Abstract
Introduction
Hypothermic brain protection has been linked to how rapidly cooling is initiated and how quickly and uniformly the therapeutic hypothermic zone (THZ) is reached. The nasopharyngeal (NP) approach is uniquely suited for preferential brain cooling due to anatomic proximity to the cerebral circulation, cavernous sinus, and carotid arteries. This study explores a novel NP cooling approach employing evaporative characteristics of aerosolized perfluorochemical (PFC).
Methods
Anesthetized, normotensive sheep (n = 30) were instrumented with temperature probes and vascular catheters, then randomized to NP approach (NP-PFC: PFC spray device; n = 24) or whole body surface (WBS: n = 6) cooling. Regional temperatures, vital signs, and blood chemistries were assessed serially. Two animals were exposed to double PFC flow rates and PFC was measured in blood during NP-PFC cooling to assess PFC uptake and elimination. Cooling rates were evaluated (ANOVA) as a function of method (NP-PFC versus WBS) and time to reach the brain THZ (i.e., ≤−3.5°C below baseline).
Results
Independent of region, brain cooling was faster during NP-PFC versus WBS (P < 0.001). During NP-PFC, brain > vascular > rectal cooling rates (P < 0.001), brain to systemic temperature gradients were maintained, the brain THZ was reached within 15 min, and the NP epithelial surface appeared histologically intact. During WBS, brain versus systemic cooling rates were not significantly different and the brain THZ could not be reached within 2 h.
Conclusions
The NP-PFC procedure more rapidly induced preferential brain cooling as compared to WBC without adverse effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The outcome of patients with neurological emergencies is largely determined by the extent of traumatic or hypoxic–ischemic cerebral injury. The duration and severity of global cerebral ischemia and secondary mechanisms of ischemia related to reperfusion contribute to the permanent extent of cerebral injury [1]. Recently, two independent randomized clinical trials have shown that lowering core temperature to 33°C for 12 or 24 h results in improved neurological outcome of comatose survivors of out-of-hospital cardiac arrest [2, 3]. Other reports on therapeutic cooling in traumatic brain injury, stroke and intracranial hemorrhage are encouraging but are less compelling possibly related to logistics of implementation, delays in initiation of cooling, adverse effects of therapeutic cooling, as well as differences in the pathophysiology of the primary insult [4].
The neuroprotective effect of cooling has been linked to how rapidly cooling is initiated, how quickly the brain is cooled, the etiology of injury, and the extent of tissue that reaches the therapeutic hypothermic zone (THZ) [5–10]. Whole body surface (WBS) devices, head caps, and intravascular methods for brain cooling are encumbered by equipment, slow response, systemic hypothermia, and concomitant systemic adverse effects [11, 12]. Preferential head cooling by helmet devices show promise in neonates; however, this approach in adults has been less encouraging [8, 13–15]. Cooling blood with intravenous catheters has gained interest because of its potential for long-term use [16, 17]. Extracorporeal cooling strategies have been devised as simple veno-venous cooling or coolant infusion systems, or as sophisticated as carotid heat exchangers for independent control of brain and core temperatures [4, 6, 18, 19]. While these approaches may be used in specialized hospitals for elective medical procedures, they have limited applicability for aiding in the recovery of neurological emergencies in the field.
Due to proximity to the cerebral circulation, the nasopharynx is uniquely suited for preferential and homogenous brain cooling; however, nasopharyngeal (NP) cooling with oxygen or liquid is limited by low heat capacity and/or respiratory compromise. As an alternative, the favorable distribution and rapid evaporative properties of NP aerosolized perfluorochemical (PFC) increases the heat capacity of respired gas; this in theory, should facilitate rapid induction and maintenance of global brain cooling without substantial reduction in systemic temperature. In the present study, we test the hypothesis that NP cooling method utilizing PFC liquid will more rapidly cool the brain as compared to WBS cooling. In addition, we sought to demonstrate that the brain will be preferentially cooled by the NP-PFC cooling approach and that intracerebral temperature gradients will be less for NP-PFC versus WBS cooling. Finally, we present histopathological sections of NP tissue, as well as, PFC uptake and elimination data as evidence of safety during NP-PFC cooling.
Methods
Animal Preparation
All animals were studied according to NIH Regulations and the approval of the Institutional Animal Care Committee. Male sheep (20–25 kg; n = 30) were shorn closely to minimize potential artifact of sheep wool insulation on heat transfer. The animals were preanesthetized with a mixture of intramuscular ketamine (10 mg/kg) and butorphanol tartrate (≤25 mg) followed by anesthesia induced by intravenous (percutaneous access to external jugular vein) administration of sodium pentobarbital (≤12.5 mg/kg). Once anesthetized, the sheep were instrumented with ECG leads, pulse oximetry, and an orogastic tube. Following local anesthesia (≤4 mg/kg of 0.5% lidocaine HCl, subcutaneous) the following cannulation procedures were performed. Peripheral vascular access was obtained through a leg vein to administer maintenance fluids (5% dextrose in 0.45% saline; 7.5 ml/kg/h) and supplemental anesthesia (≤10% loading dose/h in response to >20% increase in arterial pressure to noxious stimuli from normotensive values). Both external jugular veins (right: 7.5 Fr balloon tip thermodilution catheter; left: 7.5 Fr Cordis for thermocouple placement), and a carotid artery (8 Fr catheter) were cannulated through a ventral cervical approach. The thermodilution catheter was advanced into the proximal pulmonary artery under transduced, pressure guidance. The trachea was cannulated with an endotracheal tube (ETT) (7 mm ID HiLo Jet tube Mallinckrodt, St. Louis, MO). Following paralysis (pancuronium bromide: 0.1 mg/kg bolus followed by 0.1 mg/kg/h infusion), volume-controlled ventilation was performed with tidal volume of 8 ml/kg and positive end expiratory pressure of 10–12 cm H2O (to compensate for the role of the vocal chords to maintain functional residual capacity in the intubated animal), while maintaining PaCO2 between the clinically acceptable range of 35–60 mm Hg. Inspiratory oxygen was maintained at 100%, heated (35°C), and humidified throughout the entire study. Animals were given crystalloid volume (≤20 ml/kg) to support pulmonary artery wedge pressure of 12–15 cm H2O prior to initiating cooling.
Temperature Monitoring
Temperature probes (Type-T; Physitemp Instruments, Clifton, NY) were positioned at consistent anatomical sites across treatment groups. Following retraction of the scalp, needle microprobe thermocouples (29 ga) were placed through bone wax-sealed burr holes into three sites in the brain: (1) inferior frontal lobe, 4.5 cm deep; burr hole: 1 cm lateral to skull midline at intersection of line between supraorbital processes; (2) third ventricle, 5 cm deep; burr hole: 1 cm lateral to skull midline at intersection line between base of auricle; (3) superficial parietal, 2.5 cm deep; burr hole: 2 cm posterior from inferior frontal lobe burr hole. Flexible thermocouples were inserted through vascular catheters to two sties: (1) superior vena cava, 25 cm deep via left external jugular vein at mid-cervical neck; (2) peripheral vascular via femoral vein at femoral triangle, 45 cm deep; and rectum, 8 cm deep. Placement of brain probes was confirmed by fluoroscopy and Evans blue staining. Prior to studies, all temperature probes were standardized in ice and warm water (50°C) to establish a two-point calibration in the range of expected temperatures and standard starting point. Each probe was accurate to 0.1°C and at any given calibration temperature, readings from any two thermocouples were within 0.04°C.
Test Paradigm
Following instrumentation, animals were randomized to receive either NP-PFC cooling with a custom-designed device (see below) or WBS cooling conducted by circulating cold water (4°C; 2 l/min) through a commercially available and clinically used thermal blanket (Maxi-Therm, 24″ × 60″; Cincinnati Sub-Zero, Cincinnati, Ohio). Animals (n = 24) were studied with NP-PFC cooling as follows: (a) NP-PFC cooling induction (n = 22) was performed using continuous PFC (1 ml/min/kg)/gas (1 l/min/kg) spray for up to 1 h to target a 3.5°C decrease in temperature measured from at least one brain site; (b) in a subgroup of these animals (n = 8), once the target temperature was reached (i.e., induction) using the continuous PFC/gas spray, a maintenance strategy of PFC/gas spray was used by intermittently and manually turning the system “off” at the target temperature and “on” at 0.5°C above the target temperature for up to a total of 3 h; (c) to gain insight regarding PFC uptake and elimination with the NP-PFC cooling approach, two additional animals were studied with NP-PFC cooling for 60 min using twice PFC/gas flow delivery rate used to induce the target temperature. In these animals, blood samples were taken at 0, 5 15, 30, 45, and 60 min into delivery and 15, 30 45, and 60 min after discontinuation of NP-PFC cooling. WBS cooling (n = 6) was performed for 2 h.
Nasopharyngeal Perfluorochemical Cooling: Theory of Operation
Preferential brain cooling was performed by directing a mixture of PFC liquid and gas into the NP cavity via a specially designed nasal catheter system. Because of its chemical makeup, PFC liquid has a surface tension that is one-sixth that of water. This characteristic enables it to spread uniformly and quickly throughout the nasopharynx. Although the specific heat and heat of vaporization of PFC is less than saline or water, the vapor pressure of certain PFC liquids and density is as much as 1.7× saline or water. In addition, there is no PFC in the atmosphere. Taken together, these PFC liquids will evaporate more quickly and serve as a more effective coolant than saline or water. With these unique characteristics, aerosolized PFC significantly increases the heat carrying capacity of air passing through the nasopharynx. As such, NP-PFC approach utilizes latent heat of vaporization, hematogenous, and conductive mechanisms to induce and maintain brain cooling.
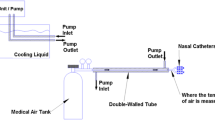
On the basis of theoretical calculations, bench top and preliminary animal studies, a NP catheter was designed (Fig. 1). The nasal catheters consist of two 4 mm tri-lumen tubes 10 cm in length that have 12 liquid/gas outlets placed along their length. These are oriented to match the features of the inside of the nasal cavity. The proprietary design of the catheter allows the optimum liquid/gas mixture to be sprayed out of each outlet. This design took into consideration selected PFC liquid properties, PFC flow, gas flow, catheter configuration, and control of local tissue temperatures (3–7°C). Briefly, the coolant was a PFC liquid (perfluorohexane, PFH; F2 Chemicals, Preston, UK) at room temperature. It has a boiling point of 57°C, vapor pressure of 363 mm Hg at 37°C, a density of 1.68 gm/ml and heat of vaporization of 21 cal/gm. PFH is currently used in an FDA approved pharmaceutical product where it is injected into the bloodstream for ultrasound contrast enhancement (Imagent, American Amersham). Theoretically, in the current design and as predicted from heat of vaporization and density, for every ml of PFC liquid coolant that evaporates, 35 calories of heat are absorbed from the nasopharynx. We have experimentally confirmed this value using a bench-top configuration consisting of a heated aluminum chamber and electronic temperature controller, and measuring the power required to maintain the chamber at 37°C while operating the NP-PFC catheter design.
For NP cooling, PFC was delivered into the nasal cannula from a pressurized reservoir-container through a mass-flow meter; gas was delivered from a universal tank through a mass-flow meter. The coolant was sprayed (1 ml/min/kg) into the nasal cavity where it evaporates and absorbs heat from the adjacent tissues and vasculature. The gas, in this case 100% oxygen, was supplied (1 l/min/kg) via the catheter at the tissue surface to enhance PFC liquid evaporation. The coolant vapor along with the oxygen exits the nasal cavity mainly via the nasopharynx to the mouth.
WBS cooling was conducted by sandwiching the sheep between two circulating cold water clinical thermal blankets, each at 4°C and 2 l/min (Maxi-Therm, 24″ × 60″; Cincinnati Sub-Zero, Cincinnati, Ohio). Every effort was made to completely surround the ventral and dorsal surfaces of the animals’ thoracic, abdominal, pelvic and cervical compartments. The choice of thermal blanket was based on practical application for use in the sheep and extensive clinical use.
Measurements
Arterial blood samples were drawn before, during and after induction, and during maintenance to assess hematocrit (Clay-Adams autocrit centrifuge), arterial blood gas tensions, acid-base status (Nova BioMedical Stat Profile 6l; temperature corrected), and hemoglobin (Radiometer OSM 3). Systemic and pulmonary arterial blood pressure, and heart rate were continuously monitored (Athena 9040 S&W Medico Teknik, Albertslund, Denmark). Pulmonary wedge pressure with cardiac output were measured by the thermodilution technique (American Edwards Laboratory; 9520 Cardiac Output Monitor) before, during and after induction, and during maintenance. Temperatures were recorded digitally on a 12 channel thermocouple scanner at sampling rate of 0.33/channel (Digisense®, Barnant Co.; Barrington, IL) integrated into a laptop computer and displayed as a function of time with each site recorded at no longer than 4 s intervals. Individual cooling curves for each measurement site were plotted over the entire duration of the respective protocols. Cooling rates were calculated from digitized temperature and time data.
At the conclusion of the protocol, NP tissue samples were obtained from consistent anatomical sites in representative animals in the NP-PFC cooling group. The samples were submersed in 10% buffered formalin, washed, then placed in ethanol. Routine techniques were used to prepare the tissues for paraffin embedding. Thin sections (<6 μm) were stained with hematoxylin and eosin.
Blood samples for PFC uptake and elimination analyses were transferred under leak-tight conditions directly into specially designed sealed vacuum headspace vials. PFH in blood samples was analyzed by a headspace-gas chromatography-flame ionization detection method with peak confirmation performed on a subset of samples by headspace-gas chromatography–mass spectrometry. Perfluorocyclohexane (PFCH) was used as internal standard for the assay. The methodology for this assay had been previously developed for determination of PFH in rat and human blood samples [20].
Statistical Analysis
Values are expressed as mean ± SD. Comparisons of vital signs and arterial blood chemistry were analyzed by analysis of variance (ANOVA) followed by Bonferroni post hoc testing to determine statistical differences as a function of time (repeated measures) and treatment group. A similar approach was used to evaluate cooling rates as a function of treatment group and region. A level of P < 0.05 was considered to be significant.
Results
Vital signs, hemodynamic and arterial blood chemistry parameters (Table 1) at baseline were comparable between groups. For both cooling techniques, animals tolerated the procedures, remaining physiologically stable over time. Cardiac output in the NP-PFC cooling group was lower (P < 0.05) than with WBS cooling; no significant differences between groups was noted for all other parameters shown.
As shown by the change from baseline of the mean value of all three brain temperature measurements in Fig. 2, there was a more rapid induction of brain cooling in the NP-PFC group versus the WBS group. The THZ was reached within approximately 15 min for NP-PFC but was not reached by 2 h in the WBS group. Figure 3 shows the maximum absolute cooling rates achieved during WBS and NP-PFC cooling as monitored in the brain (mean values of all three brain sites), vascular (mean values of both vascular sties), and core rectal compartments. Mean brain and vascular cooling rates and brain to systemic temperature gradients were significantly greater (P < 0.001) in the NP-PFC group as compared to the WBS cooling group. During NP-PFC cooling, brain > vascular > rectal cooling rates (P < 0.001); vascular and rectal cooling rates were approximately one-half and one-third of brain cooling rate, respectively. Compartmental cooling rates were not different during WBS cooling. No statistically significant relationships between brain cooling rates and arterial CO2 were found for either cooling technique.
Throughout NP-PFC cooling, brain to systemic temperature gradients demonstrated preferential brain cooling relative to vascular and rectal temperature profiles. This can be seen in Fig. 4a, where the brain to systemic temperature gradients increased during the NP-PFC cooling protocol, diminished with termination, and were no longer observed following 30 min of active cooling. Additionally, as shown by the representative tracing in Fig. 4b, during NP-PFC cooling there were no differences in temperatures between distinctive brain regions, inferior deep frontal, third ventricle, and superficial parietal lobe. In addition to rapid hypothermic induction, Fig. 4b shows that it was possible to maintain brain temperature in the target zone for several hours by selectively turning the NP-PFC spray “on” and “off”.
(a) Comparison of regional temperature gradients as a function of nasopharyngeal perfluorochemical cooling (mean ± SD; n = 22); (b) Typical tracings of temperatures within the brain (third ventricle: gray; superficial parietal lobe: blue; inferior frontal lobe: black) compared to systemic (rectal: green; peripheral vascular: red) measurements during hypothermic induction and maintenance in the same animal. During maintenance, the sinusoidal oscillations in temperature are associated with turning the nasopharyngeal perfluorochemical spary “on” and “off”
The impact of NP cooling on tissue structures and the PFC uptake and elimination profile is shown in Fig. 5. Figure 5a shows histomicrographs of NP pathway samples obtained from the region of the coldest, local temperature following 2 h of NP-PFC cooling. There was no evidence of tissue disruption or epithelial denudation at this region, or along the active range of the catheter. Furthermore as shown in Fig. 5b, PFC uptake was minimal and elimination was rapid following exposure to twice PFC flow rate. PFC uptake in blood reached a maximum of 23.5 ng/ml and an elimination half-life of less than 15 min was demonstrated.
(a) Representative histomicrographs of nasopharyngeal pathway samples obtained from the region of the coldest, local temperature following 2 h of nasopharyngeal perfluorochemical cooling (100× and 400×); (b) perfluorohexane blood concentrations (mean ± SD) during (“on”) and after (“off”) nasopharyngeal perfluorochemical cooling (n = 2)
Discussion
The principle findings of this study are that the THZ targeted for global brain neuroprotection can be rapidly reached and maintained by PFC nasopharyngeal cooling. PFC nasopharyngeal cooling maintained brain to systemic temperature gradients with selective, preferential brain cooling relative to vascular or rectal temperatures with relatively little instrumentation (nasal cannula, small supply of oxygen and 400 ml of PFC liquid) over a 2-h period. Additionally, relatively global and homogenous preferential brain cooling was achieved without cardiopulmonary or hematologic compromise. During NP-PFC cooling, cardiac output was lower than during WBS cooling without significant differences in systemic blood pressure, metabolic status, or importantly, vascular temperatures suggesting that cardioprotection was not exclusively due to myocardial cooling. This observation may be supportive functional evidence of the impact of rapid and deep brain cooling on central cardiovascular centers induced by the NP-PFC approach. Additionally, we speculate that associated reduction in myocardial work and oxygen consumption would favor cardioprotection as an adjunct to cardiac resuscitation. With respect to safety, PFC uptake is minimal and elimination is rapid. On-going studies are focused at increasing the brain to systemic temperature gradient by modulating the PFC/gas flow ratio and incorporating external warming blankets commensurate with the PFC nasopharyngeal cooling. Pre-clinical and clinical studies are ongoing to compare cooling efficacy in the sheep model with humans and to evaluate the neuroprotective effect of this approach in models of cerebral and cardiac compromise.
The brain is highly sensitive to increases in temperature; heat stress induces irreversible damage in the brain before any other organ is endangered. It has also long been known that fever makes brain injury worse and conversely, cooling ameliorates brain injury [21–23]. Adaptive brain cooling systems have evolved which in part contribute to heat transfer as well as water loss [24, 25]. One of the most primitive cooling systems involves airflow through the nasal cavities and nasopharynx, cooling blood flowing toward the brain. Nasal venous blood is therefore some degrees colder than core temperature and eventually this gradient reduces brain temperature via infraorbital veins and heat exchange with the embedded carotid artery within the cavernous sinus or rete mirabilis, respectively. In this regard, we believe that the sheep is a worse case scenario, since the sheep has a more redundant collateral circulation than the human, thus favoring less preferential brain cooling by the NP-PFC method. The anatomic differences specific to man, smaller nasopharynx and cavernous sinus, combined with a much larger brain, has evoked some controversy about the presence of a brain cooling mechanism in humans [26]. If brain cooling via the NP passages exists in man, brain temperature should be greater than normal when NP airflow is bypassed, such as with tracheostomy. This was experimentally demonstrated by Mariak et al. [27] by comparing temperatures measured in the subdural space and between the frontal lobe and cribiform plate, to esophageal temperature in conscious patients who had suffered aneurysmal subarachnoidal hemorrhages during and after removal of the endotracheal tube. Reinstitution of airflow in the upper respiratory tract coincided with a rapid drop in brain temperature (0.4–0.8°C) below core temperature, supporting the existence of this mechanism of brain cooling in man.
A focused review of systemic and regional cooling techniques is provided to evaluate the new contribution and potential assets of NP-PFC cooling over existing cooling technologies. Systemic cooling techniques can be categorized into external versus internal with or without extracorporeal methodologies. Regional cooling techniques are categorized into pulmonary and preferential head cooling approaches.
External cooling by WBS cooling has been the traditional method for therapeutic hypothermia. Although this method is effective, it is encumbered by equipment issues and slow response (core cooling rate: 0.5–1.0°C/h) [28, 29]. Other practical disadvantages include overshooting target body temperatures and discomfort. Technical progress in surface cooling has been made [30]; however, the approach still remains relatively resource intensive and time consuming requiring as long as 8 h to reach target temperature thus remaining the limiting factor [2]. Patients must be maintained adequately sedated and medicated to suppress shivering which obscures neurological assessments. Systemic cooling may cause hemodynamic effects that limit its use to the intensive care setting. Patients undergoing systemic cooling require large volumes of fluid and electrolytes immediately before and during cooling. Complications of systemic cooling, mainly arrhythmias and episodes of hypotension are largely avoidable by careful fluid balance, electrolyte substitution and attention to cardiac arrhythmias; however, junctional bradycardia, atrial fibrillation, and slow ventricular response causing hemodynamic compromise, are encountered when temperature drops close to 30°C.
Recent advances in extracorporeal and intravascular cooling have demonstrated improved cooling rates (4–8°C/h) over surface cooling [31]. Extracorporal heat exchange techniques are effective but given their vascular instrumentation, are limited to the operating room or similar controlled clinical environments. Intravascular cooling [32], while less invasive than extracorporeal systems, is less effective in reducing core temperatures (1–3°C/h) and is associated with significant shivering [16, 32]. Shivering has been the major barrier to its use in non-ICU settings. Although methods to overcome shivering have been devised, a recent study revealed significant morbidity with this approach in stroke patients [17]. Acute aortic flush of cold saline (i.e., 100 ml/kg at 4°C and 35 ml/min/kg) required pharmacologic additives to induce deep cerebral hypothermia in pigs [33]. More prolonged aortic infusion resulted in mild cerebral hypothermia [6]. Prolonged maintenance (36 h) of mild hypothermia (34°C) by cardiopulmonary bypass following profound brain hypothermia (10–15°C) induced by aortic saline flush (i.e., 2°C; 20 l) supported intact neurological outcome in a dog model of cardiac arrest following prolonged hemorrhage [34]. In a recent advancement, Vanden Hoek et al. demonstrated more rapid brain cooling using a two-phase, liquid plus ice, saline slurry than with an equal volume of cold saline [19]. Yet, all of these techniques necessarily invoke substantial systemic cooling along with brain cooling. Thus, techniques targeted toward preferential brain cooling are still needed.
The most studied regional cooling techniques include use of the lung and head. Because the entire cardiac output passes through the pulmonary circulation, the lung can serve as a core heat exchanger. Pulmonary cooling was first attempted in dogs by delivering 0°C air and achieved 2.7°C of cooling in 30 min [35]. However, insufflation of cold gas was limited by cold-gas-induced bronchoconstriction. Additional cooling studies with heliox (oxygen and helium), hyperbaric heliox, SF6/O2 and unhumidified air with 20 ppm nitric oxide highlight that while each successive step constitutes an improvement in systemic cooling in animals and humans, no gas combination alone is sufficient to achieve the amount of cooling quickly enough to confer effective neuroprotection [36–41].
As an alternative medium, PFC liquid has been shown to be a feasible pulmonary medium for induction of hypothermia. PFC liquids have high respiratory gas solubility, spreading coefficients, and thermal conductivities (e.g., 0.1 kcal/h·m·°C at 25°C with PFC RIMAR 101), which makes them excellent refrigerants for the lungs [42]. Shaffer et al. [43] and Forman et al. [44] used liquid ventilation (LV) to circulate cooled PFC (10–30°C) to induce hypothermia in small animals. Core cooling rates as fast as 9°C/h were inversely correlated with the liquid temperature and physiological gas exchange was maintained. Harris et al. used a liquid PFC (FC75; boiling point 100°C), delivered into the lungs at 4°C in dogs, as intermittent LV and demonstrated rapid core and brain cooling (>20°C/h) [45]. Using perfluorodecalin at 0°C (boiling point 142°C), administered as repeated lung lavages in rabbits during partial liquid ventilation (PLV), Hong et al. cooled at approximately 6°C/h [46]. They also showed that pulmonary cooling was comparable to systemic cooling while maintaining a narrower interorgan temperature difference. Following a similar protocol, Yang et al. used another PFC, PP2 at 4°C (boiling point 76°C), cooled at approximately 4°C/h, and show improved lung structural integrity as compared to the cooling blanket technique [47]. However, as reported in previous studies of PLV in the normal lung [48], gas exchange was impaired using PLV for pulmonary cooling as compared to systemic cooling methods. Although appropriate for neuroprotection, none of these methods allow for pulmonary cooling of a non-intubated, awake, unsedated patient in an ambulance or even emergency department setting.
The stress of systemic hypothermia and uncontrolled shivering has been shown to complicate the neuroprotective effects of hypothermia [49]. To minimize system effects, several different approaches have been used to lower head temperature to induce preferential brain cooling with less systemic hypothermia. One approach involves a cap or helmet that incorporates a coolant such as frozen liquid, solid ice, or circulating water [14, 50–54]. While successful in neonatal piglets and shown to improve survival after neonatal encephalopathy without serious adverse effects [53, 55], it has had limited success in adults [14]. This approach yields brain-cooling rates within 1–2°C/h, non-uniform intracerebral temperature gradients, and requires additional equipment and skin protection to prevent thermal insult. The NP approach using various gas combinations alone has not proved successful to induce preferential brain hypothermia in man [40, 41, 50]. Using NP delivery of cold saline in rats, Hagioka et al. demonstrated very rapid cooling rates, approximately 5 times faster than with whole body techniques [56]. This approach utilized high flow rates (100 ml/min/kg) of prechilled saline that translates to very large flows in humans and requires additional equipment for set-up and disposal. As an alternative, the NP-PFC cooling approach utilizes a clinically friendly nasal catheter design, requires much lower flows than saline, and because the PFC evaporates, no additional requirements for disposal.
PFC liquids are synthetic materials that are not naturally found in the body, are not metabolized, and are predominantly eliminated by the lung through volatilization into the expiratory gas with a small amount of PFC transpired through the skin [57–59]. Numerous safety studies have been performed regarding uptake, elimination and biodistribution following PFC exposure [60–66]. Results demonstrate that only small quantities of PFC diffuse across the tissue membranes and dissolve into blood (0.25–10 μg PFC/ml blood) [67, 68]. Follow-up of primates have shown small amounts of PFC 3 years following liquid PFC ventilation, without deleterious effects [64]. Blood levels following intratracheal PFC administration are orders of magnitude lower than those following intravascular administration (40–80 mg PFC/ml of blood) of PFC emulsion clinically approved for angioplasty in humans and under investigation as an oxygen carrier or temporary “blood substitute” [67, 69].
In the present NP-PFC cooling study, the uptake profile for PFH was unremarkable and elimination occurred rapidly (Fig. 5b). These values are in contrast to the mg/ml levels associated with PFC blood substitutes and μg/ml levels associated with PFC liquid ventilation. The maximum recorded PFH concentration in the blood (24.5 ng/ml) was reached within 15 min of terminating the PFC spray. To the degree that PFC uptake is an integrative process (balance between circulating PFC in blood, PFC reservoir in NP pathway and PFC volatization by gas flow) it was expected that there would be some uptake even after the direct PFC spray was terminated. This concentration dropped quickly to nearly non-detectable levels. Rapid elimination follows an exponential washout curve associated with high gas flow, diminishing PFC reservoirs and concentration gradients.
PFH is the active ingredient in the clinically approved ultrasound-imaging agent Imagent (Imavist) (NDA21–191) in which microbubbles of PFH gas are injected directly into the blood stream. Report Number IMUS-012-USA, from the same NDA, entitled An open-label, single-dose study to assess the pharmacokinetic parameters and rate of elimination of perfluorohexane after a 4 mg/kg bolus intravenous injection of AFO150 in healthy adult volunteers showed an average maximum blood concentration of PFH of 28.0 ng/ml without serious adverse events and that 75% of PFH was eliminated through expired air within 3 h. In the present study, with NP-PFC cooling at 10 [7] times PFH exposure as compared to intravenous Imagent (200 gm/kg versus 20 μg/kg), comparable peak blood levels were found and 75% elimination occurred more quickly, by 28 min as compared to 3 h with the imaging agent. Moreover, PFH blood values were orders of magnitude lower than reported with direct intravascular exposure of other PFC liquids used in blood substitutes (mg/ml) or intrapulmonary exposure during PFC liquid ventilation (μg/ml) [67, 69]. Summarily, comparison of these data suggest that the safety profile of NP PFC with PFH would be at least comparable to that of Imagent.
Clinical Considerations
This investigation demonstrates feasibility of a novel cooling technique that may finally allow implementation of therapeutic brain cooling in neurological emergencies in the field as well as an adjunct to more invasive procedures. Aggressive evaporative heat loss in the nasopharynx along with conductive cooling of adjacent cervicocranial vasculature is shown to cause the relevant rapid drop of 3–4° in brain temperature. This temperature reduction has been shown beneficial in various animal models of intra and early post-ischemic brain injury and several models of traumatic impact, particularly if initiated immediately after the onset of injury [5–7]. The downside of any cooling technology used to date in humans has been the late onset and very prolonged use to achieve brain hypothermia. The present technology addresses this issue in that it is relatively non-invasive, simple and quick to implement in the field, highly preferential, and rapidly targets brain cooling. On the basis of anatomical relationships, differential brain scans in patients with traumatic brain injury, skull and/or facial fractures may be warranted. While these tests may potentially delay implementation in this patient population, based on the relatively more rapid cooling rates with NP-PFC cooling technology, we suggest that it is still likely to afford greater potential for neuroprotection than existing technologies. With these limitations in mind and based on these positive preclinical findings, clinical trials are currently ongoing.
References
Krieger DW, Yenari MA. Therapeutic hypothermia for acute ischemic stroke: what do laboratory studies teach us? Stroke 2004;35:1482–9.
The Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549–56.
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557–63.
Schwab S, Schwarz S, Spranger M, Keller E, Bertram M, Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke 1998;29:2461–6.
Marion DW, Leonov Y, Ginsberg M, Katz LM, Kochanek PM, Lechleuthner A, Nemoto EM, Obrist W, Safar P, Sterz F, Tisherman SA, White RJ, Xiao F, Zar H. Resuscitative hypothermia. Crit Care Med 1996;24:S81–9.
Behringer W, Prueckner S, Safar P, Radovsky A, Kentner R, Stezoski SW, Henchir J, Tisherman SA. Rapid induction of mild cerebral hypothermia by cold aortic flush achieves normal recovery in a dog outcome model with 20-minute exsanguination cardiac arrest. Acad Emerg Med 2000;7:1341–8.
Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med 1993;21:1348–58.
Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Mullner M. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med 2005;33:414–8.
Maclellan CL, Davies LM, Fingas MS, Colbourne F. The influence of hypothermia on outcome after intracerebral hemorrhage in rats. Stroke 2006;37:1266–70.
Maclellan CL, Girgis J, Colbourne F. Delayed onset of prolonged hypothermia improves outcome after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab 2004;24:432–40.
Gupta R, Jovin TG, Krieger DW. Therapeutic hypothermia for stroke: do new outfits change an old friend? Expert Rev Neurother 2005;5:235–46.
Plattner O, Kurz A, Sessler DI, Ikeda T, Christensen R, Marder D, Clough D. Efficacy of intraoperative cooling methods. Anesthesiology 1997;87:1089–95.
Thoresen M, Whitelaw A. Therapeutic hypothermia for hypoxic-ischaemic encephalopathy in the newborn infant. Curr Opin Neurol 2005;18:111–6.
Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001;51:275–81.
Wang H, Olivero W, Lanzino G, Elkins W, Rose J, Honings D, Rodde M, Burnham J, Wang D. Rapid and selective cerebral hypothermia achieved using a cooling helmet. J Neurosurg 2004;100:272–7.
De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, Koroshetz WJ, Rordorf G, Warach S. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology 2004;63:312–7.
Krieger DW, De Georgia MA, Abou-Chebl A, Andrefsky JC, Sila CA, Katzan IL, Mayberg MR, Furlan AJ. Cooling for acute ischemic brain damage (cool aid): an open pilot study of induced hypothermia in acute ischemic stroke. Stroke 2001;32:1847–54.
Mori K, Saito J, Kurata Y, Takeyama Y, Itoh Y, Kaneko M, Asai Y, Renzi FP, Dickson EW. Rapid development of brain hypothermia using femoral-carotid bypass. Acad Emerg Med 2001;8:303–8.
Vanden Hoek TL, Kasza KE, Beiser DG, Abella BS, Franklin JE, Oras JJ, Alvarado JP, Anderson T, Son H, Wardrip CL, Zhao D, Wang H, Becker LB. Induced hypothermia by central venous infusion: saline ice slurry versus chilled saline. Crit Care Med 2004;32:S425–31.
Straub JA, Chickering DE, Hartman TG, Gloff CA, Bernstein H. AI-700 pharmacokinetics, tissue distribution and exhaled elimination kinetics in rats. Int J Pharm 2007;328:35–41.
Ginsberg MD, Busto R. Combating hyperthermia in acute stroke: a significant clinical concern. Stroke 1998;29:529–34.
Baena RC, Busto R, Dietrich WD, Globus MY, Ginsberg MD. Hyperthermia delayed by 24 hours aggravates neuronal damage in rat hippocampus following global ischemia. Neurology 1997;48:768–73.
Kim Y, Busto R, Dietrich WD, Kraydieh S, Ginsberg MD. Delayed postischemic hyperthermia in awake rats worsens the histopathological outcome of transient focal cerebral ischemia. Stroke 1996;27:2274–80.
Maloney SK, Mitchell D, Blache D. The contribution of carotid rete variability to brain temperature variability in sheep in a thermoneutral environment. Am J Physiol Regul Integr Comp Physiol 2007;292:R1298–305.
Mitchell D, Maloney SK, Jessen C, Laburn HP, Kamerman PR, Mitchell G, Fuller A. Adaptive heterothermy and selective brain cooling in arid-zone mammals. Comp Biochem Physiol B Biochem Mol Biol 2002;131:571–85.
Cabanac M. Selective brain cooling in humans: “fancy” or fact? FASEB J 1993;7:1143–6.
Mariak Z, White MD, Lewko J, Lyson T, Piekarski P. Direct cooling of the human brain by heat loss from the upper respiratory tract. J Appl Physiol 1999;87:1609–13.
Schwab S, Spranger M, Aschoff A, Steiner T, Hacke W. Brain temperature monitoring and modulation in patients with severe MCA infarction. Neurology 1997;48:762–7.
Zeiner A, Holzer M, Sterz F, Behringer W, Schorkhuber W, Mullner M, Frass M, Siostrzonek P, Ratheiser K, Kaff A, Laggner AN. Mild resuscitative hypothermia to improve neurological outcome after cardiac arrest. A clinical feasibility trial. Hypothermia After Cardiac Arrest (HACA) Study Group. Stroke 2000;31:86–94.
Mayer SA, Kowalski RG, Presciutti M, Ostapkovich ND, McGann E, Fitzsimmons BF, Yavagal DR, Du YE, Naidech AM, Janjua NA, Claassen J, Kreiter KT, Parra A, Commichau C. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med 2004;32:2508–15.
Furuse M, Preul MC, Kinoshita Y, Nishihara K, Isono N, Kuroiwa T. Rapid induction of brain hypothermia by endovascular intra-arterial perfusion. Neurol Res 2007;29:53–7.
Steinberg GK, Ogilvy CS, Shuer LM, Connolly ES Jr, Solomon RA, Lam A, Kassell NF, Baker CJ, Giannotta SL, Cockroft KM, Bell-Stephens TE, Allgren RL. Comparison of endovascular and surface cooling during unruptured cerebral aneurysm repair. Neurosurgery 2004;55:307–14.
Janata A, Holzer M, Bayegan K, Frossard M, Sterz F, Losert UM, Laggner AN, Behringer W. Rapid induction of cerebral hypothermia by aortic flush during normovolemic cardiac arrest in pigs. Crit Care Med 2006;34:1769–74.
Wu X, Drabek T, Kochanek PM, Henchir J, Stezoski SW, Stezoski J, Cochran K, Garman R, Tisherman SA. Induction of profound hypothermia for emergency preservation and resuscitation allows intact survival after cardiac arrest resulting from prolonged lethal hemorrhage and trauma in dogs. Circulation 2006;113:1974–82.
Zikria BA, Ferrer JM, Malm JR. Pulmonary hypothermia in dogs. J Appl Physiol 1968;24:707–10.
Harrison MR, Hysing ES, Bo G. Control of body temperature: use of the respiratory tract as a heat exchanger. J Pediatr Surg 1977;12:821–8.
Beran AV, Sperling DR. An improved method for inducing hypothermia and rewarming. Aviat Space Environ Med 1979;50:844–6.
Piantadosi CA, Thalmann ED. Thermal responses in humans exposed to cold hyperbaric helium-oxygen. J Appl Physiol 1980;49:1099–106.
Ingenito E, Solway J, Lafleur J, Lombardo A, Drazen JM, Pichurko B. Dissociation of temperature-gradient and evaporative heat loss during cold gas hyperventilation in cold-induced asthma. Am Rev Respir Dis 1988;138:540–6.
Andrews PJ, Harris B, Murray GD. Randomized controlled trial of effects of the airflow through the upper respiratory tract of intubated brain-injured patients on brain temperature and selective brain cooling. Br J Anaesth 2005;94:330–5.
Harris BA, Andrews PJ, Murray GD. Enhanced upper respiratory tract airflow and head fanning reduce brain temperature in brain-injured, mechanically ventilated patients: a randomized, crossover, factorial trial. Br J Anaesth 2007;98:93–9.
Corno C, Fiore GB, Martelli E, Dani C, Costantino ML. Volume controlled apparatus for neonatal tidal liquid ventilation. ASAIO J 2003;49:250–8.
Shaffer TH, Forman DL, Wolfson MR. Physiological effects of ventilation with liquid fluorocarbon at controlled temperatures. Undersea Biomed Res 1984;11(3):287–98.
Forman DL, Bhutani VK, Tran N, Shaffer TH. A new approach to induced hypothermia. J Surg Res 1986;40:36–42.
Harris SB, Darwin MG, Russell SR, O’Farrell JM, Fletcher M, Wowk B. Rapid (0.5 degrees C/min) minimally invasive induction of hypothermia using cold perfluorochemical lung lavage in dogs. Resuscitation 2001;50:189–204.
Hong SB, Koh Y, Shim TS, Lee SD, Kim WS, Kim DS, Kim WD, Lim CM. Physiologic characteristics of cold perfluorocarbon-induced hypothermia during partial liquid ventilation in normal rabbits. Anesth Analg 2002;94:157–62.
Yang SS, Jeng MJ, McShane R, Chen CY, Wolfson MR, Shaffer TH. Cold perfluorochemical-induced hypothermia protects lung integrity in normal rabbits. Biol Neonate 2005;87:60–5.
Mates EA, Hildebrandt J, Jackson JC, Tarczy-Hornoch P, Hlastala MP. Shunt and ventilation-perfusion distribution during partial liquid ventilation in healthy piglets. J Appl Physiol 1997;82:933–42.
Thoresen M, Satas S, Loberg EM, Whitelaw A, Acolet D, Lindgren C, Penrice J, Robertson N, Haug E, Steen PA. Twenty-four hours of mild hypothermia in unsedated newborn pigs starting after a severe global hypoxic-ischemic insult is not neuroprotective. Pediatr Res 2001;50:405–11.
Mellergard P. Changes in human intracerebral temperature in response to different methods of brain cooling. Neurosurgery 1992;31:671–7.
Gunn AJ, Gunn TR. The ‘pharmacology’ of neuronal rescue with cerebral hypothermia. Early Hum Dev 1998;53:19–35.
Gunn AJ, Gluckman PD, Gunn TR. Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics 1998;102:885–92.
Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–70.
Horn AR, Woods DL, Thompson C, Eis I, Kroon M. Selective cerebral hypothermia for post-hypoxic neuroprotection in neonates using a solid ice cap. S Afr Med J 2006;96:976–81.
Thoresen M, Simmonds M, Satas S, Tooley J, Silver IA. Effective selective head cooling during posthypoxic hypothermia in newborn piglets. Pediatr Res 2001;49:594–9.
Hagioka S, Takeda Y, Takata K, Morita K. Nasopharyngeal cooling selectively and rapidly decreases brain temperature and attenuates neuronal damage, even if initiated at the onset of cardiopulmonary resuscitation in rats. Crit Care Med 2003;31:2502–8.
Shaffer TH, Foust R, Wolfson MR, Miller TF Jr. Analysis of perfluorochemical elimination from the respiratory system. J Appl Physiol 1997;83:1033–40.
Kechner N. Factors effecting perfluorochemical uptake into the blood following exposure to liquid assisted ventilation. Temple University School of Medicine; 1997. p. 1–209.
Miller TF, Milestone B, Stern R, Shaffer TH, Wolfson MR. Effects of perfluorochemical distribution and elimination dynamics on cardiopulmonary function. J Appl Physiol 2001;90:839–49.
Wolfson MR, Kechner NE, Roache RF, Dechadarevian JP, Friss HE, Rubenstein SD, Shaffer TH. Perfluorochemical rescue after surfactant treatment: effect of perflubron dose and ventilatory frequency. J Appl Physiol 1998;84:624–40.
Cox CA, Stavis L, Wolfson MR, Shaffer TH. Long-term tidal liquid ventilation in premature lambs: physiologic, biochemical and histological correlates. Biol Neonate 2003;84:232–42.
Shaffer TH, Wolfson MR, Greenspan JS, Hoffman RE, Davis SL, Clark LC Jr. Liquid ventilation in premature lambs: uptake, biodistribution and elimination of perfluorodecalin liquid. Reprod Fertil Dev 1996;8:409–16.
Wolfson MR, Greenspan JS, Shaffer TH. Liquid-assisted ventilation: an alternative respiratory modality. Pediatr Pulmonol 1998;26:42–63.
Modell JH, Calderwood HW, Ruiz BC, Tham MK, Hood CI. Liquid ventilation of primates. Chest 1976;69(1):79–81.
Holaday DA, Fiserova-Bergerova V, Modell JH. Uptake, distribution, and excretion of fluorocarbon FX-80 (perfluorobutyl perfluorotetrahydrofuran) during liquid breathing in the dog. Anesthesiology 1972;37:387–94.
Hood CI, Modell JH. A morphologic study of long-term retention of fluorocarbon after liquid ventilation. Chest 2000;118:1436–40.
Riess JG. Overview of progress in the fluorocarbon approach to in vivo oxygen delivery [Review]. Artif Cells Blood Substit Immobil Biotechnol 1992;20:20–4.
Riess JG, LeBlanc M. Solubility and transport phenomeno in perfluorochemical relevant to blood substitution and other biomedical applications. Pure Appl Chem 1982;54:2383–406.
Keipert PE, Otto S, Flaim SF, Weers JG, Schutt EA, Pelura TJ, Klein DH, Yaksh TL. Influence of perflubron emulsion particle size on blood half-life and febrile response in rats. Artic Cells Blood Substit Immobil Biotechnol 1994;22:1169–74.
Acknowledgments
The authors thank Becky Inderbitzen, MS.E. for her review and insight on the manuscript; BeneChill, Inc. and National Institutes of Health COBRE 1P20RR20173-03 for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Work was performed at Temple University School of Medicine, Department of Physiology.
Rights and permissions
About this article
Cite this article
Wolfson, M.R., Malone, D.J., Wu, J. et al. Intranasal Perfluorochemical Spray for Preferential Brain Cooling in Sheep. Neurocrit Care 8, 437–447 (2008). https://doi.org/10.1007/s12028-008-9064-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-008-9064-0