleeding complications. Goals for anticoagulation are anti-Xa levels between 0.25 and 0.35 U/ml. Their use as an anticoagulant for CRRT is sparse and no recommendation can be made about their use [47, 48].
Prostacyclins
Prostacyclins exert their effects by platelet inhibition. There is inadequate data to suggest use of prostaglandins for anticoagulation in CRRT. However, a few small studies should have efficacy [49, 50]. Disadvantages include cost, vasodilatation, increased ICP, and difficulty in monitoring.
Hirudin
Hirudin is a specific thrombin inhibitor. The safety and efficacy of hirudin as an anticoagulant strategy in CRRT has been demonstrated [51]. Disadvantages include difficulty in monitoring and no effective antidote. It also requires complete dependence on renal function for elimination.
Argatroban
Argatroban is a direct thrombin inhibitor that is cleared by liver. Argatroban has rapid onset and elimination and appears to have a low risk of bleeding. Argatroban is an appropriate alternative in patients with HIT [52]. More studies are being done to look at the safety and efficacy of argatroban. Dosing range is 0.5–2 mcg/kg/min with goal of PTT 1–3 times normal range.
aPC
aPC inhibits and reduces the expression of tissue factor. Anecdotal reports and small studies in patients with sepsis who received aPC and CRRT suggest filter life was equivalent to other forms of anticoagulation [53].
Nafamostat
Nafamostat is a synthetic serine protease inhibitor and works as an anticoagulant by acting on factors IIa, Xa, XIIa and inhibiting TF–VIIa complex [54]. Disadvantages include agranulocytosis, hyperkalemia, and anaphylaxis. It is currently available only in Japan.
Regional Anticoagulation
Heparin–Protamine
In this form of anticoagulation, heparin is administered pre-filter and protamine given post-filter. Overall this has not proved to be an effective strategy [47]. Protamine also has risk of hypotension and anaphylaxis-type reactions.
Citrate-calcium
Regional anticoagulation with citrate is emerging as the most promising of all anticoagulation regimes [55]. Citrate chelates calcium, which decreases extracorporeal iCa++. Citrate is partially removed by CRRT and partially enters the systemic circulation. In systemic circulation iCa++ levels rise due to liberation of calcium when citrate is metabolized and from replacement of calcium. Therefore, there is no net effect of systemic coagulation. Administration of citrate is usually based on titration of citrate based on post-filter iCa++ (with goal <0.35 mmol/l) or giving a fixed dose of citrate with no monitoring iCa++ in the circuit but titrating systemic calcium administration to a serum iCa++ of 1.0–1.1 mM [56].
Accumulation of citrate can occur when the ability to metabolize citrate is decreased (i.e., liver failure and decreased tissue perfusion). Clinical consequence includes decreased iCa++, metabolic acidosis, increased anion gap and increased total/iCa++ concentration [57]. It can also occur with unintended citrate over-infusion or decreased removal in case of a decline in membrane performance at constant citrate infusion. Clinical consequences include decreased iCa++ and metabolic alkalosis. Other complications of citrate include hypernatremia, hypo- or hypercalcemia, hypomagnesemia, alkalosis, and metabolic acidosis.
No Anticoagulation
CRRT can be successfully performed without the use of anticoagulation. Methods to reduce clotting without using anticoagulation include saline infusions and periodic saline flushes along with reduction in filtration fraction and pre-dilution fluid substitution [58]. Interventions that can improve success of CRRT without anticoagulation include adequate access flow, minimization of machine stoppage and reduction in filtration fraction [59]. Performing CRRT without anticoagulation can be performed with good results in patients at high risk for bleeding [60].
Filter Technology
Dialysis membranes need to be efficient at clearing wastes, but must also be biocompatible with human blood. Compatibility means that exposure of blood to the dialysis membrane produces minimal adverse effects. Pore size, the number of pores and the thickness of the membrane influence filter permeability. Generally, high-flux membranes which have more or larger pores allow more solutes and ultrafitrate to move across the membrane. Thinner membranes offer less resistance to solute movement by decreasing the distance the solute must travel across the membrane and also favors increased filtration [61].
Solutes are passed through the membrane according to solute size. Small and mid sized molecules pass across the membrane, without the loss of larger proteins. High-flux membranes that have a larger pore size increase clearance by allowing larger molecules to pass through the membrane, and by allowing more ultrafiltrate flow. The AN69 filter used with some CRRT circuits is a high-flux membrane.
Sieving properties of a membrane describe the membrane’s permeability to solutes during ultrafiltration. Permeability of solutes decreases as the molecular size increases. The cut-off point for a membrane is defined by the molecular weight where only 10% of the solute is filtered. Sieving properties are usually expressed as sieving co-efficients (Sc). The equation is shown in Fig. 4. Both the AN69 and the polysulfone filters have similar Sc [62].
Adsorption is the ability of larger solutes to adhere to the surface of the dialysis membrane [63]. AN69 filters have strong adsorptive properties including inflammatory mediators [64]. The greatest benefit appears to occur in the first few hours; once the filter becomes saturated with proteins, further removal from the serum is limited [65].
TMP is the pressure exerted on the dialysis membrane during operation and reflects the difference between blood and fluid compartments or essentially the hydrostatic pressure exerted from the blood interface within the filter. An increased TMP and the rate of TMP increase contribute to filter clotting. The greater the pressure drop the more likely there is filter clotting.
Conclusion
This is a review of the technical aspects of CRRT. This is only a primer and not an exhaustive review. In Table 2 is a list of references that explore this technology further and also discuss drug dosing and nutritional support during CRRT. Also, there are references that discuss and review debates that exist between CRRT and IHD.
References
Litmathe J, Kurt M, Feindt P, Gams E, Boeken U. Predictors and outcome of ICU readmission after cardiac surgery. Thorac Cardiovasc Surg. 2009;57(7):391–4.
Andrikos E, Tseke P, Balafa O, Cruz DN, Tsinta A, Androulaki M, Pappas M, Ronco C. Epidemiology of acute renal failure in ICUs: a multi-center prospective study. Blood Purif. 2009;28(3):239–44.
Charbonney E, Saudan P, Triverio PA, Quinn K, Mentha G, Martin PY. Prognosis of acute kidney injury requiring renal replacement therapy in solid organ transplanted patients. Transpl Int. 2009.
Cislaghi F, Condemi AM, Corona A. Predictors of prolonged mechanical ventilation in a cohort of 5123 cardiac surgical patients. Eur J Anaesthesiol. 2009;26(5):396–403.
Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten HM, Ronco C, Kellum JA. Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care. 2009;24(1):129–40.
Zarbock A, Singbartl K, Kellum JA. Evidence-based renal replacement therapy for acute kidney injury. Minerva Anestesiol. 2009;75(3):135–9.
Lins RL, et al. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant. 2009;24(2):512–8.
Vege SS, Gardner TB, Chari ST, Munukuti P, Pearson RK, Clain JE, Petersen BT, Baron TH, Farnell MB, Sarr MG. Low mortality and high morbidity in severe acute pancreatitis without organ failure: a case for revising the Atlanta classification to include “moderately severe acute pancreatitis”. Am J Gastroenterol. 2009;104(3):710–5.
Ronco C, Bellomo R, Ricci Z. Continuous renal replacement therapy in critically ill patients. Nephrol Dial Transplant. 2001;16(Suppl 5):67–72.
Meyer MM. Renal replacement therapies. Crit Care Clin. 2000;16(1):29–58.
Ronco C, Bellomo R, Kellum JA. Continuous renal replacement therapy: opinions and evidence. Adv Ren Replace Ther. 2002;9(4):229–44.
Subach RA, Marx MA. Drug dosing in acute renal failure: the role of renal replacement therapy in altering drug pharmacokinetics. Adv Ren Replace Ther. 1998;5(2):141–7.
Veltri MA, Neu AM, Fivush BA, Parekh RS, Furth SL. Drug dosing during intermittent hemodialysis and continuous renal replacement therapy: special considerations in pediatric patients. Paediatr Drugs. 2004;6(1):45–65.
Böhler J, Donauer J, Keller F. Pharmacokinetic principles during continuous renal replacement therapy: drugs and dosage. Kidney Int Suppl. 1999;56(72):S24–8.
Joy MS, Matzke GR, Armstrong DK, Marx MA, Zarowitz BJ. A primer on continuous renal replacement therapy for critically ill patients. Ann Pharmacother. 1998;32(3):362–75.
Li AM, Gomersall CD, Choi G, Tian Q, Joynt GM, Lipman J. A systematic review of antibiotic dosing regimens for septic patients receiving continuous renal replacement therapy: do current studies supply sufficient data? J Antimicrob Chemother. 2009;64(5):929–37.
Pea F, Viale P, Pavan F, Furlanut M. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin Pharmacokinet. 2007;46(12):997–1038.
Bellomo R, Ronco C. How to feed patients with renal dysfunction. Curr Opin Crit Care. 2000;6(4):239–46.
Story DA, Ronco C, Bellomo R. Trace element and vitamin concentrations and losses in critically ill patients treated with continuous venovenous hemofiltration. Crit Care Med. 1999;27(1):220–3.
Arzuaga A, et al. Elimination of piperacillin and tazobactam by renal replacement therapies with AN69 and polysulfone hemofilters: evaluation of the sieving coefficient. Blood Purif. 2006;24(4):347–54.
Ronco C. Continuous renal replacement therapies for the treatment of acute renal failure in intensive care patients. Clin Nephrol. 1993;40(4):187–98.
Manns M, Sigler MH, Teehan BP. Continuous renal replacement therapies: an update. Am J Kidney Dis. 1998;32(2):185–207.
Davenport A. The management of renal failure in patients at risk of cerebral edema/hypoxia. New Horiz. 1995;3(4):717–24.
Fletcher JJ, Bergman K, Feucht EC, Blostein P. Continuous renal replacement therapy for refractory intracranial hypertension. Neurocrit Care. 2009;11(1):101–5.
Yu C, Liu ZH, Chen ZH, Gong DH, Ji DX, Li LS. Improvement of monocyte function and immune homeostasis by high volume continuous venovenous hemofiltration in patients with severe acute pancreatitis. Int J Artif Organs. 2008;31(10):882–90.
Fortenberry JD, Paden ML. Extracorporeal therapies in the treatment of sepsis: experience and promise. Semin Pediatr Infect Dis. 2006;17(2):72–9.
Peng Y, Yuan Z, Li H. Removal of inflammatory cytokines and endotoxin by veno-venous continuous renal replacement therapy for burned patients with sepsis. Burns. 2005;31(5):623–8.
Druml W. Metabolic aspects of continuous renal replacement therapies. Kidney Int Suppl. 1999;(72):S56–61.
Ronco C, et al. Management of fluid balance in CRRT: a technical approach. Int J Artif Organs. 2005;28(8):765–76.
Davenport A. Continuous renal replacement therapy for liver disease. Hemodial Int. 2003;7(4):348–52.
Clark WR, Mueller BA, Kraus MA, Macias WL. Extracorporeal therapy requirements for patients with acute renal failure. J Am Soc Nephrol. 1997;8(5):804–12.
Soni SS, Nagarik AP, Adikey GK, Raman A. Using continuous renal replacement therapy to manage patients of shock and acute renal failure. J Emerg Trauma Shock. 2009;2(1):19–22.
Joannidis M. Continuous renal replacement therapy in sepsis and multisystem organ failure. Semin Dial. 2009;22(2):160–4.
Bouchard J, Mehta RL. Acid-base disturbances in the intensive care unit: current issues and the use of continuous renal replacement therapy as a customized treatment tool. Int J Artif Organs. 2008;31(1):6–14.
Bellomo R, Ronco C. Blood purification in the intensive care unit: evolving concepts. World J Surg. 2001;25(5):677–83.
Dunham CM. Clinical impact of continuous renal replacement therapy on multiple organ failure. World J Surg. 2001;25(5):669–76.
Wei Q, Baihai S, Ping F, Xiaolei C, Jing L, Rong Z. Successful treatment of crush syndrome complicated with multiple organ dysfunction syndrome using hybrid continuous renal replacement therapy. Blood Purif. 2009;28(3):175–80.
Brunet S, Leblanc M, Geadah D, Parent D, Courteau S, Cardinal J. Diffusive and convective solute clearances during continuous renal replacement therapy at various dialysate and ultrafiltration flow rates. Am J Kidney Dis. 1999;34(3):486–92.
Messer J, Mulcahy B, Fissell WH. Middle-molecule clearance in CRRT: in vitro convection, diffusion and dialyzer area. ASAIO J. 2009;55(3):224–6.
Kumar VA, Craig M, Depner TA, Yeun JY. Extended daily dialysis: a new approach to renal replacement for acute renal failure in the intensive care unit. Am J Kidney Dis. 2000;36(2):294–300.
Zaccaria R, Ronco C. Dose and efficiency of renal replacement therapy: continuous renal replacement therapy versus intermittent hemodialysis versus slow extended daily dialysis. Cril Care Med. 2008;36 Suppl 4.
Marshall M, Ma T, Galler D, et al. Sustained low-efficiency daily diafiltration for critically ill patients requiring renal replacement therapy: towards an adequate therapy. Nephrol Dail Transplant. 2004;19:877–84.
Vanholder R, Biesen WV, Lameire N. What is the renal replacement method of first choice for intensive care patients? J Am Soc Nephrol. 2001;12:S40–3.
Ronco C, Bellomo R. New CRRT systems: impact on dose delivery. Am J Kidney Dis. 1997;30(5 Suppl 4):S15–9.
Alarabi AA, Danielson BG, Wikström B. Continuous arteriovenous hemodialysis: outcome in intensive care acute renal failure patients. Nephron. 1993;64(1):58–62.
Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten H, Ronco C, Kellum JA. Continuous renal replacement therapy: a worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med. 2007;33(9):1563–70.
Tolwani AJ, Wille KM. Anticoagulation for continuous renal replacement therapy. Semin Dial. 2009;22(2):141–5.
Sagedal S, Hartmann A. Low molecular weight heparins as thromboprophylaxis in patients undergoing hemodialysis/hemofiltration or continuous renal replacement therapies. Eur J Med Res. 2004;9(3):125–30.
Oudemans-van Straaten HM, Wester JP, de Pont AC, Schetz MR. Anticoagulation strategies in continuous renal replacement therapy: can the choice be evidence based? Intensive Care Med. 2006;32(2):188–202.
Gainza FJ, Quintanilla N, Pijoan JI, Delgado S, Urbizu JM, Lampreabe I. Role of prostacyclin (epoprostenol) as anticoagulant in continuous renal replacement therapies: efficacy, security and cost analysis. J Nephrol. 2006;19(5):648–55.
Birnbaum J, Spies CD, Klotz E, Hein OV, Morgera S, Schink T, Ziemer S, Grund MS, Saalmann R, Kox WJ, Lehmann C. Iloprost for additional anticoagulation in continuous renal replacement therapy—a pilot study. Ren Fail. 2007;29(3):271–7.
Vargas Hein O, von Heymann C, Lipps M, Ziemer S, Ronco C, Neumayer HH, Morgera S, Welte M, Kox WJ, Spies C. Hirudin versus heparin for anticoagulation in continuous renal replacement therapy. Intensive Care Med. 2001;27(4):673–9.
Link A, Girndt M, Selejan S, Mathes A, Böhm M, Rensing H. Argatroban for anticoagulation in continuous renal replacement therapy. Crit Care Med. 2009;37(1):105–10.
de Pont AC, Bouman CS, de Jonge E, Vroom MB, Büller HR, Levi M. Treatment with recombinant human activated protein C obviates additional anticoagulation during continuous venovenous hemofiltration in patients with severe sepsis. Intensive Care Med. 2003;29(7):1205.
Amanzadeh J, Reilly RF. Anticoagulation and continuous renal replacement therapy. Semin Dial. 2006;19(4):311–6.
Joannidis M, Oudemans-van Straaten HM. Clinical review: patency of the circuit in continuous renal replacement therapy. Crit Care. 2007;11(4):218.
Palsson R, Niles JL. Regional citrate anticoagulation in continuous venovenous hemofiltration in critically ill patients with a high risk of bleeding. Kidney Int. 1999;55(5):1991–7.
Burry LD, Tung DD, Hallett D, Bailie T, Carvalhana V, Lee D, Ramganesh S, Richardson R, Mehta S, Lapinsky SE. Regional citrate anticoagulation for PrismaFlex continuous renal replacement therapy. Ann Pharmacother. 2009;43(9):1419–25.
Davies H, Leslie G. Maintaining the CRRT circuit: non-anticoagulant alternatives. Aust Crit Care. 2006;19(4):133–8.
Mehta RL. Anticoagulation during continuous renal replacement therapy. ASAIO J. 1994;40(4):931–5.
Tan HK, Baldwin I, Bellomo R. Continuous veno-venous hemofiltration without anticoagulation in high-risk patients. Intensive Care Med. 2000;26(11):1652–7.
Morabito S, Pierucci A, Marinelli R, Cicciarelli A, Guzzo I, Cinotti GA, Marino B, Chiavarelli R. Efficiency of different hollow-fiber hemofilters in continuous arteriovenous hemodiafiltration. Am J Nephrol. 2000;20(2):116–21.
Isla A, Gascón AR, Maynar J, Arzuaga A, Sánchez-Izquierdo JA, Pedraz JL. In vitro AN69 and polysulphone membrane permeability to ceftazidime and in vivo pharmacokinetics during continuous renal replacement therapies. Chemotherapy. 2007;53(3):194–201.
Leypoldt JK, Gilson JF, Blindauer KM, Cheung AK. Macromolecule adsorption to hemodialysis membranes depends on molecular size. Blood Purif. 1992;10(1):53–60.
Bouman CS, van Olden RW, Stoutenbeek CP. Cytokine filtration and adsorption during pre- and postdilution hemofiltration in four different membranes. Blood Purif. 1998;16(5):261–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, P., Nandwani, V., McCarthy, P.J. et al. Continuous Renal Replacement Therapies: A Brief Primer for the Neurointensivist. Neurocrit Care 13, 286–294 (2010). https://doi.org/10.1007/s12028-010-9386-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-010-9386-6