Abstract
Analgesic therapy following intracranial procedures remains a source of concern and controversy. Although opioids are the mainstay of the “balanced” general anesthetic techniques frequently used during intracranial procedures, neurosurgeons and others have been reluctant to administer opioid analgesics to patients following such procedures. This practice is supported by the concern that the sedation and miosis associated with opioid administration could mask the early signs of intracranial catastrophe, or even exacerbate it through decreased ventilatory drive, elevated arterial carbon dioxide levels, and increased cerebral blood flow. This reluctance to use opioids following intracranial surgery is enabled by decades of training and anecdote emphasizing that pain is minimal following these procedures. However, recent data suggests otherwise, and raises the question of how to provide safe and effective analgesia for these patients. Here, this data is reviewed along with the relevant pain pathways, analgesic drugs and techniques, and the available data on their use following intracranial surgery. Although pain following intracranial surgery appears to be more intense than initially believed, it is readily treated safely and effectively with techniques that have proven useful following other types of surgery, including patient-controlled administration of opioids. The use of multimodal analgesic therapy is emphasized not only for its effectiveness, but to reduce dosages and, therefore, side effects, primarily of the opioids, that could be of legitimate concern to physicians and affect the comfort of their patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Historically, the pain associated with intracranial surgery has been undertreated because of a presumed lack of need and a fear that use of opioids, the analgesics most often used to treat moderate to severe pain, may interfere with the neurologic examination or lead to its deterioration [1]. Even with the immediate availability of modern imaging technology, the neurologic exam remains the primary instrument for perioperative evaluation of patients following intracranial surgery. Opioids may produce sedation, miosis, nausea and vomiting, symptoms that mask or mimic signs of intracranial catastrophe. Furthermore, opioids, even when administered in therapeutic doses, may depress minute ventilation leading to hypercapnia, increased intracerebral blood volume, and potentially increased intracranial pressure and cerebral edema [2]. Therefore, it is understandable why physicians involved with the care of these patients are reluctant to administer opioids. Moreover, why expose a patient to these risks when there is a presumed lack of need? Decades of training and anecdote have reinforced a widely held belief that patients do not experience intense pain following intracranial surgery, a belief supported by the fact that surgery on the brain parenchyma per se is not painful.
Pain Assessment
Pain assessment and management are interdependent and one is essentially useless without the other. The goal of pain assessment is to provide accurate data about the location and intensity of pain, as well as the effectiveness of measures used to alleviate or abolish it. For the most part, with the exceptions described later on for special populations, acute pain can be assessed by self-report with simple numeric rating scales such as the discrete 0 (no pain) to 10 (worst imaginable pain) scale that is commonly used [3]. Direct patient observation is generally insufficient since even experienced nurses and physicians tend to underestimate pain particularly when it is most severe [4]. Unpublished data indicates that, at least at the authors’ institution, few physicians involved in the care of neurosurgical patients make the necessary formal assessments of patients’ pain [5]. When patients describe past painful experience they tend to recall pain intensity as less than what they reported when they were experiencing the pain [6, 7]. Furthermore, for reasons which remain unclear, patients hesitate to make medical staff aware that they are in pain [8]. Finally, satisfaction with pain-relieving therapy does not necessarily relate to the effectiveness of that therapy. As a rule, patients expect perioperative pain [9] and value even less effective efforts at providing analgesia [10]. Given these findings, it is easy to see how pain following intracranial surgery might be perceived by surgeons as less than actually experienced by patients, providing reassurance to those who would prefer to limit analgesic therapy for the reasons elucidated above [11].
Pain and Intracranial Surgery
The literature on pain following intracranial surgery is just beginning to present a sufficiently coherent picture to reconsider its quality, intensity, and duration. Several small, early studies demonstrated a period of moderate to severe pain in 41% [12] to 84% [13] of patients in the first 24 postoperative hours. Studies like these supported a growing recognition that craniotomy pain was more intense than physicians believed [1, 14, 15]. On the other hand, a larger, more recent study was inconsistent with this view [16]. In this study of pain during admission to the postanesthesia care unit for periods of time averaging less than 2 h, pain scores on a discrete 0–10 scale averaged less than 1. Whether this was due to residual effects of the opioid-based anesthetic techniques common during intracranial surgery or some other factor, these observations fueled the belief that patients were comfortable following craniotomy. For many patients in another study, the pain accompanying intracranial surgery was found to be greater than expected [17]. Most recently, a large prospective study of pain following major intracranial surgery demonstrated some period of moderate to severe pain (≥4 on a 0–10 scale) in 69% of patients on the first postoperative day and in 48% of patients on the second [18]. In contrast to studies with other types of pain [10], patient satisfaction varied significantly with the quality of pain relief. Similar rates of moderate to severe pain were also observed in another more recent study [19]. Demographic and clinical factors associated with increased pain following intracranial surgery include sex [13, 18], age [13, 18, 19], surgical site [18–20], surgical approach [21, 22], and use of nerve blocks [18, 23] or local anesthetic infiltration of the incision site [24]. Pain intensity is also a significant factor in studies evaluating the quality of recovery from intracranial surgery [25].
Although the primary focus of intensivists is the acute pain which accompanies intracranial surgery, a full appreciation of the pain associated with intracranial surgery requires recognition of the chronic pain syndromes that can also occur. This recognition mirrors what is occurring with other types of surgery, where an increasing number of surgical approaches are linked with procedure-specific pain syndromes of varying incidence and impact [26]. Common factors associated with other types of surgery include the intensity of postoperative pain and nerve injury. After supratentorial surgery, the prevalence of headache one year following surgery was 11% [27]. This is lower than seen with posterior fossa procedures, where the prevalence of headache one year after surgery is reported to be about 30% [28, 29]. Whether aggressive perioperative analgesia could reduce the incidence of long-term pain following craniotomy, as has been speculated for other types of surgery [30], remains unanswered.
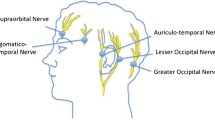
Ironically the brain is insensate, and this fact may also contribute to the notion that pain following intracranial surgery should be limited. However, the muscles which attach to the skull, the scalp, the periosteum, and the dura, can be quite sensitive to noxious stimuli. An understanding of their innervation can be useful for developing a perioperative analgesic regimen to reduce postoperative pain, and for appreciating the limitations of particular approaches for certain types of surgery. The key nerves (Fig. 1) arise from all three divisions of the trigeminal nerve and the first three spinal nerves. They contribute sensation to the frontalis, temporalis, occipitalis, and cervical muscles which attach to the occiput, all of which may be manipulated or whose origin on the skull may be interrupted during intracranial surgery. These same nerves provide sensation to the scalp and periosteum [31]. Importantly, all of these nerves are readily accessible for nerve block (Fig. 1).
Innervation of the scalp and underlying muscles. Sensation is provided by all three divisions of the trigeminal nerve and the ventral rami of the 2nd and 3rd cervical nerve roots. The ophthalmic branch of the trigeminal nerve gives rise to the supraorbital and supratrochlear nerves, the maxillary division gives rise to the zygomaticotemoral nerve, and the posterior trunk of the mandibular division gives rise to the auriculotemporal nerve. The lesser occipital nerve originates from the ventral ramus of the 2nd cervical nerve root, whereas the greater occipital nerve originates from the ventral rami of the 2nd and 3rd cervical nerve roots. A scalp block sufficient for awake craniotomy or as an adjunct to general anesthesia can be performed by injections at the crosshatched regions on the figure. In practice, a bead of local anesthetic over the medial half of the brow and one initiated just lateral to the orbit and continued to the occipital prominence is effective. The specific choice of nerves to block should be individualized once the surgeon has specified the incision. In addition to blockade of the nerves supplying sensation to the surgical site, local anesthetic blockade of the pin sites not otherwise contained by prior nerve blocks should be performed. A long-acting local anesthetic (e.g., bupivacaine 0.5%) will maximize postoperative analgesia, but efforts to avoid potentially toxic doses should be made as large bilateral incisions can often require 30 ml or more of local anesthetic. Some surgeons have expressed concern about local anesthetic injection in the temporal region prior to vascular procedures out of concern about potential damage to the superficial temporal artery. Local anesthetic administration in the temporal region close to the zygoma can also produce an inadvertent block of branches of the facial nerve and this should be considered in the postoperative neurologic assessment
Although the same cranial and spinal nerves provide sensation to the dura, the innervation is less straightforward and less accessible for neural blockade (Fig. 2) [32]. Catecholaminergic fibers from the dura also originate from the superior cervical ganglion of the sympathetic chain [33]. Although the innervation of the dura cannot readily be interrupted with extracranial nerve blocks, knowledge of these pathways is still important for relieving pain during awake intracranial surgery. Furthermore, mechanical and chemical irritation of the dura remaining after surgery can contribute to painful postoperative sensation, even after an effective scalp block. Since this pain is referred to the corresponding somatic distribution of the associated nerves, this may help to identify the intracranial region of concern.
Intracranial innervation of the dura is provided by branches from all three divisions of the trigeminal nerve and the first three spinal nerves. As might be anticipated, the pain associated with noxious stimulation of the dura is referred to the corresponding somatic distribution of the nerve involved. A tentorial branch of the ophthalmic division of the trigeminal supplies the tentorium cerebelli and falx cerebri, coursing posteriorly along the tentorium, ascending along the falx and traveling anteriorly. These nerves tend to follow venous structures, providing sensation to the sinuses and the terminal portion of the veins which drain into them. The most anterior portion of the falx and base of the anterior cranial fossa are supplied by meningeal branches of the anterior and posterior ethmoidal branches of the ophthalmic division of the trigeminal nerve. The remainder of the supratentorial dura is supplied by a meningeal branch of the maxillary division of the trigeminal nerve, the nervus meningeus medius, and a branch of the mandibular division, the nervus spinosus. The nervus meningeus medius originates just prior to the exit of the maxillary division of the trigeminal nerve through the foramen rotundum, whereas the nervus spinosus is a recurrent branch of the mandibular division which enters the skull through the foramen spinosum along with the middle meningeal artery. Both of these meningeal nerves course with the branches of the middle meningeal artery, and the dura tends to be most sensitive where these vessels are the most plentiful. The dura of the posterior fossa is supplied by branches of the first three spinal nerves which enter the cranial vault through the anterior portion of the foramen magnum and through the jugular foramen and hypoglossal canal. Although extracranial access to these nerves for the purpose of neural blockade is not generally possible, intracranial blockade of the nervus spinosus can be performed by injecting the dura where the middle meningeal artery exits the foramen. When pain is experienced during awake craniotomies by traction of the dura and associated vascular structures, identification of the nerve responsible and its blockade is possible by recalling the anatomy of the tentorial and meningeal nerves, their association with veins and arteries, respectively, and injecting small amounts of local anesthetic between the dural layers at an appropriate location
Patient, Anesthetic, and Surgical Factors
Treatment of the pain accompanying intracranial surgery begins prior to the procedure itself through recognition of patient-specific issues which influence perioperative management. As indicated above, a number of demographic factors such as female sex, younger age, and posterior fossa approach may predispose patients toward more intense perioperative pain. Patients with chronic painful conditions incidental to or associated with the anticipated surgery are more likely to experience increased perioperative pain [34] and may already be taking opioid analgesics [35]. For certain procedures, pain may not simply be a symptom of the underlying disease, but is the criterion which dictates that surgery should take place. Examples of this are decompressions of type-I Chiari malformations or microvascular decompression of the trigeminal nerve.
Effective perioperative analgesia also requires knowledge of the anesthetic course and how it could affect the pain experienced upon the conclusion of surgery. Opioid-based “balanced” anesthetics are often used for intracranial procedures [36] because of the hemodynamic stability they confer, their minimal effects on cerebral blood flow, and their contribution to a prompt and smooth emergence. The specific opioid, the amount administered, and the timing of administration with respect to the duration of surgery and emergence all impact on the level of opioid analgesia present upon emergence.
Paradoxically, intraoperative opioids may increase postoperative analgesic requirements, a phenomenon known as opioid-induced hyperalgesia [37]. This may be prevented with concurrent administration of the dissociative analgesic ketamine which is an N-methyl-d-aspartate (NMDA) antagonist [38]. However, ketamine is rarely used in neurosurgical patients because it is a hallucinogen that alters the postoperative sensorium. Recently, it was demonstrated that preoperative gabapentin can also prevent opioid-induced hyperalgesia [39]. Opioid-induced hyperalgesia may be a particular problem with remifentanil, whose short elimination half-life of 3–10 min permits a large intraoperative opioid effect capable of inducing hyperalgesia, but with virtually no residual opioid effect once emergence is complete. Certainly, in one study, when remifentanil was used as a component of a balanced anesthetic technique for supratentorial craniotomy, even in conjunction with small amounts of morphine sulfate, the number of patients reporting severe pain in the immediate postoperative period doubled compared to the fentanyl group [40]. Another study comparing remifentanil and fentanyl, as components of a balanced anesthetic technique for supratentorial craniotomy, demonstrated earlier requests for analgesics in the remifentanil group [41]. Comparisons of intraoperative remifentanil–propofol to sufentanil–propolfol for supratentorial craniotomy revealed only that patients who received remifentanil requested analgesics sooner [42]. Furthermore, as nitrous oxide enjoys another period of disfavor [43] and with remifentanil infusion already advocated as a means of achieving a brisk emergence without use of nitrous oxide [44], remifentanil use is becoming more common. Apart from creating a vacuum that may be filled by other drugs with their specific impact on analgesia, avoiding nitrous oxide may also avoid its beneficial analgesic effects [45], some of which may stem from its properties as an NMDA antagonist [46]. A newer drug, dexmedetomidine, an α2-agonist administered by continuous intravenous infusion, was recently introduced, is currently approved for sedation during awake craniotomy and is also used for sedation in the setting of the intensive care unit [47]. It may be useful to recognize the perioperative opiate-sparing effects of this class of drug [48].
Large doses of corticosteroids, generally dexamethasone, are frequently administered during intracranial surgery. Consequently, patients experience a powerful antiinflammatory effect that may reduce pain as well as an antiemetic effect [49]. However, the antihyperalgesic effect from cyclooxygenase-2 (COX-2) inhibition in the spinal cord that is seen with nonsteroidal antiinflammatory drugs is not realized [50]. Finally, whether a patient receives a scalp block or local anesthetic infiltration of the incision site will influence the intensity and time course of postoperative pain [18, 23, 24]. Preoperative use of local anesthetic in this manner can decrease intraoperative anesthetic requirements, leading to a brisker emergence and a more cooperative patient in the immediate postoperative period.
Apart from the actual location of the surgery, a number of additional surgical factors may influence the pain experienced afterwards. For example, in the approach to the posterior fossa, use of a craniotomy as opposed to a craniectomy [21] and performance of a cranioplasty after a craniectomy [51] have both been reported to reduce pain, perhaps by preventing the traction that occurs with postoperative cervical muscle attachment to the dura. A translabyrinthine as opposed to a suboccipital approach was reported to reduce the incidence of pain following acoustic neuroma resection [22], but these differences were no longer appreciated one year after surgery [52]. Other studies were not as favorable concerning the translabyrinthine approach [53]. Another surgical factor which may affect perioperative pain is the extent that branches of the greater occipital nerve are divided in the approach to the posterior fossa. It has also been hypothesized that the amount of muscle damage from resection of the temporalis muscle for supratentorial craniotomy or splitting of the posterior cervical muscles for posterior fossa surgery may largely determine the amount of pain experienced [14].
Overall Analgesic Strategy
Acute pain management in adults and children is increasingly characterized by a multimodal approach in which smaller doses of opioid and nonopioid analgesics, such nonsteroidal antiinflammatory drugs, local anesthetics, NMDA antagonists, α2-adrenergic agonists, and other drugs are combined to target pain at multiple pathways. A multimodal approach can also utilize nonpharmacologic complimentary and alternative medicine therapies as well. These include distraction, guided imagery, massage, transcutaneous nerve stimulation, and acupuncture. Combining analgesic techniques and drugs has an additive or synergistic effect which maximizes pain control, minimizes opioid-induced side effects and, therefore, facilitates recovery and rehabilitation.
Another important concept about acute pain therapy is that of “preemptive analgesia” [54–56]. Intense nociceptor stimulation can lead to central sensitization, the process whereby neurons of the central nervous system adjust their dynamic range so that subsequent stimuli are experienced with greater intensity. Importantly, insofar as perioperative pain is concerned, this process is ongoing despite otherwise adequate levels of volatile anesthetics such as isoflurane [57]. Preemptive analgesic strategies seek to limit central sensitization during surgery by modulating the response to noxious input during the perioperative period, thereby reducing subsequent pain and the level of analgesic therapy necessary to control it. Of the analgesic modalities relevant for pain therapy following intracranial surgery, local anesthetic infiltration, NSAIDs and NMDA antagonists, but not systemic opioids can lead to measurable preemptive analgesic effects [30]. It is debatable whether the phenomenon of opioid-induced hyperalgesia described earlier has, in some circumstances, masked the benefits of preemptive systemic opioid administration. Of the available analgesic therapies, local anesthetic use, as detailed below and in Fig. 1, is the only one with a demonstrative preemptive analgesic effect following intracranial surgery, although it is the only one evaluated explicitly for this effect in this population, thus far.
In the next sections, we will review some of the drugs and techniques used postoperatively for multimodal pain treatment and treatment algorithms for many of the most common opioid-induced side effects. The tools and fundamental questions revolving about analgesic therapy following intracranial surgery are the choice of specific systemic adjuvants (Table 1), systemic opioids (Table 2), and nerve blocks (Figs. 1 and 2) along with the route and method of their administration, timing, and dosage. Suggestions for managing opioid-induced side effects can be found in the algorithms of Fig. 3 and in Table 3.
Algorithm for relief of common opioid side effects. See Table 3 for drug dosages
Nonopioid Analgesics
The “weaker” or “milder” analgesics with antipyretic activity, of which acetaminophen [paracetamol] (Tylenol®), salicylate (aspirin), ibuprofen (Motrin®), naproxen (Aleve®, Naprosyn®), diclofenic are the classic examples, comprise a heterogenous group of nonsteroidal antiinflammatory drugs (NSAIDs) and nonopioid analgesics (Table 1). They provide pain relief primarily by blocking peripheral and central prostaglandin production by inhibiting COX-1 and COX-2. These analgesic agents are administered enterally via the oral or, on occasion, the rectal route and are particularly useful for inflammatory, bony, or rheumatic pain. Parenterally administered NSAIDs, such as ketorolac (Toradol®), are available for use in patients when oral or rectal administration is not possible. Unfortunately, regardless of dose, the nonopioid analgesics reach a “ceiling effect” above which pain can not be relieved by these drugs alone. Because of this, these weaker analgesics are often administered in oral combination forms with opioids such as codeine, oxycodone, or hydrocodone.
Aspirin has been largely abandoned in postoperative pain management because it permanently inhibits platelet function, which could have catastrophic consequences in a patient who has recently undergone intracranial surgery. On the other hand, choline-magnesium trisalicylate (Trilisate®) is an unique aspirin-like compound that does not bind to platelets and therefore has minimal, if any, effects on platelet function [58]. This makes choline-magnesium trisalicylate a potentially useful adjunct in postoperative analgesia.
The most commonly used nonopioid analgesic in neurosurgical practice remains acetaminophen (paracetamol). Unlike aspirin and the other NSAIDs, acetaminophen works primarily centrally and has minimal, if any, antiinflammatory activity. It is also thought to have an analgesic effect by antagonizing NMDA and substance P in the spinal cord. When administered in normal doses (10–15 mg/kg, PO), acetaminophen is extremely safe and has very few serious side effects. It is an antipyretic and like all enterally administered NSAIDs, takes about 30 min to provide effective analgesia. It is often administered alone or in combination with oral opioids such as codeine, hydrocodone, or oxycodone (see below). The ubiquitousness and sense of safety of acetaminophen sets up the possibility of excessive and potentially hepatotoxic acetaminophen dosing, particularly when combination preparations such as Tylox® or numbered Tylenol® are given for uncontrolled pain or when they are ordered in addition to previously prescribed acetaminophen. This problem was seen in our study of analgesic use following craniotomy [18]. Regardless of preparation(s), the daily adult maximum acetaminophen dose is 4000 mg/day (Table 1). Finally, an intravenous formulation of acetaminophen is now available in Europe and can be used in patients in whom the enteral route is unavailable. This formulation has been associated with better analgesia than oral acetaminophen in clinical trials in adult patients and is equally effective and less painful than the “pro” formulation of the drug in children [59]. It is under investigation in the United States and hopefully will be available for widespread use shortly.
The discovery of at least two COX isoenzymes has updated our knowledge of NSAIDs [60–63]. The two COX isoenzymes share structural and enzymatic similarities, but are specifically regulated at the molecular level and may be distinguished apart in their functions. Protective prostaglandins, which preserve the integrity of the stomach lining and maintain normal renal function in a compromised kidney, are synthesized by COX-1 [61, 62, 64]. COX-2 is inducible, and the inducing stimuli include pro-inflammatory cytokines and growth factors, which implies a role for COX-2 in both inflammation and control of cell growth. In addition to the induction of COX-2 in inflammatory lesions, it is present constitutively in the brain and spinal cord, where it may be involved in nerve transmission, particularly that for pain and fever. Although the discovery of COX-2 has made possible the design of drugs that reduce inflammation without removing the protective prostaglandins in the stomach and kidney made by COX-1, the growing controversy regarding the potential adverse cardiovascular risks of prolonged use of the COX-2 inhibitors has dampened much of the initial enthusiasm for this drug class [65, 66].
Opioid Drug Selection
The potent analgesic drugs are commonly referred to as “narcotics” (from the Greek “narco”—to deaden), “opiates” (from the Greek “opion”—poppy juice, for drugs derived from the poppy plant), “opioids” (for all drugs with morphine-like effects, whether synthetic or naturally occurring) or, euphemistically, “strong analgesics.” Opioids, the preferred terminology, produce analgesia by binding to G-protein-coupled receptors (μ, κ, and δ) located throughout the central and peripheral nervous system as well as in the gut [67, 68]. The opioids most commonly used in the management of pain are μ agonists and include morphine, meperidine, methadone, codeine, oxycodone, and the fentanyls. Many factors should be considered when deciding which is the appropriate opioid analgesic to administer to a patient in pain. These include pain intensity, patient age, co-existing disease, potential drug interactions, prior treatment history, physician preference, patient preference, and route of administration. The idea that some opioids are “weak” (e.g., codeine) and others are “strong” (e.g., morphine) is outdated. All are capable of treating pain regardless of its intensity if the dose is adjusted appropriately. For the most part, at equipotent doses, opioids have similar effects and side effects, particularly where respiratory depression is concerned. Characteristics of selected μ opioid agonist drugs are listed for quick reference in Table 2.
The use of meperidine requires some additional discussion. An entire generation of physicians believes that meperidine causes less respiratory depression and less biliary spasm than morphine. This was based on a study of postoperative adult patients in which half received 10 mg morphine and the other half 10 mg of meperidine. The meperidine group had less respiratory depression and biliary spasm than morphine. They also had more pain. The equianalgesic dose of meperidine is 100 mg. When the study was repeated with appropriate dosing the investigators found that meperidine had the same side effect profile as morphine [69]. Meperidine has a neurotoxic metabolite, normeperidine, that possesses no analgesic properties and relies on the kidney for its excretion. Normeperidine accumulation causes CNS excitation, resulting in a range of toxic reactions from anxiety and tremors to grand mal seizures.
Commonly Used Intravenous Opioids
Intravenous opioids are the primary drugs used in the treatment of moderate to severe pain. Morphine (from Morpheus, the Greek God of Sleep) is the gold standard for analgesia against which all other opioids are compared. When small doses, 0.1 mg/kg (i.v., i.m., s.c.), are administered to otherwise unmedicated patients in pain, analgesia usually occurs without loss of consciousness. The relief of tension, anxiety, and pain usually may result in drowsiness and sleep as well. Once steady-state pharmacokinetics are achieved, morphine has a duration of action that is approximately 3–4 h. Fentanyl is a shorter acting alternative which has become a favored analgesic for intraoperative anesthesia, and can also be used for patient-controlled analgesia (PCA) and breakthrough pain. Fentanyl is approximately 100 (50–100) times more potent than morphine (the equianalgesic dose is 0.001 mg/kg) and is largely devoid of hypnotic or sedative activity.
Finally, methadone is increasingly being used for postoperative pain relief and for the treatment of intractable pain. Primarily thought of as a drug to treat or wean opioid-addicted or -dependent patients, methadone’s long half-life of elimination and high oral bioavailability provides very long duration of effective analgesia (Table 2). Additionally, methadone is unique in that it is also an NMDA receptor antagonist which makes it useful for treatment of chronic and neuropathic pain and may prevent opioid-induced hyperalgesia.
Commonly Used Oral Opioids
Codeine, oxycodone (the opioid in Tylox® and Percocet®) and hydrocodone (the opioid in Vicodin® and Lortab®) are opioids which are frequently used to treat pain in children and adults, particularly for less severe pain or when patients are being converted from parenteral opioids to enteral ones (Table 2). Oral morphine is commonly used in regimens for chronic pain (e.g., cancer). Codeine, oxycodone, and hydrocodone are most commonly administered in the oral form, usually in combination with acetaminophen or aspirin [70].
In equipotent doses, codeine, oxycodone, hydrocodone, and morphine are equal both as analgesics and respiratory depressants (Table 2). In addition, they share with other opioids common effects on the central nervous system including sedation, respiratory depression, and nausea through stimulation of the chemoreceptor trigger zone in the brain stem. This last attribute of the opioids is particularly true for codeine. There are many fewer problems with nausea and vomiting with oxycodone. Codeine, hydrocodone, and oxycodone have a bioavailability of approximately 60% following oral ingestion. The analgesic effects occur as early as 20 min following ingestion and reach a maximum at 60–120 min. The plasma half-life of elimination is 2.5–4 h.
Codeine is the most popularly prescribed enteral and parenteral opioid in neurosurgical practice and is frequently administered intramuscularly [1]. Although it is an effective analgesic when administered parenterally, intramuscular codeine has no advantage over morphine or any other opioid. If it has any use (and we do not think it does), it is as an oral analgesic. Codeine undergoes nearly complete metabolism in the liver prior to its final excretion in urine. Approximately 10% of codeine is metabolized into morphine (cytochrome P450 2D6) and it is this 10% that is responsible for codeine’s analgesic effect. Interestingly, approximately 10% of the population are “slow” metabolizers of codeine into morphine and, in these patients, codeine will have little analgesic effect. Additionally, 10% of the population are “rapid” metabolizers in whom a “standard” dose may produce excessive sedation and respiratory depression.
Like oxycodone, codeine, and hydrocodone, morphine is also very effective when given orally, but only about 20–30% of an oral dose of morphine reaches the systemic circulation. In the past, this led many to conclude that morphine was ineffective when administered orally. In fact, this was the result of failing to provide sufficient morphine. Therefore, when converting a patient’s intravenous morphine requirement to oral maintenance, one must multiply the intravenous dose by 3–4.
Whereas oral morphine is prescribed alone, oral codeine, hydrocodone, and oxycodone are usually prescribed in combination with either acetaminophen or aspirin (Tylenol® and codeine elixir, Percocet®, Tylox®, Vicodin®, Lortab®). Acetaminophen and aspirin potentiate the analgesia produced by opioids, and permit satisfactory analgesia with less opioid. Typically, codeine is prescribed in a dose of 0.5–1 mg/kg. As stated previously, in all “combination preparations”, beware of inadvertently administering an hepatotoxic acetaminophen dose when increasing opioid doses for uncontrolled pain [71]. Acetaminophen toxicity may result from a single toxic dose, from repeated ingestion of large doses of acetaminophen (e.g., in adults, 7.5–10 g daily for 1–2 days, children 60–420 mg/kg/day for 1–2 days) or from chronic ingestion. Codeine elixirs are available in virtually every pharmacy and contain 120 mg acetaminophen and 12 mg codeine per teaspoon (5 ml) [70]. Codeine and acetaminophen are also available as “numbered” tablets, e.g., Tylenol® number 1, 2, 3, or 4. The number refers to how much codeine is in each tablet. Tylenol® number 4 has 60 mg codeine, number 3 has 30 mg, number 2 has 15 mg, and number 1 has 7.5 mg.
Hydrocodone is prescribed in a dose of 0.05–0.1 mg/kg. The elixir is available as 2.5 mg/5 ml combined with acetaminophen 167 mg/5 ml. As a tablet, it is available in hydrocodone doses between 2.5 and 10 mg, combined with 500–650 mg acetaminophen. Oxycodone is prescribed in a dose of 0.05–0.1 mg/kg. Unfortunately, the elixir is not available in most pharmacies. When it is, it comes either as 1 mg/ml or 20 mg/ml. This can obviously result in catastrophic dispensing errors. In tablet form, oxycodone is commonly available as a 5 mg tablet or as Tylox® (500 mg acetaminophen and 5 mg oxycodone) or Percocet® (325 mg acetaminophen and 5 mg oxycodone).
Oxycodone is also available without acetaminophen in a sustained-release tablet for use in chronic pain. Like many other time-release tablets, it must not be crushed and therefore cannot be administered through a gastric tube since breaking the tablet results in the immediate release of an extremely large amount of oxycodone. Like sustained-release morphine (see below), sustained-release oxycodone is intended only for use in opioid-tolerant patients with chronic pain, and not for routine postoperative pain. Furthermore, in patients with rapid GI transit, sustained-release preparations may not be absorbed at all (liquid methadone may be an alternative).
Oral morphine is available as a liquid in various concentrations (as much as 20 mg/ml), a tablet (such as MSIR, for “morphine sulfate immediate release”; available in 15 and 30 mg tablets), and as a sustained-release preparation (MSContin and Oramorph tablets, and Kadian “sprinkle capsules,” which may be opened and sprinkled on applesauce). Because it is so concentrated, the liquid is particularly easy to administer to severely debilitated patients. Indeed, in terminal patients who cannot swallow, liquid morphine will provide analgesia when simply dropped into the patient’s mouth [70].
Pain Management Adjuvants
Several drugs that are used in chronic and sympathetically mediated pain are increasingly being used in the multimodal management of acute pain. Apart from their ability to limit opioid-induced hyperalgesia as described earlier [39], gabapentin and pregabalin, when given preoperatively, have been shown to decrease postoperative pain and opioid consumption in many surgical procedures [72]. Whether this family of drugs may be useful in patients undergoing intracranial surgery or provides better opioid sparing and/or improved pain relief is presently unknown. As indicated above, the α2-agonist dexmedetomidine is being used for sedation during “awake” craniotomy and in the intensive care unit [47]. The resulting adrenergic modulation of spinal cord activity [73] can reduce subsequent pain and opioid consumption [48]. Clonidine, which is often administered to reduce hypertension, is also an α2-agonist with similar analgesic properties [74]. However, because of their sedative nature, the α2-agonists are unlikely to be used primarily for their analgesic effects when intracranial surgery is the focus. The NMDA antagonists ketamine and dextromethorphan are analgesics with significant preemptive analgesic effects [30, 75, 76] whose use can also limit opioid-induced hyperalgesia [38]. The dissociative nature of ketamine may render it less desirable for use in association with intracranial surgery, and dextromethorphan can produce sedation and other types of CNS symptoms. Tramadol is a nonopioid analgesic which, in contrast to the drugs already described in this section, has actually been evaluated as an analgesic in association with intracranial surgery and is, therefore, described in more detail below.
Tramadol is a synthetic 4-phenyl-piperidine analog of codeine, is a centrally acting synthetic analgesic that has been used for 30 years in Europe and was approved by the FDA for adult use in the U.S. in 1995 [77, 78] It is a racemic mixture of two enantiomers, (+)-tramadol and (−)-tramadol [78, 79]. The (+)-enantiomer has a moderate affinity for the μ-opioid receptor, greater than that of the (−)-enantiomer. In addition, the (+)-enantiomer inhibits serotonin uptake and the (−)-enantiomer blocks the reuptake of norepinephrine, complementary properties which result in a synergistic antinociceptive interaction between the two enantiomers. Tramadol may also produce analgesia as an α2-agonist [80]. A metabolite (O-desmethyltramadol) binds to opioid receptors with a greater affinity than the parent compound and could contribute to tramadol’s analgesic effects as well. However, in most animal tests and human clinical trials, the analgesic effect of tramadol is only partially blocked by the opioid antagonist naloxone, suggesting an important nonopioid mechanism as well.
Tramadol’s intravenous analgesic effect has been reported to be 10–15 times less than that of morphine and is roughly equianalgesic with NSAIDs [78, 81]. Unlike NSAIDs and opioid-mixed agonist/antagonists (e.g., butorphanol, nalbuphine), the therapeutic use of tramadol has not been associated with clinically important side effects such as respiratory depression, constipation, or sedation. In addition, analgesic tolerance has not been a serious problem during repeated administration, and neither psychological dependence nor euphoric effects are observed in long-term clinical trials. Thus, tramadol may offer significant advantages in the management of pain following intracranial surgery by virtue of its dual mechanism of action, its lack of a ceiling effect, and its minimal respiratory depression. Tramadol may be administered orally, rectally, or intravenously [82, 83]. Oral and intravenous tramadol is administered in doses of 1–2 mg/kg; the higher dose provides a longer duration of action without increasing side effects.
Complications and Side Effects
Regardless of the method of administration, all opioids commonly produce unwanted side effects, such as pruritus, nausea and vomiting, constipation, urinary retention, cognitive impairment, tolerance, and dependence [84]. The most common in patients with both acute and chronic opioid administration is bowel dysfunction. Opioid-induced bowel dysfunction (OBD), often described as constipation in patients taking opioids chronically and as postoperative ileus in patients taking opioids acutely, is virtually universal [85, 86]. Historically, opioids were used to treat diarrhea prior to their use as analgesics. Many patients suffer needlessly from pain because they would rather suffer than experience these opioid-induced side effects [87], and because physicians are reluctant to prescribe opioids because of these common side effects and because of their fear of respiratory depression.
Rather than reacting to side effects, we recommend that anticipatory best practice treatment protocols and algorithms be put into practice at the initiation of opioid therapy. These protocols outlined in Fig. 3 and Table 3 are used in our practice. For example, all patients being treated with opioids, even for short periods of time, will become constipated and should be treated with senna or other stool bulking and softeners at the initiation of therapy. Further, several clinical and laboratory studies have demonstrated that low-dose naloxone infusions (0.25–1 mcg/kg/h) can prophylactically treat or prevent opioid-induced side effects without affecting the quality of analgesia or opioid requirements [88]. This was confirmed in a study in children and adolescents, and our institution now routinely initiates a simultaneous low-dose naloxone infusion whenever PCA is initiated in children [89]. Finally, a peripheral opioid antagonist, methylnaltrexone (Relistor®), has recently been approved by the United States Food and Drug Administration and may dramatically alter how we deal with unwanted opioid-induced side effects, particularly OBD.
Additional Considerations
Clearly, patients presenting for intracranial surgery are far from uniform with respect to age, mental capacity, and underlying comorbidities. Pediatric, elderly, and critically ill or injured patients can all differ in their ability to participate in pain assessment and the pharmacokinetics and pharmacodynamics underlying their response to analgesics. Extremes of age, prior or acquired cognitive limitations, and language barriers can interfere with pain assessment and, therefore, analgesic therapy. Since, most pharmacokinetic studies are performed in healthy adult volunteers, those with limited illness or those with stable chronic disease, these data are of limited use in patients at extremes of age or those who are critically ill. These sources of variability can be offset by careful titration of analgesics, something that is possible only if pain can be adequately assessed.
Analgesic therapy in pediatric patients often requires pain assessment tools different from the discrete 0–10 scale so useful with cognitively intact adults, and is further burdened by lingering beliefs that some groups of pediatric patients do not experience pain despite almost two decades of data demonstrating the contrary [90, 91]. Pain assessment in pediatric populations is still possible with appropriate pain assessment tools, the use of which will make clear the capacity of these patients to experience pain. These include additional tools that permit self-report (e.g., cartoon images or colors that represent different pain levels) as well as observational scales based on physiologic variables (e.g., heart rate and blood pressure) and behavioral criteria (e.g., posture and grimacing) [91, 92]. These tools may be useful for any age group where cognitive impairment or language barriers could interfere with the assessment of pain. Unique pharmacokinetic considerations, if any, and specific dosing regimens for pediatric patients are given above and in the associated tables for each of the analgesics discussed. However, even for patients without conditions that could alter pharmacokinetics, pain assessment and analgesic titration are essential. Interestingly, patient-controlled analgesic administration can be used safely and effectively by relatively young patients or their proxies (e.g., family member or nurse) [93].
In elderly patients, analgesic therapy is complicated by differing analgesic requirements, preexisting and postoperative cognitive decline, and altered pharmacokinetics. In this population, perioperative pain assessment may be more difficult due to preexisting or acquired cognitive decline, situations for which observational pain assessment tools have been developed [94]. For reasons yet to be elucidated, elderly patients often perceive acute pain to a lesser extent than younger patients [95, 96], and this is consistent with what has been observed following intracranial surgery [18]. Postoperative delirium is often observed in elderly patients [97], is more likely to occur in those with preexisting cognitive decline, may be exacerbated by opioids, and may also be exacerbated by the pain and stress associated with surgery, perhaps through activation of the glucocorticoid system [98, 99]. The increased proportion of adipose tissue in elderly patients can alter the volume of distribution of the opioids and other analgesics, and decreases in hepatic and renal function can affect their elimination [100].
Analgesic therapy in critically ill patients is fraught with constraints. Analgesic therapy may exacerbate hemodynamic instability or even contribute to it through histamine release when morphine is administered more than a few milligrams at a time. Individual alterations in renal or hepatic function must be considered. The opioids interfere with normal sleep architecture [101, 102] and are though to play the major roll in the decline of restorative sleep known to occur postoperatively [103]. This probably contributes to the disrupted sleep experienced by patients in intensive care settings [104], but opioids should not be withheld because untreated pain can also disrupt normal sleep patterns [105].
Analgesic Therapy Following Intracranial Surgery—Current Practice
A recent survey revealed that intramuscular codeine phosphate is still the primary analgesic in the majority (70%) of centers [36]. Only 13% used morphine as the primary analgesic. Only 4% used PCA. Thus, not only does the traditional teaching regarding the minimal nature of perioperative pain following intracranial surgery persist [1], so does the traditional teaching regarding its management.
Although it now clear that better analgesic therapy is required following intracranial surgery, the available literature on how to accomplish this remains sparse. Certainly, for supratentorial craniotomy using a propofol–remifentanil anesthetic, acetaminophen alone is inadequate [106], but when combined with tramadol or nalbuphine administered on an “as needed” (PRN) basis both combinations were equally effective, resulting in mild pain for the first 24 postoperative hours. Several studies have compared intramuscular codeine phosphate with morphine sulfate. When intramuscular morphine sulfate PRN is compared to intramuscular codeine phosphate PRN, morphine was found to be equally safe with a more sustained period of effectiveness [107]. When intramuscular codeine was compared with PCA morphine administration, there was a slight reduction in pain in the PCA group without any differences in side effects or adverse events [108]. Intramuscular codeine phosphate was also compared with intramuscular tramadol, each administered at the conclusion of surgery, with minimal differences in analgesic effects, but greater side effects in the tramadol group [109]. Oxycodone was administered by PCA to patients following supratentorial craniotomy who received either paracetamol or ketoprofen (an NSAID), with few differences observed and no adverse events related to opioid administration [110]. A recent study sought to demonstrate the safety and efficacy of PCA opioid administration following supratentorial craniotomy [111]. Following a balanced anesthetic technique with fentanyl and a preincisional scalp block with bupivacaine, patients were randomized to receive fentanyl either PRN or via PCA. Patients in the PCA group used significantly more fentanyl and achieved significantly better pain control. Nonetheless, sedation scores and respiratory parameters were identical between the two groups. Collectively, these studies indicate that opioid administration by PCA following supratentorial craniotomy is safe and effective.
Scalp block and local anesthetic infiltration tailored to the given surgical approach can be an important adjunct to a balanced anesthetic technique by reducing intraoperative anesthetic requirements, and can also reduce perioperative pain. When the incision site is infiltrated with a long-acting local anesthetic such as bupivacaine 0.25% some intraoperative hemodynamic responses were blunted and pain in the immediate postoperative period was reduced [24]. When a scalp block (Fig. 1) is performed with a long-acting local anesthetic prior to supratentorial craniotomy, pain throughout the first postoperative day is reduced [18, 23]. Some of this reduction in pain is likely due to a preemptive analgesic effect [30].
Recommendations
The authors currently embrace a multimodal approach that combines the benefits of a balanced anesthetic technique with fentanyl, nitrous oxide, and a volatile anesthetic with a scalp block using bupivacaine 0.5% and placed prior to surgery. If no prior scalp incision has been made, then the scalp block is supplemented by the surgeons with bupivacaine 0.25% at the incision site. Postoperatively, all patients receive acetaminophen at regular intervals (650 mg pr, while initially NPO then 1 g PO q 6 h in an adult). Care is taken to avoid oral opioids in combination with acetaminophen due to the potential for prescribing potentially toxic doses [18]. Patients typically receive fentanyl via PCA with no background infusions, a bolus dose of 0.25–0.50 μg/kg with a lockout of 6 min and up to 10 boluses per hour. If only PRN opiate administration is permitted, then fentanyl 25–50 μg iv q 10 min is suggested for adults. On the first postoperative day patients transition to oral analgesics which include, along with the acetaminophen, oxycodone 5–10 mg q 3–4 h in an adult.
Conclusions
Future studies will be required to determine whether the amount of opioid necessary to keep patients undergoing posterior fossa procedures comfortable can be safely administered by PCA. It would also be beneficial to know whether adjuncts such as gabapentin should be administered prior to craniotomy as is done with other types of surgery [112]. Overall, analgesic therapy for intracranial surgery now stands on a new foundation with data demonstrating the necessity of treating pain in these patients and that, in contrast to traditional teaching, safe and effective analgesic therapy is possible with drugs and techniques commonly used to treat the pain associated with other types of surgical procedures.
References
Stoneham MD, Walters FJ. Post-operative analgesia for craniotomy patients: current attitudes among neuroanaesthetists. Eur J Anaesthesiol. 1995;12(6):571–5.
Cold GE, Felding M. Even small doses of morphine might provoke “luxury perfusion” in the postoperative period after craniotomy. Neurosurgery. 1993;32(2):327.
Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008;101(1):17–24.
Grossman SA, Sheidler VR, Swedeen K, Mucenski J, Piantadosi S. Correlation of patient and caregiver ratings of cancer pain. J Pain Symptom Manage. 1991;6(2):53–7.
Berkow LC, Erdek M, Gottschalk A, Thompson RE, White ED, Yaster M. Pain assessment in adult post-craniotomy patients: a preliminary prospective study. Anesth Analg. 2005;100:S-248.
Eich E, Reeves JL, Jaeger B, Graff-Radford SB. Memory for pain: relation between past and present pain intensity. Pain. 1985;23(4):375–80.
Hunter M, Philips C, Rachman S. Memory for pain. Pain. 1979;6(1):35–46.
Juhl IU, Christensen BV, Bulow HH, Wilbek H, Dreijer NC, Egelund B. Postoperative pain relief, from the patients’ and the nurses’ point of view. Acta Anaesthesiol Scand. 1993;37(4):404–9.
Svensson I, Sjostrom B, Haljamae H. Influence of expectations and actual pain experiences on satisfaction with postoperative pain management. Eur J Pain. 2001;5(2):125–33.
Dawson R, Spross JA, Jablonski ES, Hoyer DR, Sellers DE, Solomon MZ. Probing the paradox of patients’ satisfaction with inadequate pain management. J Pain Symptom Manage. 2002;23(3):211–20.
Durieux ME, Himmelseher S. Pain control after craniotomy: off balance on the tightrope? J Neurosurg. 2007;106:207–9.
De Benedittis G, Lorenzetti A, Migliore M, Spagnoli D, Tiberio F, Villani RM. Postoperative pain in neurosurgery: a pilot study in brain surgery. Neurosurgery. 1996;38(3):466–9.
Quiney N, Cooper R, Stoneham M, Walters F. Pain after craniotomy. A time for reappraisal? Br J Neurosurg. 1996;10(3):295–9.
Talke PO, Gelb AW. Postcraniotomy pain remains a real headache! Eur J Anaesthesiol. 2005;22:324–6.
de Gray LC, Matta BF. Acute and chronic pain following craniotomy: a review. Anaesthesia. 2005;60(7):693–704.
Dunbar PJ, Visco E, Lam AM. Craniotomy procedures are associated with less analgesic requirements than other surgical procedures. Anesth Analg. 1999;88(2):335–40.
Klimek M, Ubben JF, Ammann J, Borner U, Klein J, Verbrugge SJ. Pain in neurosurgically treated patients: a prospective observational study. J Neurosurg. 2006;104(3):350–9.
Gottschalk A, Berkow LC, Stevens RD, et al. A prospective evaluation of pain and analgesic use following major elective intra-cranial surgery. J Neurosurg. 2007;106:210–6.
Thibault M, Girard F, Moumdjian R, Chouinard P, Boudreault D, Ruel M. Craniotomy site influences postoperative pain following neurosurgical procedures: a retrospective study. Can J Anaesth. 2007;54(7):544–8.
Irefin SA, Schubert A, Bloomfield EL, DeBoer GE, Mascha EJ, Ebrahim ZY. The effect of craniotomy location on postoperative pain and nausea. J Anesth. 2003;17(4):227–31.
Koperer H, Deinsberger W, Jodicke A, Boker DK. Postoperative headache after the lateral suboccipital approach: craniotomy versus craniectomy. Minim Invasive Neurosurg. 1999;42(4):175–8.
Schessel DA, Nedzelski JM, Rowed D, Feghali JG. Pain after surgery for acoustic neuroma. Otolaryngol Head Neck Surg. 1992;107(3):424–9.
Nguyen A, Girard F, Boudreault D, et al. Scalp nerve blocks decrease the severity of pain after craniotomy. Anesth Analg. 2001;93(5):1272–6.
Bloomfield EL, Schubert A, Secic M, Barnett G, Shutway F, Ebrahim ZY. The influence of scalp infiltration with bupivacaine on hemodynamics and postoperative pain in adult patients undergoing craniotomy. Anesth Analg. 1998;87(3):579–82.
Leslie K, Troedel S, Irwin K, et al. Quality of recovery from anesthesia in neurosurgical patients. Anesthesiology. 2003;99(5):1158–65.
Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93(4):1123–33.
Kaur A, Selwa L, Fromes G, Ross DA. Persistent headache after supratentorial craniotomy. Neurosurgery. 2000;47(3):633–6.
Hanson MB, Glasscock MEIII, Brandes JL, Jackson CG. Medical treatment of headache after suboccipital acoustic tumor removal. Laryngoscope. 1998;108(8 Pt 1):1111–4.
Ryzenman JM, Pensak ML, Tew JM Jr. Headache: a quality of life analysis in a cohort of 1,657 patients undergoing acoustic neuroma surgery, results from the acoustic neuroma association. Laryngoscope. 2005;115(4):703–11.
Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg. 2005;100(3):757–73.
Gray’s Anatomy. 37th ed. Edinburgh: Churchill Livingstone; 1989. p. 1129.
Feindel W, Penfield W, McNaughton F. The tentorial nerves and localization of intracranial pain in man. Neurology. 1960;10:555–63.
Cavallotti D, Artico M, De SS, Iannetti G, Cavallotti C. Catecholaminergic innervation of the human dura mater involved in headache. Headache. 1998;38(5):352–5.
Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–25.
Swenson JD, Davis JJ, Johnson KB. Postoperative care of the chronic opioid-consuming patient. Anesthesiol Clin North America. 2005;23(1):37–48.
Roberts GC. Post-craniotomy analgesia: current practices in British neurosurgical centres—a survey of post-craniotomy analgesic practices. Eur J Anaesthesiol. 2005;22(5):328–32.
Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570–87.
Celerier E, Rivat C, Jun Y, et al. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92(2):465–72.
Van Elstraete AC, Sitbon P, Mazoit JX, Benhamou D. Gabapentin prevents delayed and long-lasting hyperalgesia induced by fentanyl in rats. Anesthesiology. 2008;108(3):484–94.
Gelb AW, Salevsky F, Chung F, et al. Remifentanil with morphine transitional analgesia shortens neurological recovery compared to fentanyl for supratentorial craniotomy. Can J Anaesth. 2003;50(9):946–52.
Guy J, Hindman BJ, Baker KZ, et al. Comparison of remifentanil and fentanyl in patients undergoing craniotomy for supratentorial space-occupying lesions. Anesthesiology. 1997;86(3):514–24.
Gerlach K, Uhlig T, Huppe M, et al. Remifentanil-propofol versus sufentanil-propofol anaesthesia for supratentorial craniotomy: a randomized trial. Eur J Anaesthesiol. 2003;20(10):813–20.
Hopf HW. Is it time to retire high-concentration nitrous oxide? Anesthesiology. 2007;107(2):200–1.
Mathews DM, Gaba V, Zaku B, Neuman GG. Can remifentanil replace nitrous oxide during anesthesia for ambulatory orthopedic surgery with desflurane and fentanyl? Anesth Analg. 2008;106(1):101–8.
Petersen-Felix S, Luginbuhl M, Schnider TW, Curatolo M, Rendt-Nielsen L, Zbinden AM. Comparison of the analgesic potency of xenon and nitrous oxide in humans evaluated by experimental pain. Br J Anaesth. 1998;81(5):742–7.
Georgiev SK, Kohno T, Ikoma M, Yamakura T, Baba H. Nitrous oxide inhibits glutamatergic transmission in spinal dorsal horn neurons. Pain. 2008;134(1–2):24–31.
Cormack JR, Orme RM, Costello TG. The role of alpha2-agonists in neurosurgery. J Clin Neurosci. 2005;12(4):375–8.
Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth. 2006;53(7):646–52.
Apfel CC, Korttila K, Abdalla M, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350(24):2441–51.
Yaksh TL, Dirig DM, Conway CM, Svensson C, Luo ZD, Isakson PC. The acute antihyperalgesic action of nonsteroidal, anti-inflammatory drugs and release of spinal prostaglandin E2 is mediated by the inhibition of constitutive spinal cyclooxygenase-2 (COX-2) but not COX-1. J Neurosci. 2001;21(16):5847–53.
Harner SG, Beatty CW, Ebersold MJ. Impact of cranioplasty on headache after acoustic neuroma removal. Neurosurgery. 1995;36(6):1097–9.
Ruckenstein MJ, Harris JP, Cueva RA, Prioleau G, Alksne J. Pain subsequent to resection of acoustic neuromas via suboccipital and translabyrinthine approaches. Am J Otol. 1996;17(4):620–4.
van Leeuwen JP, Braspenning JC, Meijer H, Cremers CW. Quality of life after acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1996;105(6):423–30.
Gottschalk A, Ochroch EA. Is preemptive analgesia clinically effective? In: Fleisher L, editor. Evidence-based practice of anesthesia. Philadelphia: Saunders; 2008.
Kelly DJ, Ahmad M, Brull SJ. Preemptive analgesia II: recent advances and current trends [L’analgesie preventive II : progres recents et nouvelle orientation]. Can J Anaesth. 2001;48(11):1091–101.
Kelly DJ, Ahmad M, Brull SJ. Preemptive analgesia I: physiological pathways and pharmacological modalities. Can J Anaesth. 2001;48(10):1000–10.
Abram SE, Yaksh TL. Morphine, but not inhalation anesthesia, blocks post-injury facilitation. The role of preemptive suppression of afferent transmission. Anesthesiology. 1993;78(4):713–21.
Yaster M. Non-steroidal antiinflammatory drugs. In: Yaster M, Krane EJ, Kaplan RF, Cote CJ, Lappe DG, editors. Pediatric pain management and sedation handbook. St. Louis: Mosby Year Book, Inc; 1997. p. 19–28.
Murat I, Baujard C, Foussat C, et al. Tolerance and analgesic efficacy of a new i.v. paracetamol solution in children after inguinal hernia repair. Paediatr Anaesth. 2005;15(8):663–70.
Cashman JN. The mechanisms of action of NSAIDs in analgesia. Drugs. 1996;52 Suppl 5:13–23.
Vane JR, Botting RM. Mechanism of action of aspirin-like drugs. Semin Arthritis Rheum. 1997;26(6 Suppl 1):2–10.
Vane JR, Botting RM. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104(3A):2S–8S. discussion 21S–2.
Jouzeau JY, Terlain B, Abid A, Nedelec E, Netter P. Cyclo-oxygenase isoenzymes. How recent findings affect thinking about nonsteroidal anti-inflammatory drugs. Drugs. 1997;53(4):563–82.
Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120.
Levesque LE, Brophy JM, Zhang B. The risk for myocardial infarction with cyclooxygenase-2 inhibitors: a population study of elderly adults. Ann Intern Med. 2005;142(7):481–9.
Johnsen SP, Larsson H, Tarone RE, et al. Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: a population-based case-control study. Arch Intern Med. 2005;165(9):978–84.
Snyder SH, Pasternak GW. Historical review: opioid receptors. Trends Pharmacol Sci. 2003;24(4):198–205.
Standifer KM, Pasternak GW. G proteins and opioid receptor-mediated signalling. Cell Signal. 1997;9(3–4):237–48.
Radnay PA, Duncalf D, Novakovic M, Lesser ML. Common bile duct pressure changes after fentanyl, morphine, meperidine, butorphanol, and naloxone. Anesth Analg. 1984;63(4):441–4.
Krane EJ, Yaster M, Yaster M. Transition to less invasive therapy. In: Yaster M, Krane EJ, Kaplan RF, Cote CJ, Lappe DG, editors. Pediatric pain management and sedation handbook. St. Louis: Mosby Year Book, Inc; 1997. p. 147–62.
Heubi JE, Barbacci MB, Zimmerman HJ. Therapeutic misadventures with acetaminophen: hepatoxicity after multiple doses in children [see comments]. J Pediatr. 1998;132(1):22–7.
Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol. 2007;20(5):456–72.
Pertovaara A. Antinociception induced by alpha-2-adrenoceptor agonists, with special emphasis on medetomidine studies. Prog Neurobiol. 1993;40(6):691–709.
Boyd RE. Alpha2-adrenergic receptor agonists as analgesics. Curr Top Med Chem. 2001;1(3):193–7.
Himmelseher S, Durieux ME. Ketamine for perioperative pain management. Anesthesiology. 2005;102(1):211–20.
Siu A, Drachtman R. Dextromethorphan: a review of N-methyl-d-aspartate receptor antagonist in the management of pain. CNS Drug Rev. 2007;13(1):96–106.
Minto CF, Power I. New opioid analgesics: an update. Int Anesthesiol Clin. 1997;35(2):49–65.
Raffa RB. A novel approach to the pharmacology of analgesics. Am J Med. 1996;101(1A):40S–46S.
Raffa RB, Friderichs E, Reimann W, et al. Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J Pharmacol Exp Ther. 1993;267(1):331–40.
Desmeules JA, Piguet V, Collart L, Dayer P. Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br J Clin Pharmacol. 1996;41(1):7–12.
Naguib M, Seraj M, Attia M, Samarkandi AH, Seet M, Jaroudi R. Perioperative antinociceptive effects of tramadol. A prospective, randomized, double-blind comparison with morphine. Can J Anaesth. 1998;45(12):1168–75.
Bozkurt P. Use of tramadol in children. Paediatr Anaesth. 2005;15(12):1041–7.
Gunes Y, Gunduz M, Unlugenc H, Ozalevli M, Ozcengiz D. Comparison of caudal vs intravenous tramadol administered either preoperatively or postoperatively for pain relief in boys. Paediatr Anaesth. 2004;14(4):324–8.
Yaster M, Kost-Byerly S, Maxwell LG. Opiod agonists and antagonists. In: Schechter NL, Berde CB, Yaster M, editors. Pain in infants, children, and adolescents. Philadelphia: Lippincott Williams and Wilkins; 2003. p. 181–224.
Fallon MT, Hanks GW. Morphine, constipation and performance status in advanced cancer patients. Palliat Med. 1999;13(2):159–60.
Glare P, Lickiss JN. Unrecognized constipation in patients with advanced cancer: a recipe for therapeutic disaster. J Pain Symptom Manage. 1992;7(6):369–71.
Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology treatment, and prevention. Anesthesiology. 1992;77(1):162–84.
Gan TJ, Ginsberg B, Glass PS, Fortney J, Jhaveri R, Perno R. Opioid-sparing effects of a low-dose infusion of naloxone in patient-administered morphine sulfate. Anesthesiology. 1997;87(5):1075–81.
Maxwell LG, Kaufmann SC, Bitzer S, et al. The effects of a small-dose naloxone infusion on opioid-induced side effects and analgesia in children and adolescents treated with intravenous patient-controlled analgesia: a double-blind, prospective, randomized, controlled study. Anesth Analg. 2005;100(4):953–8.
Maxwell LG, Yaster M. Perioperative management issues in pediatric patients. Anesthesiol Clin North America. 2000;18(3):601–32.
American Academy of Pediatrics. Committee on Psychosocial Aspects of Child and Family Health; Task Force on Pain in Infants, Children, and Adolescents. The assessment and management of acute pain in infants, children, and adolescents. Pediatrics. 2001;108(3):793–7.
McGrath PA. An assessment of children’s pain: a review of behavioral, physiological and direct scaling techniques. Pain. 1987;31(2):147–76.
Monitto CL, Greenberg RS, Kost-Byerly S, et al. The safety and efficacy of parent-/nurse-controlled analgesia in patients less than six years of age. Anesth Analg. 2000;91(3):573–9.
Murdoch J, Larsen D. Assessing pain in cognitively impaired older adults. Nurs Stand. 2004;18(38):33–9.
Li SF, Greenwald PW, Gennis P, Bijur PE, Gallagher EJ. Effect of age on acute pain perception of a standardized stimulus in the emergency department. Ann Emerg Med. 2001;38(6):644–7.
Gagliese L, Katz J. Age differences in postoperative pain are scale dependent: a comparison of measures of pain intensity and quality in younger and older surgical patients. Pain. 2003;103(1–2):11–20.
Bryson GL, Wyand A. Evidence-based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction. Can J Anaesth. 2006;53(7):669–77.
Gaudreau JD, Gagnon P. Psychotogenic drugs and delirium pathogenesis: the central role of the thalamus. Med Hypotheses. 2005;64(3):471–5.
Brown TM. Drug-induced delirium. Semin Clin Neuropsychiatry. 2000;5(2):113–24.
ElDesoky ES. Pharmacokinetic-pharmacodynamic crisis in the elderly. Am J Ther. 2007;14(5):488–98.
Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3(1):33–6.
Shaw IR, Lavigne G, Mayer P, Choiniere M. Acute intravenous administration of morphine perturbs sleep architecture in healthy pain-free young adults: a preliminary study. Sleep. 2005;28(6):677–82.
Knill RL, Moote CA, Skinner MI, Rose EA. Anesthesia with abdominal surgery leads to intense REM sleep during the first postoperative week. Anesthesiology. 1990;73(1):52–61.
Friese RS, Az-Arrastia R, McBride D, Frankel H, Gentilello LM. Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping? J Trauma. 2007;63(6):1210–4.
Onen SH, Onen F, Courpron P, Dubray C. How pain and analgesics disturb sleep. Clin J Pain. 2005;21(5):422–31.
Verchere E, Grenier B, Mesli A, Siao D, Sesay M, Maurette P. Postoperative pain management after supratentorial craniotomy. J Neurosurg Anesthesiol. 2002;14(2):96–101.
Goldsack C, Scuplak SM, Smith M. A double-blind comparison of codeine and morphine for postoperative analgesia following intracranial surgery. Anaesthesia. 1996;51(11):1029–32.
Stoneham MD, Cooper R, Quiney NF, Walters FJ. Pain following craniotomy: a preliminary study comparing PCA morphine with intramuscular codeine phosphate. Anaesthesia. 1996;51(12):1176–8.
Jeffrey HM, Charlton P, Mellor DJ, Moss E, Vucevic M. Analgesia after intracranial surgery: a double-blind, prospective comparison of codeine and tramadol. Br J Anaesth. 1999;83(2):245–9.
Tanskanen P, Kytta J, Randell T. Patient-controlled analgesia with oxycodone in the treatment of postcraniotomy pain. Acta Anaesthesiol Scand. 1999;43(1):42–5.
Morad A, Winters BD, Yaster M, et al. Intravenous patient controlled analgesia safely and effectively treats pain after supratentorial intracranial surgery: a prospective randomized controlled trial. J Neurosurg. 2008; Accepted for publication.
Mathiesen O, Moiniche S, Dahl JB. Gabapentin and postoperative pain: a qualitative and quantitative systematic review, with focus on procedure. BMC Anesthesiol. 2007;7:6.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Gottschalk and M. Yaster are without conflict with respect to the interventions described in this review.
Rights and permissions
About this article
Cite this article
Gottschalk, A., Yaster, M. The Perioperative Management of Pain from Intracranial Surgery. Neurocrit Care 10, 387–402 (2009). https://doi.org/10.1007/s12028-008-9150-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-008-9150-3