Abstract
Analgesic therapy following intracranial procedures remains a source of concern. For many years, it was assumed that there was minimal pain following a craniotomy. However, several surveys have shown that postoperative pain in these patients is more common than generally assumed. The study of post-craniotomy pain can be challenging because of several variables such as intraoperative opioids, subjectivity of pain assessment techniques and the patient’s neurological status. The analgesic of choice in neurosurgical units was codeine phosphate for many years due to the concern that potent opioids will adversely affect neurological status.
However, many high-quality trials have shown that patient-controlled analgesia with morphine, oxycodone or fentanyl can be used to provide effective pain control with not many adverse effects. These techniques have been used as part of multimodal analgesia along with paracetamol, regional scalp blocks and/or tramadol. Adequate pain control not only improves patient comfort but can also reduce the incidence of chronic headache following intracranial surgery.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
It had been a common belief in the past that patients undergoing craniotomy experience minimal pain in the postoperative period. This probably goes back to a study conducted in the 1970s [1]. However, several surveys in the last two decades have shown that many patients suffer from moderate to severe pain following craniotomy. Pain following craniotomy is common. De Benedittis et al. from the Institute of Neurosurgery in Milan assessed important pain variables in 37 consecutive patients who underwent various brain neurosurgical procedures [2]. Pain intensity was recorded regularly over 48 h following surgery. Postoperative pain was more common than generally assumed (60%), and the intensity was moderate to severe. Pain was predominantly superficial (86%) suggesting that it was somatic rather than visceral in origin. Subtemporal and suboccipital surgical routes yielded the highest incidence of postoperative pain. That site of surgery as an important variable was shown by Thibault et al. in a retrospective study of 299 patients, designed to assess the intensity of postoperative pain in relation to the location of craniotomy [3]. Frontal craniotomy was associated with the lower pain scores than those who underwent posterior fossa craniotomy and required significantly less opioid analgesics.
Post-operative pain management following intracranial surgery has not been well studied and there is a lack of good evidence-based guidelines to provide appropriate postoperative analgesia protocols for these neurosurgical patients. The study of post-craniotomy pain can be challenging because of several variables such as intraoperative opioids, subjectivity of pain assessment techniques and primarily the patient’s neurological status. Opioid administration after major intracranial surgery is limited by both a presumed lack of need and a concern that opioids will adversely affect the postoperative neurological status. This attitude can be seen in the surveys of post-craniotomy analgesic practices in neurosurgical centres. In a 1996 survey, Stoneham and Walters sent a postal questionnaire to 183 consultant members of Neuroanaesthesia Society of Great Britain [4]. They received responses from 110 neuroanaesthetists from 37 different centres. Intramuscular (IM) codeine phosphate or dihydrocodeine was the mainstay of postoperative analgesia for 97% of neuroanaesthetists despite the fact that over half of them thought that analgesia was inadequate. Only four of them would consider using stronger opioids because of fears of respiratory depression and sedation, yet all except one used opioids intraoperatively. Postoperative analgesia was perceived to be inadequate, yet traditional prejudice against opioids prevented its use.
Ten years later, another survey of current practices in British neurosurgical centres showed not much difference in the way pain was addressed following craniotomy [5]. A postal questionnaire was sent to every neurosurgical unit within the UK enquiring about the current, standard analgesic practices following craniotomy. The response rate was 70%. Intramuscular codeine phosphate, a weak opioid, was found to be the principal first-line analgesic post-craniotomy. Only three centres used morphine as the first-line analgesic and only one centre used patient-controlled analgesia (PCA). This survey demonstrates that codeine phosphate continues to be the first-line analgesic of choice, at least in the UK. Codeine is a weak opioid and only 5–15% is converted to morphine. The enzyme that catalyses the demethylation of codeine to morphine exhibits genetic polymorphism resulting in about 15% of Caucasians experiencing no analgesic effect with it [6]. In addition, analgesics were prescribed regularly in only half the units surveyed.
In a more recent survey of Canadian neurosurgeons, with regard to pain management in post-craniotomy patients, codeine was also the most prescribed first-line analgesic (59%) followed by morphine (38%) [7]. The use of a second-line opioid was significantly higher among codeine prescribers compared to morphine. The majority of respondents reported a high level of satisfaction with their current choice of analgesia; they predominantly described their practice as personal preference or protocol driven rather than evidence based.
2 Treatment of Acute Postoperative Pain After Intracranial Surgery
2.1 Opioids and Patient-Controlled Analgesia
With the increasing realization that patients were suffering from inadequate analgesia following craniotomy, there have been several studies in the last two decades to improve it, by including opioids as first line in pain management. In an early study, nearly 20 years ago, Stoneham et al. compared patient-controlled analgesia (PCA) with morphine with the traditional intramuscular (IM) codeine phosphate [8]. In a prospective randomized trial of 30 patients, they compared PCA morphine, 1 mg bolus with 10 min lockout and no background infusion with IM codeine. There was a wide variation in the amounts of morphine requested in the PCA group with some reduction in pain scores. There were no significant differences between the two groups with respect to nausea and vomiting, sedation scores and respiratory rate and no major adverse effects in either group. They concluded that PCA morphine can be an alternative to IM codeine. Tanskanen and group in Finland evaluated the feasibility and safety of PCA with oxycodone in neurosurgical patients and compared the efficacy of paracetamol with ketoprofen [9]. In a group of 45 patients who were randomized to receive either paracetamol 1000 mg or ketoprofen 100 mg three times, all patients were allowed to use PCA oxycodone boluses of 0.03 mg/kg with a maximum of three times an hour with a lockout time of 10 min. The ketoprofen group required less oxycodone with comparable pain scores and both groups were satisfied with pain relief. There was no progressive hypoventilation, desaturation or excessive sedation with the use of oxycodone.
The above two studies showed that PCA morphine/oxycodone did not produce any adverse effects. However, the effect on arterial CO2 levels was not known. Hence, Sudheer and colleagues compared the analgesic efficacy and respiratory effects of morphine, tramadol and codeine after craniotomy [10]. Sixty patients were randomly allocated to receive morphine PCA, tramadol PCA or codeine phosphate 60 mg intramuscularly following craniotomy. Baseline values of pain and sedation scores and arterial CO2 tension were recorded at the time of first analgesic administration and at 30 min, 1, 4, 8, 12, 18 and 24 h. Patient satisfaction was assessed at 24 h. There were no differences in PaCO2 or sedation scores between groups at any time, but in all three groups, some patients had increased PaCO2 levels greater than 1 kPa. Morphine produced significantly better analgesia than tramadol at all time points (p < 0.005) and better analgesia than codeine at 4, 12 and 18 h. Patients were more satisfied with morphine than codeine and tramadol (p < 0.001). In addition, vomiting and retching was noted in 50% of patients on tramadol. Tramadol has been advocated by some due to its dual mechanism of action and less incidence of sedation, but it showed that it had a higher incidence of vomiting, which is not suitable for patients following craniotomy.
Despite this, tramadol continued to be evaluated for postoperative pain management. Rahimi et al. conducted a randomized, blinded prospective study to evaluate the efficacy of tramadol as an alternative strategy for post-craniotomy pain management [11]. They found the group of patients assigned to tramadol and opioids had better pain control, reduced length of hospital stay than the group assigned to paracetamol and opioids. Besides morphine, the shorter-acting fentanyl has been used recently to evaluate its efficacy for neurosurgical patients who have undergone intracranial surgery. Morad et al. hypothesized that intravenous PCA would safely and more effectively treat postoperative craniotomy pain than conventional as needed (PRN) therapy [12]. Following a standardized course of general anaesthesia, adult patients who underwent elective supratentorial intracranial surgery were randomized to receive either PRN intravenous fentanyl 5–50 μg every 30 min or PCA fentanyl 0.5 μg/kg every 15 min (maximum 50 μg fentanyl/dose; four doses per hour). The authors measured pain (self-reported pain scores), sedation (Ramsay Sedation Scale scores), Glasgow Coma Scale scores, fentanyl use and major adverse events (excessive sedation, respiratory rate, nausea, vomiting hourly). Sixty-four patients with a mean age of 48 years were randomized to receive PCA or PRN fentanyl. Patients receiving PCA had significantly lower pain scores than those receiving IV, PRN fentanyl. They also received significantly more fentanyl than the PRN group. There were no differences between the two groups regarding the number of patients with adverse events. They concluded that IV PCA with fentanyl was an effective method of pain relief.
Following their success, this same group studied patients who underwent posterior fossa surgery, which often produces more intense postoperative pain [13]. They therefore designed a prospective, randomized controlled trial, with a 1:1 allocation ratio to evaluate whether IV PCA would lead to reductions in postoperative pain when compared to nurse-administered PRN therapy. Eighty patients were randomized to two arms. One group to receive PCA fentanyl 0.5 μg/kg/dose with a maximal dose limit of 50 μg and a 15-min lockout interval, while the other group received fentanyl 25–50 μg every 30 min, PRN. Patients in the PCA group reported less pain at rest (p = 0.003) and received more fentanyl than the PRN group (p = 0.002). There were no differences in side effects and no adverse effects related to analgesic therapy. All patients were also treated with paracetamol, local anaesthetic nerve blockade and anti-inflammatory steroids as well as opioids. The limitation of this study is that it was done at a single centre with a dedicated critical care unit, and it was not specifically designed to access the safety of IV PCA.
2.2 Paracetamol
Paracetamol is used regularly as part of multimodal analgesia following craniotomy to reduce opioid requirements. Paracetamol alone is not adequate to control postoperative pain in these patients. Verchere et al. randomized patients into three groups: one group was given paracetamol, nalbuphine was added to the second group and tramadol was added to the third group [14]. Inclusions into the paracetamol group were stopped after eight patients, as pain relief was insufficient with paracetamol alone. Addition of either nalbuphine or tramadol was deemed to be necessary to achieve adequate analgesia. Paracetamol continues to be part of multimodal analgesia and one needs to be careful not to exceed 4 g/day.
2.3 Coxibs/Non-selective NSAIDs
The addition of ketoprofen was shown to be more effective than paracetamol in reducing PCA opioids following surgery [9]. Diclofenac sodium has also been used to improve pain relief and improve patient comfort after major intracranial surgery [15]. There is, however, a concern about using non-selective NSAIDs following neurosurgery as the cyclooxygenase enzyme-1 (COX-1) inhibitor component of the drug can lead to intracranial bleeding. In a large, single-centre, retrospective cohort study of 6668 cases over 5 years, there was an association between the development of postoperative haematoma and the use of aspirin or non-selective NSAIDs, which the authors concluded was an avoidable risk factor in 75% of the cases [16].
With the availability of the COX-2 selective drugs (coxibs), there was renewed enthusiasm to use them to reduce pain following surgery. However, a single dose of parecoxib did not show any benefit over placebo in the first 24 h regarding pain scores, morphine consumption or analgesia-related adverse effects in one study [17], when scalp infiltration, paracetamol and morphine was used in both groups. In an earlier study, Jones and colleagues from Melbourne administered a single dose of parecoxib 40 mg at dural closure and only found some pain reduction at 6 and 12 h with a only a modest impact on overall postoperative analgesia [18]. Perhaps a single dose is not adequate, and it may need to be repeated at 12 h for better efficacy.
2.4 Scalp Infiltration with Local Anaesthetics (Regional Scalp Blocks)
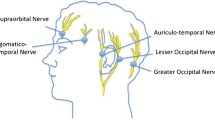
In efforts to reduce the need for opioids in the postoperative period, regional scalp blocks have been tried to improve pain relief. Most neurosurgeons routinely infiltrate the scalp prior to incision. Scalp blocks have been used extensively as it is intuitive that they will be found to be useful. Guilfoyle et al. recently published a systematic review and meta-analysis on regional scalp block (RSB) for post-craniotomy analgesia [19]. They identified seven high-quality RCTs which met their criteria with a total recruitment of 320 patients. All studies used standard local anaesthetic drugs (lignocaine, bupivacaine, ropivacaine), with three studies combining LA with adrenaline. RSB was done preoperatively in three studies and after wound closure in four studies. Meta-analysis found a pooled reduction on pain scores at 1 h. Both pre-and post-groups showed significant reductions in pain scores at 2, 4, 6, 8 and 12 h with an overall reduction in opioid requirements in the first 24 h. There were no complications attributable to RSB. This meta-analysis has confirmed the standard protocols in most neurosurgical units to include RSB as part of multimodal analgesia.
In a more recent study, Hwang et al. showed that inclusion of scalp blocks with levobupivacaine improved the recovery profile of patients undergoing aneurysm clipping [20]. In this study, 52 patients scheduled for elective frontoparietal craniotomy for unruptured aneurysm clipping were randomized to receive scalp blocks with either normal saline or levobupivacaine 0.75%. Postoperative pain scores and PCA consumption were recorded for 72 h. The time from patient recovery to first use of PCA, requirements for vasoactive drugs and adverse effects of PCA and LA were recorded. Scalp blocks lowered postoperative pain and PCA consumption without severe adverse events and reduced the requirements for antihypertensive agents. Besides RSB, superficial cervical plexus blocks have been successfully used as transitional analgesia for infratentorial and occipital craniotomy [21].
3 Post-craniotomy Pain in Children
Children from infancy to adolescents need intracranial surgery for a variety of causes which include tumours, epilepsy, vascular malformations and craniofacial reconstruction. To evaluate the incidence of pain after craniotomy in children, a multicentre observational study was conducted in nine Italian hospitals [22]. After IRB approval, 213 infants and children <10 years old undergoing major craniotomy were enrolled. Pain intensity, analgesic therapy and adverse effects were evaluated for the first 2 days. Moderate to severe pain was defined as a median FLACC or NRS score ≥4; severe pain was defined as a median FLACC or NRS score of ≥7. The overall postoperative median FLACC/NRS score was 1. Twenty-one children (16%) presented with moderate to severe pain in the recovery room and 14(6%) on the first and second days after surgery. Rectal codeine was the weak opioid used, while remifentanil and morphine were widely used in the paediatric ICU. They concluded that children receiving multimodal analgesia experienced minimum pain, while longer surgical procedures correlated with increased risk of having postoperative pain.
In another prospective observational cohort study, 200 children undergoing intracranial surgery in three academic children’s hospitals were evaluated to determine the incidence of pain, prescribed analgesics, method of analgesic delivery and patient/parent satisfaction [23]. Neither intraoperative anaesthetic management nor postoperative pain management was standardized. The age of the children ranged from 2 months to 18.5 years with an average of 7.8 ± 5.8 years. Despite considerable variation in mode and route of administration, there were no differences in average pain score, length of hospital stay and parental satisfaction with care. Parenteral opioids were used along with paracetamol and this was changed to oral oxycodone and/or paracetamol once oral intake was allowed. The most common opioid-induced side effect was vomiting, and it did not relate to the total daily opioid consumption.
The safety and efficacy of continuous morphine infusions following paediatric cranial surgery was reported from a hospital in British Columbia, Canada [24]. The medical records of 71 children were retrospectively reviewed. The outcome measures included pain control and adverse events. Thirty-seven children received continuous morphine infusion and 34 received paracetamol and codeine. There was no statistical difference in pain control between the two groups, but there was a significant increase in nausea in the morphine group. They recommend the use of continuous morphine infusion if pain is poorly controlled with non-opioid analgesics.
In the absence of trials comparing different analgesic regimes in children, these observational cohort studies indicate that multimodal analgesia including potent opioids can be safely used in the setting of high dependency units, in which these children will be nursed in the postoperative period.
4 Chronic Pain Following Craniotomy
Following intracranial surgery, patients not only have acute pain lasting several days but can develop chronic post-craniotomy headaches. One of the risk factors for the development of chronic pain is unrelieved acute pain [25]. Diagnostic criteria according to the International Headache Society is that it is of variable intensity, maximum in the area of the craniotomy performed for other than head trauma, occurs within 7 days and persists >3 months after craniotomy [26]. Batoz et al. conducted a prospective single-blinded study to evaluate if scalp infiltration with local anaesthetic will reduce postoperative pain [27]. Fifty-two patients were enrolled and half of them received infiltration of the surgical site with 20 mL of 0.75% ropivacaine at the end of surgery. The VAS pain scores were significantly higher in the control group with a trend towards lower consumption of nalbuphine in the infiltration group. In addition, 2 months post-surgery, persistent pain was significantly lower than in the control group (p = 0.0003). Scalp infiltration may be relevant for the rehabilitation of neurosurgical patients and their quality of life by limiting the development of persistent pain.
Conclusions
Postoperative pain following intracranial surgery is an area of clinical concern that is receiving increasing attention in the last decade. Studies show that craniotomy leads to significant pain in the early postoperative period. There have been no large-scale studies to definitively draw up guidelines and protocols for pain management in this group of patients. However, the judicious use of opioids (morphine/fentanyl), along with non-opioid analgesics such as paracetamol/coxibs and regional scalp blocks, have all provided effective pain relief after craniotomy. Unrelieved acute pain after intracranial surgery can lead to chronic persistent headaches, which can diminish the quality of life.
References
Geevarghese K. Postoperative care of the patient undergoing neurological surgery. Int Anaesthesiol Clin. 1977;15:309–20.
De Benedittis G, Lorenzetti A, Migliore M, Spagnoli D, Tiberio F, Villani R. Postoperative pain in neurosurgery: a pilot study in brain surgery. Neurosurgery. 1996;38(3):466–70.
Thibault M, Girard F, Moumdjian R, Chouinard P, Boudreault D, Ruel M. Craniotomy site influences postoperative pain following neurosurgical procedures: a retrospective study. Can J Anaesth. 2007;54(7):544–8.
Stoneham MD, Walters FJ. Post-operative analgesia for craniotomy patients: current attitudes among neuroanaesthetists. Eur J Anaesthesiol. 1996;12(6):571–5.
Roberts GC. Post-craniotomy analgesia: current practices in British neurosurgical centres – a survey of post-craniotomy analgesic practices. Eur J Anaesthesiol. 2005;22:328–32.
Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, Haider CE, et al. Clinical pharmacogenetics implementation consortium (CPIC) guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther. 2014;95(4):376–82.
Hassouneh B, Centofanti JE, Reddy K. Pain management in post-craniotomy patients: a survey of Canadian neurosurgeons. Can J Neurol Sci. 2011;38(3):456–60.
Stoneham MD, Cooper R, Quiney NF, Walters FJM. Pain following craniotomy: a preliminary study comparing PCA morphine with intramuscular codeine phosphate. Anaesthesia. 1996;51:1176–8.
Transkanen P, Kytta J, Randell T. Patient-controlled analgesia with oxycodone in the treatment of post craniotomy pain. Acta Anaesthesiol Scand. 1999;43(1):42–5.
Sudheer PS, Logan SW, Terblanche C, Ateleanu B, Hall JE. Comparison of the analgesic efficacy and respiratory effects of morphine, tramadol and codeine after craniotomy. Anaesthesia. 2007;62(6):555–60.
Rahimi SY, Alleyne CH, Vernier E, Witcher MR, Vender JR. Postoperative pain management with tramadol after craniotomy: evaluation and cost analysis. J Neurosurg. 2010;112(2):268–72.
Morad AH, Winters BD, Yaster M, Stevens RD, White ED, Thompson RE, Weingart JD, Gottschalk A. Efficacy of intravenous patient-controlled analgesia after supratentorial intracranial surgery: a prospective randomized controlled trial. J Neurosurg. 2009;111(2):343–50.
Morad A, Winters B, Stevens R, White E, Weingart J, Yaster M, Gottschalk A. The efficacy of intravenous patient-controlled analgesia after intracranial surgery of the posterior fossa: a prospective, randomized controlled trial. Anesth Analg. 2012;114:416–23.
Verchere E, Grenier B, Mesli A, Siao D, Sesay M, Maurette P. Postoperative pain management after supratentorial craniotomy. J Neurosurg Anesthesiol. 2002;14(2):96–101.
Gunes Y, Unlugenc H, Yilmaz D, Ozcengiz D. Management of acute craniotomy pain; the analgesic effect of diclofenac sodium-tramadol or paracetamol-tramadol. Neurosurg Quarterly. 2011;21(4):236–9.
Palmer JD, Sparrow OC, Iannotti F. Postoperative hematoma: a 5-year survey and identification of avoidable risk factors. Neurosurgery. 1994;35(6):1061–4.
Williams DL, Pemberton E, Leslie K. Effect of intravenous parecoxib on post-craniotomy pain. Br J Anaesth. 2011;107(3):398–403.
Jones SJ, Cormack J, Murphy MA, Scott DA. Parecoxib for analgesia after craniotomy. Br J Anaesth. 2009;102(1):76–9.
Guilfoyle MR, Helmy A, Duane D, Hutchinson PJ. Regional scalp block for postcraniotomy analgesia: a systematic review and meta-analysis. Anesth Analg. 2013;116(5):1093–102.
Hwang JY, Bang JS, Oh CW, Joo JD, Park SJ, Do SH, Yoo YJ, Ryu JH. Effect of scalp blocks with levobupivacaine on recovery profiles after craniotomy for aneurysm clipping: a randomized, double-blind, and controlled study. World Neurosurg. 2015;83(1):108–13.
Girard F, Quentin C, Charbonneau S, Ayoub C, Boudreault D, Chouinard P, Ruel M, Moumdijian R. Superficial cervical plexus block for transitional analgesia in infratentorial and occipital craniotomy: a randomized trial. Can J Anaesth. 2010;57(12):1065–70.
Bronco A, Pietrini D, Lamperti M, et al. Incidence of pain after craniotomy in children. Paediatr Anaesth. 2014;24(7):781–7.
Maxwell LG, Buckley GM, Kudchadkar SR, Ely E, Stebbins EL, Dube C, Morad A, et al. Pain management following major intracranial surgery in pediatric patients: a prospective cohort study in three academic children’s hospitals. Paediatr Anaesth. 2014;24(11):1132–40.
Warren DT, Bowen-Roberts T, Ou C, Purdy R, Steinbok P. Safety and efficacy of continuous morphine infusions following pediatric cranial surgery in a surgical ward setting. Childs Nerv Syst. 2010;26(11):1535–41.
De Gray LC, Matta BF. Acute and chronic pain following craniotomy. A review. Anaesthesia. 2005;60(7):693–704.
Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache disorders. 2nd ed. Cephalalgia. 2004;24(Suppl 1):9–160.
Batoz H, Verdonck O, Roux G, Maurette P. The analgesic properties of scalp infiltrations with ropivacaine after intracranial tumoral resection. Anesth Analg. 2009;109(7):240–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Vijayan, R., San, L.P. (2017). Postoperative Pain Management After Craniotomy. In: Khan, Z. (eds) Challenging Topics in Neuroanesthesia and Neurocritical Care. Springer, Cham. https://doi.org/10.1007/978-3-319-41445-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-41445-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-41443-0
Online ISBN: 978-3-319-41445-4
eBook Packages: MedicineMedicine (R0)