Abstract
Background
The Neuroform stent can help in the treatment of difficult, wide-necked intracranial aneurysms. The objective of our study is to report some of the challenges associated with the Neuroform stent in the treatment of intracranial aneurysms.
Methods
From January 2003 to August 2006, consecutive patients treated with Neuroform stent for intracranial aneurysms were prospectively enrolled. Information on patient demographics, cerebrovascular risk factors, aneurysm size and location were collected. Technical and clinical complications as well as clinical outcomes were measured. Data were analyzed retrospectively using SPSS software version 11.5.
Results
Successful deployment of the stent, in the target artery, was achieved in 65/67 (97%) patients. Stent deployment failed in two cases and the migration of stent developed in one during coiling. Postoperative thromboembolic events developed in three patients. These three patients possessed hyperactive platelets, and were treated with intravenous eptifibatide. Intraoperative rupture of aneurysm developed in one patient, which was secured by subsequent coiling. Majority of the patients had good outcomes GOS (Glasgow Outcome Score) 1 or NIHSS (National Institute of Health Stroke Scale) 0 in 63/67 (94%), GOS 2 or NIHSS 2 in one patient and GOS 3 or NIHSS 4 was observed in three cases.
Conclusion
Despite a low rate of intraoperative complications, post-procedural thromboembolic events were common in Neuroform stent-treated patients, which might be associated with hyperactive platelets. Further studies are warranted to identify any potential relationship between post-stent hyperactive platelets and thromboembolism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endovascular treatment of intracranial aneurysms has gained increasing popularity, compared to an open craniotomy, due to its having equivalent safety, efficacy profile [3, 17–20, 34, 40] and for being a lesser invasive approach. Endovascular therapy, in the treatment of vertebro-basilar system aneurysm and high-grade aneurysmal SAH, is considered a superior technical and therapeutic option [5, 9, 11, 12, 21, 22, 37]. Endovascular coiling of aneurysms is found to be a safe and superior alternative to surgical treatment in the selected group of patients by a recent randomized trial (International Subarachnoid Aneurysm Trial (ISAT)) [29].
The wide-necked, large and giant aneurysms might have posed challenges on the traditional endovascular treatment of aneurysms [3, 13]. In a study [13], where both ruptured and unruptured aneurysms were treated with GDC coil, complete coil occlusion of the aneurysm was achieved in 70.8% for small aneurysms (<10 mm) with small necks, 31.2% for small aneurysms with wide necks, 35% for large aneurysms (≥10 mm ≤ 25 mm), and 50% of giant aneurysms (≥25 mm). The obstacles to the primary, complete occlusion of an aneurysm, by the endovascular approach had been overcome significantly due to the evolution of new devices and techniques such as three-dimensional coils and balloon-assisted remodeling of the aneurysms [14, 23, 25, 28, 30, 32, 36, 39]. Additionally, a neck-bridge device TriSpan (Target Therapeutic/Boston Scientific, Fremont, CA) had been found to be useful in coil embolization of wide-necked bifurcation aneurysm [43]. However, the long-term durability after coiling and neck residual remained as a barrier [4, 6, 13, 26, 27, 39] to endovascular coiling of aneurysms. The use of stents to overcome the challenge of current endovascular technique had been addressed [42] in a limited fashion, and yet needed further evaluation and elaboration. The widespread use of balloon-mounted coronary stents had been limited due to its less flexible, high profile nature, poor navigation capabilities in the tortuous cerebral blood vessels, and potential risk of vessel dissection or rupture, as well as delayed stenosis [1, 24, 38, 41]. Therefore, to minimize these challenges, focus had been shifted to develop a self-deployed stent, which would be flexible enough to navigate through the tortuous cerebral vascular architecture and tough enough to hold its configuration when encountered by a coil mass. The Neuroform stent (Boston Scientific Target, Fremont, CA), a nickel–titanium alloy (Nitinol)—possessed a high degree of elasticity and deformability and had been approved under a humanitarian device exemption by Food and Drug Administrations (FDA) for the treatment of wide-necked aneurysms. Since the approval of Neuroform stent, few case series had been published [2, 8, 10, 15, 16], where the complications of Neuroform stent had been elucidated. The objective of this study was to report on our experience of the clinical and technical challenges associated with Neuroform-assisted treatment of intracranial aneurysms.
Methods
All patients who underwent placement of a Neuroform stent for the treatment of an intracranial wide-necked and fusiform aneurysm were registered in a prospectively maintained database. From this database, consecutive patients from January 2003 to August 2006 were selected, and the data were retrospectively analyzed. Institutional Review Board (IRB) approval was obtained prior to the treatment and retrieval of the data. Similarly, informed consent was obtained from all patients prior to placement of a Neuroform stent for the treatment of their aneurysms. The architecture of the aneurysm was elucidated by digital subtraction cerebral angiography and a three-dimensional reconstruction of aneurysm. Wide-necked aneurysms were defined as having dome to neck ratios <2 or a neck >4 mm in diameter (Fig. 1). An intracranial aneurysm was defined as fusiform if the aneurysm was an out-pouching dilatation of the parent blood vessel affecting at least 270° of circumference of the lumen and possessing no discernible neck. A total of 355 patients with the diagnosis of intracranial aneurysm (unruptured 201, ruptured 154) were treated, from which 67 patients (wide-necked 52, fusiform 5) were enrolled for Neuroform stent-assisted treatment of intracranial aneurysm. The decision of microsurgical versus endovascular Neuroform stent-assisted treatment of aneurysm was made upon the agreement between a vascular neurosurgeon and an endovascular neuro-endovascular specialist. The outcomes were predefined on the basis of radiographic and clinical criteria. The radiographic outcomes were defined as the degree or type of aneurysm occlusion (Complete occlusion (100%), partial occlusion (>95% < 100%) or subtotal occlusion <95%) after the completion of Neuroform stent-assisted coiling of the aneurysm. The clinical outcomes were measured using National Institute of Health Stroke Scale (NIHSS) and Glasgow Outcome Scale (GOS). The outcome was defined as good if the obtained GOS had been 2 or less and NIHSS 2 or less within 90 days or at a later follow-up visit.
A thromboembolic event was defined as the clinical development of a transient or permanent neurological deficit after the procedure. A periprocedural thromboembolic event was defined as the angiographic partial or complete occlusion of an artery or a perfusion defect in the territory of an arterial supply. Patients with a suspected thromboembolic event underwent confirmatory imaging including magnetic resonance imaging (MRI) with diffusion-weighted sequences. Routine imaging to identify a silent event in an asymptomatic patient was not performed in our study.
All Neuroform stent treated patients were treated with both aspirin 325 mg/day and Clopidogrel 75 mg/day at least 5 days prior to their treatment. Patients were continued on both aspirin and Plavix daily for 4 weeks after the stenting procedure, and thereafter 325 mg aspirin daily. For wide-necked ruptured aneurysms, the stenting procedure was modified due to the risk of aggressive antiplatelet therapy and anticoagulation required for the procedure. In this instance, if feasible, coiling (>90%) to secure the aneurysm was attempted first, followed by Neuroform stent-assisted coiling 4–6 weeks after the initial procedure. In our study, four ruptured wide-necked aneurysm patients were treated with Neuroform stent-assisted coiling in the same setting. For all ruptured aneurysms, an extra ventricular catheter was placed prior to the procedure and initiation of antithrombotic medications in order to prevent potential bleeding complication related to ventriculostomy catheter. We did not use routine systemic anticoagulation in our subarachnoid hemorrhage patients who underwent stent-assisted coiling of the aneurysm. However, routine deep vein thrombosis prophylaxis using subcutaneous heparin was mandatory for all of our subarachnoid hemorrhage patients and considered as a standard of practice in our institution.
Selection Criteria
All patients with the diagnosis of a wide-necked aneurysm or fusiform aneurysm were enrolled. Based on the results of the International Study of Unruptured Intracranial Aneurysms Investigators [44], we empirically chose 7 mm as our cutoff for the unruptured intracranial aneurysms. Predefined exclusion criteria were active bleeding diatheses and or a platelet count <100,000/dl and patients exhibiting reluctance to take aspirin and Clopidogrel. Based on the non-compliance criteria no patient was excluded from the study.
Procedures
A complete neurological examination was performed prior to the procedure. A 6 French (6F) introducer sheath was placed in the femoral artery and was flushed continuously with heparinized saline. Baseline serum activated coagulation time (ACT) was obtained and intravenous heparin was administered to achieve ACT between 250 s to 300 s prior to the introduction of a guiding catheter. A 6F-guiding catheter (Boston Scientific Target, Fremont, CA), which was flushed with continuous heparinized saline, was placed in the proximal part of the vessels of interest (internal carotid, vertebral artery). A pre-shaped 150 cm microcatheter (SL 10, Boston Scientific Target, Fremont, CA) was loaded over a microwire (Synchro 14, Boston Scientific Target, Fremont, CA, or Transcend 14, Cordis, Miami, FL) and advanced through the guiding catheter under the guidance of fluoroscope and roadmaps. The aneurysm was crossed with the negotiation between a microwire and a microcatheter, and the tip of the microcatheter was placed in the artery distal to the neck of the aneurysm. The microcatheter was swapped with an exchange length microwire (X-celerator 300 cm, eV3, Irvine, CA). The Neuroform stent delivery system (stent delivery microcatheter and stent stabilizer) was prepared and continuously flushed with heparin mixed saline. The stent delivery system was then advanced over the exchange length microwire as a unit and subsequently deployed after a suitable position was achieved to cover the neck of the aneurysm. Recently, the authors have begun to use a direct approach to navigate the stent delivery system without using an exchange length microwire. In the direct technique, a 200 cm 0.014-inch compatible microwire was backloaded through the stent delivery catheter prior to the introduction into the guide catheter. The stent-delivery system with the microwire was advanced as a unit through the guiding catheter to cross the neck of the aneurysm. To cover the neck of the aneurysm adequately, the stent was deployed at least 4 mm proximal and 4 mm distal to the neck on the parent artery (Figs. 2, 3). In our study, endovascular coiling of the aneurysm was performed after the Neuroform stent was placed. Patient femoral arteriotomy sites were closed either by Perclose (Abbott Vascular, Redwood city, CA), Angio-Seal (St. Jude Medical, Minnetonka, MN), closure pad (Medtronic, Minneapolis, MN), or by manual compression, depending on the result of a femoral artery angiogram.
An artistic drawing of a basilar artery bifurcation widenecked aneurysm and Neuroform stent assisted aneurysm Y-shaped neck-reconstruction by a professional artist. (A) Demonstrates that the 1st stent was implanted between BA and left posterior cerebral artery (PCA). The 2nd stent has been placed between BA and right posterior cerebral artery (B)
Patients were extubated immediately after the procedure and a complete neurological examination was performed in order to identify any new deficits. Patients were sent to the neurointensive care unit for observation and subsequently discharged home. The angiographic and clinical follow-up was planned for each patient at 3, 12, 18, and 36 months.
Statistical Analysis
A prospective database was created and maintained for all patients treated with Neuroform stent for their intracranial aneurysms in a tertiary-care facility. Consecutive patients, who underwent treatment of intracranial wide-necked and fusiform aneurysms, using Nitinol self-expandable stent from July 2003 to August 2006, were enrolled and the data were analyzed retrospectively. Information on patient demographics, cerebrovascular risk factors, aneurysm size and location were collected. Technical complications including intraoperative rupture, vessel injury, thromboembolic events, and access site complications were also included. Additionally, clinical outcomes were measured using NIHSS and GOS scores at 90 days. The data were analyzed using SPSS software version 11.5 for analyzing mean value with standard deviation, frequencies and percentages of different parameters of these patients’ populations.
Results
A total of 67 patients were included in this study, and were provided with treatment for Neuroform stent for their intracranial aneurysms. The mean age of the patients was 50.51 ± 13.8 years, including females 57 (85.1%), whites 54 (80.6%), and African Americans 11 (16.4%), Hispanics 1, and Asians 1. With regard to the locations of the aneurysm (Table 1), the manifestations were 13 basilar artery (basilar bifurcation 11 cases, basilar trunk 2), 29 internal carotid artery (ICA) (ICA bifurcation five cases, cavernous carotid five cases, ophthalmic carotid 14 cases and supraclinoid carotid five cases), six middle cerebral artery (MCA) (MCA—bifurcation 5 and MCA-M1 in 1), 16 posterior communicating artery (PComA) and vertebro-basilar junction 3. The mean diameter of the aneurysm was 10.28 ± 5.9 mm and ranged from 5 mm to 30 mm.
Successful deployment of stent in the target artery (Fig. 3) was achieved in 65–67 (97%). Two patients required an additional stent for the Y reconstruction of the aneurysm neck (Figs. 2, 4) (Patient No. 3 and 48 on Table 1). The deployment of stent failed in two cases (one in a middle cerebral artery bifurcation, and one in carotid/ophthalmic artery) due to the extreme tortuous nature of the parent artery. These two patients underwent open craniotomy for the treatment of their aneurysm and their outcomes were continuously monitored. Stent prolapsed into the aneurysm without any event during coiling procedure in one patient with a giant BA—trunk aneurysm (Case 61, Table 1). Clinical complications including thromboembolic events and intraoperative rupture of aneurysm were observed in 4/67 cases (Table 1). There was no mortality reported as being a consequence of the procedures. Intraoperative rupture of aneurysm occurred in one patient (unruptured symptomatic BA bifurcation aneurysm: transient episodes of dizziness, imbalances, and visual field cut) during the final stages of the packing of the aneurysm (Case No 48, Table 1). This patient required a Y-neck reconstruction of her BA bifurcation aneurysm before the aneurysm was coiled. No abnormalities were identified in the angiographies obtained before and after the placement of each stent. There was no hemodynamic instability before the rupture. There were no extravasations after aneurysm catheterization or after detachment of first six GDC 360 coils (Boston Scientific Target, Fremont, CA). The angiography obtained prior to the detachment of the seventh coil (3 mm × 6 cm, GDC 360 coil) demonstrated extravasations from the aneurysm in the late phase of the run. There was sudden rise of blood pressure with increased heart rate observed initially, followed by hypertension and bradycardia. Since the patient was under general anesthesia, the only positive neurological examination that could be detected was bilateral dilatation of pupils with poor light responses. An urgent resuscitation was initiated with the hyperventilation, intravenous administration of manitol and extraventricular drainage of cerebrospinal fluid. Additionally, a loading dose, followed by a maintenance dose of intravenous thiopental sodium, was initiated to reduce the metabolic demand of the brain. The aneurysm was rapidly secured, and completely excluded from the circulation with subsequent placement of coils (Fig. 4). Post-procedure computed tomography (CT) of head demonstrated massive intraventricular hemorrhage predominantly presented in the perimesencephalic cisterns. The follow-up serial CT scans revealed progressive resolution of intraventricular blood. This patient was neurologically intact but required a prolonged hospital stay, and achieved a GOS 1 or NIHSS 0 on day 30.
Thromboembolic events were observed in three patients. One patient (Patient No. 15) developed symptomatic stent thrombosis on day 14 after the stent placement, which resolved completely without stroke after intravenous administration of eptifibatide. Another patient suffered from a minor stroke (NIHSS 4) on day 7 after the placement of a Neuroform stent to reconstruct a wide-necked right MCA aneurysm (Table 1, Case 3). This patient was also treated with intravenous eptifibatide for 24 h, and her NIHSS returned to 0 at the time of discharge. The third patient developed a transient ischemic event on post-stenting day 6. These three patients were found to have suboptimal platelet inhibition measured by the platelet aggregation studies despite being on both aspirin and Clopidogrel for more than 7 days. The platelet aggregation studied was performed by light transmission aggregometry using adenosine diphosphate as agonist in our university hospital lab (Accumetrics, San Diego, CA), and considered a reliable test. Routine platelet function testing was not performed for all of our patients.
Technical failure was observed in three cases during procedures; stent stabilizer failed in two cases, and in one case a coil pusher was required to deploy stent. In one case, the stent delivery system could not be navigated due to the extreme tortuosity of the artery; therefore, a medium balanced weight 0.014-inch compatible microwire (Guidant, Santa Clara, CA) was used to provide extra support during navigation.
The immediate and complete occlusion of aneurysms was observed in 32 patients (47.8%) (Figs. 4, 5), neck remnant was present in 25 (37.3%) (Fig. 3), and subtotal occlusion was achieved in 8 (12%) (Fig. 6).
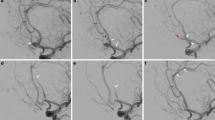
45-years-old man with a symptomatic right carotid artery bifurcation wide-necked aneurysm who required Neuroform stent-assisted coiling of the aneurysm. Anterior–posterior view of the right internal carotid artery angiogram demonstrates that the wide-necked internal carotid artery bifurcation aneurysm has been excluded from the circulation
With regard to the 90 days clinical outcome (Table 1), GOS 1 or NIHSS 0 was observed in 63 (94%) cases. GOS 2 or NIHSS 2 was observed in one patient, who had prior disability from a ruptured aneurysm. GOS 3 or NIHSS 4–6 was observed in another three patients, two of which (Cases 7 and 55, Table 1) were ruptured aneurysms (presented with H&H III and Fisher 4) and the other was a symptomatic unruptured aneurysm (NIHSS 6, GOS 3). The unruptured aneurysm patient presented with a pontine stroke with NIHSS 6 from a BA trunk giant aneurysm (Table 1, Case 63); her symptoms remained unchanged after treatment.
Discussion
The thromboembolic complications associated with Neuroform stent in the treatment of intracranial aneurysms have been described in previous case series [10, 2, 15, 8]. In a study [10], technical difficulties in the deployment of stent occurred in six, inability to deploy the stent in one, stent displacement in two, and inadvertent deployment in one case. Total thromboembolic events were observed in 4/19 cases due to the thrombosis of Neuroform stent. Symptomatic thrombosis of the stent occurred in two patients whereas asymptomatic thrombosis occurred in just one. There were two deaths due to the intracranial hemorrhage in their study [10]. In another study [2], stent deployment failed in 8/56 (14.3%) of patients and thromboembolic events were observed in seven cases. One death and four symptomatic thromboembolic events were reported to be related to the procedures. The majority of the patients were reported to have a good outcome (GOS 1 in 41, GOS 2 in three), and GOS 5 in four, all ruptured aneurysms. There was no short-term or long-term clinical or radiographic outcome data available for these two studies [10, 2]. In a recent study [7], symptomatic thromboembolic events were observed in 3/17, with two being transient and one presenting permanent deficit, which appeared to have a higher incidence than previous studies [10, 2]. No mortality was observed and the clinical outcome appeared appreciable [7]. Our clinical and technical challenges were compared (Table 2) to the studies published in previous reports [2, 10], which demonstrated no significant differences.
It is likely that the recent improvements in the microcatheter of Neuroform stent delivery system, with an improved stent stabilizer device (Neuro 2 and Neuro 3), might have facilitated navigation and deployment of Neuroform stent. In the first generation stent, the microcatheter was upbraided, and therefore subject to obtaining an ovoid shape while navigating through tortuous vessels [15]. As the radius of the curve grew tighter in a vessel, the Microdelivery Catheter got prone to being more oval-shaped. Such distortions could cause the microcatheter to lose up to 60% of its luminal diameter. This reduction in luminal diameter, effectively, locks the stent in place within the catheter causing a significant amount of force to be required to unsheathe the stent. The second generation full-length braiding of the microdelivery catheter had improved its structural integrity and had demonstrated a significant decrease in the ovalization of the catheter when it approached a tight angle of a blood vessel. These modifications had reduced the force needed to deploy the stent by 26% [15]. In the third generation Neuroform stent, the fully braided stabilizer catheter improved the stability of the stabilizer in the deployment of the stent from the microcatheter more successfully and with less friction. Continuous flush of heparinized saline with pressure bag through a stabilizer catheter, as well as through stent delivery catheter, in our study, might also have reduced the friction between hydrophilic microwire and stabilizer catheter. The hydrophilic coatings attracted water molecules, resulting in the formation of a slimy and watery coating on the surface of the material when it was in an aqueous solution. Devices with a hydrophilic coating retain a nonslip surface when dried.
Mechanism of intraoperative rupture of aneurysm in our patient was not clear. Since there were no signs of extravasations on the angiographies obtained after the placement of each stent or catheterization of the aneurysm, or before the detachment of each coil for the first six coils, therefore, the rupture was felt not to be related to the stent, microwire or microcatheter. The possible mechanism of rupture of the aneurysm in our study might be the following: during packing, the seventh coil might have deployed between the framing coils and the weak wall of the aneurysm, which resulted in the rupture, or the Y-neck reconstruction (Fig. 4) might have changed the anatomical conformation with stretching of the wall of the aneurysm, and subsequent coiling of the aneurysm in the same setting may result in the rupture. It was also likely that the Y-stenting might have changed the hemodynamics of the aneurysm blood flow, which might be a contributory factor for the rupture. We hypothesize that the combination effects of the above postulated factors might be responsible for the rupture of the aneurysm. Therefore, the authors recommend that Y-stent aneurysm neck reconstruction should be staged in case of unruptured aneurysm if clinically feasible. Additionally, caution should be made to prevent potential coil perforation of the aneurysm by avoiding unwanted placement of coils between the framing coils and the wall of the aneurysm.
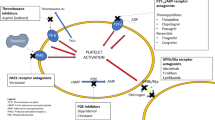
It is likely that the thromboembolic events, associated with the Neuroform stent, are platelet-mediated. Neuroform stent, like any other stent, is a thrombogenic material and acts as a foreign body when entered into blood, leading to platelet activation and aggregation. A platelet monolayer may quickly be formed on the struts of the stent, eventually forming a thrombus if aggregation is left unchecked [33, 10]. Therefore, adequate antiplatelet therapy with clopidogrel (75 mg/d) in conjunction with aspirin (325 mg/d) for at least 7 days before the procedure and then continuing this regimen for at least 1 month after the procedure may be warranted [31, 35]. Antiplatelet therapy with clopidogrel prevents the mitogenic effect of platelet secretion that leads to fibrin production and cellular proliferation and acts synergistically with aspirin significantly reduces the chance of stent related to thromboembolic events during and after the procedure. It is possible to administer a loading (375–600 mg) dose of clopidogrel and 325 mg of aspirin in an urgent situation for complex wide-necked aneurysm patients who may require stent-assisted coiling. This loading dose provides platelet inhibition of 55% in 1 h and 80% within 5 h of administration [31]. Authors do not recommend routine use of loading dose of antiplatelet and use of Neuroform stent in all acute subarachnoid hemorrhage patients, but instead recommend preserving this option for the selected patients who are poor candidates for the surgical clipping of the aneurysm. Additionally, an urgent decompressive craniotomy, indicated for the progressive mass effect induced either by the hematoma or by the cerebral edema in subarachnoid hemorrhage patients, might also be interrupted by the administration of a large dose of the antithrombotic medications. In ruptured aneurysms, authors recommend placement of a ventriculostomy catheter prior to a potential Neuroform stent case to prevent bleeding complications associated with the interaction between an urgent ventriculostomy and therapeutic antithrombotic required for Neuroform stent.
Balloon-assisted temporary aneurysm neck-remodeling and coiling of the aneurysm is an alternative technique used in the treatment of the wide-necked intracranial aneurysms [45–49]. In an experimental study [45], balloon-assisted coiling of the wide-necked intracranial aneurysm had been associated with sudden rise of pressure in the aneurysm. Therefore, it was recommended that the balloon must be inflated and deflated slowly to minimize potential risk of aneurysm rupture in the clinical setting. Studies with small case series demonstrated that balloon-assisted aneurysm neck-remodeling with temporary parent artery occlusion during coiling of the aneurysm were safe and posed no increased risk of aneurysm rupture or thromboembolism [46–48]. However, a recent large-scale study of rupture intracranial aneurysms [49] had revealed that the intraoperative rupture of aneurysms and thromboembolic events are associated with temporary balloon-assisted neck remodeling techniques. The mechanism of thromboembolic events in balloon-assisted coiling of the aneurysm might share the similar pathway as had been observed in balloon angioplasty, which was usually induced by an injury to the vessel wall and subsequent activation of platelets and thrombin cascades [50].
The advantage of Neuroform stent-assisted coiling of the wide-necked intracranial aneurysm might be explained by the following properties of the Neuroform stent. First, the stent provided durable protection to the parent vessel, especially in the case of broad-necked or giant aneurysms. The stent also practically facilitated more complete packing of the aneurysm [10]. Second, the stent redirected flow, disrupting the aneurysm inflow and outflow zones, resulting in “hemodynamic uncoupling” of the parent vessel–aneurysm complex [10]. Subsequently, the flow within the aneurysm became disordered, facilitating aneurysm thrombosis and coil compaction in the inflow zone. Third, a stent provided a physical matrix for endothelial growth, promoting remodeling of the aneurysm neck as well as the parent vessel.
The limitation of our study was that we have not had a true control arm. However, we tried to overcome this by comparing our study with the historic control groups. A comparison study which evaluated the incidence of complications between Neuroform stent and other neck bridging devices such as a balloon would have been ideal. The other limitation was the number of patients and events, which might not have been large enough to draw a significant statistical conclusion.
In conclusion, the challenges of Neuroform stents were thromboembolic events, intra-operative rupture of aneurysm, and technical difficulty in deploying stent in the tortuous blood vessels. Thromboembolic complications in our study were only observed in the postoperative period, which might be associated with poor platelet inhibitions. Further study may be warranted to discover any potential relationship between hyperactive platelets, induced by the Neuroform stent and thromboembolism. If such relationship exists, then a rigorous monitoring of platelet function and antiplatelet regimen might prevent potential thromboembolic events in patients treated with the Neuroform stent.
References
Anderson PG, Boerth NJ, Liu M, McNamara DB, Cornwell TL, Lincoln TM. Cyclic GMP-dependent protein kinase expression in coronary arterial smooth muscle in response to balloon catheter injury. Arterioscler Thromb Vasc Biol 2000;20:2192–7.
Benitez RB, Silva MT, Klem J, Veznedaroglu E, Rosenwasser RH. Endovascular occlusion of wide-necked aneurysm with a new intracranial microstent and detachable coils. Neurosurgery 2004;54:1359–68.
Brilstra EH, Rinkel GJE, Van Der Graaf Y, van Rooij WJJ, Algra A. Treatment of intracranial aneurysms by embolization with coils: systemic review. Stroke 1999;30:470–6.
Byrne JV, Sohn MJ, Molyneux AJ, Chir B. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg 1999;90:656–63.
Casasco AE, Aymard A, Gobin YP, Houdart E, Rogopoulos A, George B, Hodes JE, Cophignon J, Merland JJ. Selective endovascular treatment of 71 intracranial aneurysms with platinum coils. J Neurosurg 1993;79:3–10.
Cognard C, Weill A, Castaings L, Rey A, Moret J. Intracranial berry aneurysms: angiographic and clinical results after endovascular treatment. Radiology 1998;206:499–510.
dos Santos Souza MP, Agid R, Willinsky RA, Cusimano M, Montanera W, Wallace MC, terBrugge KG, Marotta TR. Microstent-assisted coiling for wide-necked intracranial aneurysms. Can J Neurol Sci 2005;32(1):71–81.
Perez-Arjona E, Gordon V, Fessler RD. Basilar artery to bilateral posterior cerebral artery ‘Y stenting’ for endovascular reconstruction of wide-necked basilar apex aneurysms: report of three cases. Neurol Res 2004;26:276–81.
Eskridge JM, Song JK. Endovascular embolization of 150 basilar tip aneurysms with Guglielmi detachable coils: results of the Food and Drug Administration multicenter clinical trial. J Neurosurg 1998;89:81–6.
Fiorella D, Albuquerque FC, Han P, McDougall CG. Preliminary experience using the Neuroform stent for the treatment of cerebral aneurysms. Neurosurgery 2004;54:6–17.
Graves VB, Stother CM, Duff TA, Perl J II. Early treatment of ruptured aneurysms with Guglielmi detachable coils: effect on subsequent bleeding. Neurosurgery 1995;37:640–8.
Gruber DP, Zimmerman GA, Tomsick TA, van Loveren HR, Link MJ, Tew JM Jr. A comparison between endovascular and surgical management of basilar artery apex aneurysms. J Neurosurg 1999;90:868–74.
Hayakawa M, Murayama Y, Duckwilre GR, Gobin YP, Gugliemi G, Vinuela F. Natural history of the neck remnant of a cerebral aneurysms treated with the Gugliemi detachable coil system. J Neurosurg 2000;93:561–8.
Higashida RT, Smith W, Gress D, Uwin R, Dowd CF, Balousek PA, Halbach VV. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of he basilar artery: case report and review of the literature. J Neurosurg 1997;87:944–9.
Howington JU, Hanel RA, Harrigan MR, Levy EI, Guterman LR, Hopkins LN. The neuroform stent, the first microcatheter-delivered stent for use in the intracranial circulation. Neurosurgery. 2004;54:1.
Jobbour P, Kobbe C, Veznedaroglu E, Benitez RP, Rosenwasser R. Stent-assisted coil placement for unruptured cerebral aneurysms. Neurosurgery Focus 2004;17(5):E10 Nov 15.
Johnston HC, Higashida RT, Barrow DL, Caplan LR, Dion JE, Hademenos G, Hopkins LN, Molyneux A, Rosenwasser RH, Vinuela F, Wilson CB. Committee on cerebrovascular imaging of the American heart association council on cardiovascular radiology: recommendation for the endovascular treatment of the intracranial aneurysms: a statement for healthcare professionals from the committee on cerebrovascular imaging of the America heart association council on cardiovascular radiology. Stroke 2002;33:2536–44.
Johnston SC, Wilson CB, Halbach VV, Higashida RT, Dowd CF, McDermott MW, Applebury CB, Farley TL, Gress DR. Endovascular and surgical treatment of unruptured cerebral aneurysms: comparison of risks. Ann Neurol 2000;48:11–9.
Johnston SC, Zhao S, Dudley RA, Berman MF, Gress DR. Treatment of unruptured cerebral aneurysms in California. Stroke 2001;32:597–605.
Koivisto T, Vanninen R, Hurskainen H, Saari T, Hernesniemi J, Vapalathi M. Outcome of early endovascular versus surgical treatment of ruptured cerebral aneurysms: a prospective randomized study. Stroke 2000;31:2369–77.
Kremer C, Groden C, Hansen HC, Gzyska U, Zeumer H. Outcome after endovascular treatment of Hunt and Hess Grade IV or V aneurysms: comparison of anterior versus posterior circulation. Stroke 1999;30:2617–22.
Lempert TE, Malek AM, Halbach VV, Phatouros CC, Meyers PM, Dowd CF, Higashida RT. Endovascular treatment of ruptured posterior circulation cerebral aneurysms: clinical and angiographic outcomes. Stroke 2000;31:100–10.
Levy DI, Ku A. Balloon-assisted coil placement in wide-necked aneurysms: technical note. J Neurosurg 1997;86:724–7.
Levy EI, Hanel RA, Bendok BR, Boulos AS, Hartney ML, Guterman LR, Qureshi AI, Hopkins LN. Staged stent-assisted angioplasty for symptomatic intracranial vertebro-basilar artery stenosis. J Neurosurg 2002;97:1294–301.
Malek AM, Higashida RT, Phatouros CC, Dowd CF, Halbach VV. Treatment of and intracranial aneurysm using a new three-dimensional shape Guglielmi of detachable coil: technical case report. Neurosurgery 1999;44:1142–5.
Malisch TW, Gugliemi G, Vinuela F, Dickwiler G, Gobin YP, Martin NA, Frazee JG. Intracranial aneurysms treated with the Guglielmi detachable coil: midterm clinical results in a consecutive series of 100 patients. J Neurosurg 1997;87:176–83.
Mawad M. Subarachnoid hemorrhage due to late recurrence of previously unruptured aneurysms after complete endovascular occlusion. AJNR Am J Neuroradiol 1998;19:1810–11.
Mericle RA, Wakhloo AK, Rodriguez R, Guterman LR, Hopkins LN. Temporary balloon protection as an adjunct to endovascular soiling of wide-necked cerebral aneurysms: technical note. Neurosurgery 1997;41:975–8.
Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R. International Subarachnoid Aneurysms Trial (ISAT) Collaborative Group: International Subarachnoid Aneurysms Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet 2002;360:1267–74.
Moret J, Cognard C, Weill A, Castaings L, Rey A. Reconstruction technique in the treatment of wide-neck intracranial aneurysms: long-term angiographic and clinical results – report of 56 cases [in French]. J Neuroradiol 1997;24:30–44.
Moshfegh K, Redondo M, Julmy F, Wuillemin WA, Gebauer MU, Haeberli A, Meyer BJ. Antiplatelet effect of clopidogrel compared with aspirin after myocardial infarction: enhanced inhibitory effect of combination therapy. J Am Coll Cardiol 2000;36:699–705.
Phatoursos CC, Sasaki TY, Higashida RT, Malek AM, Meyers PM, Dowd CF, Halbach VV. Stent-supported coil embolization: the treatment of fusiform and wide-neck aneurysms and pseudoaneurysms. Neurosurgery 2000;47:107–15.
Qureshi AI, Luft AR, Sharma M, Guterman LR, Hopkins LN. Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures: part 1 – pathophysiological and pharmacological features. Neurosurgery 2000;46:1344–59.
Raftopoulos C, Mathurin P, Boscherini D, Billa RF, Van Boven M, Hantson P. Prospective analysis of aneurysm treatment in a series of 103 consecutive patients when endovascular embolization is considered the first option. J Neurosurgery 2000;93:175–82.
Savcic M, Hauert J, Bachman F, Wyld PJ, Geudelin B, Cariou R. Clopidogrel loading dose regiments: kinetic profile of pharmacodynamic response in healthy subjects. Semin Thromb Hemost 1999;25(suppl 2):15–9.
Sekhon LH, Morgan MK, Sorby W, Grinnell V. Combined endovascular stent implantation and endovascular coil placement for the treatment of a wide-necked vertebral artery aneurysm: technical case report. Neurosurgery 1998;43:380–4.
Sturaitis MK, Rinne J, Chaloupka JC, Kaynar M, Lin Z, Awad IA. Impact of Guglielmi detachable coils on outcomes of patients with intracranial aneurysms treated by a multidisciplinary team at a single institution. J Neurosurg 2000;93:569–580.
Tanaka H, Sukhova GK, Swanson SJ, Clinton SK, Ganz P, Cybulsky MI, Libby P. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation 1993;88:1788–803.
Uda K, Goto K, Ogata N, Izumi N, Nagata S, Matsuno H. Embolization of cerebral aneurysms using Guglielmi detachable coils: problems and treatment plans in the acute stage after subarachnoid hemorrhage and long-term efficiency. Neurol Med Chir (Toyko) 1998;38:143–54.
Vannien R, Koivisto T, Saari T, Hermesniemi J, Vapalahti M. Ruptured intracranial aneurysms: acute endovascular treatment with electrolytically detachable coils – a prospectively randomized study. Radiology 1999;211:325–36.
Wainwright CL, Miller AM, Wadsworth RM. Inflammation as a key event in the development of neointima following vascular balloon injury. Clin Exp Pharmacol Physiol 2001;28:891–5.
Wakhloo AK, Lanzino G, Lieber BB, Hopkins LN. Stents for intracranial aneurysms: the beginning of a new endovascular era? Neurosurgery 1998;43:377–9.
Raymond J, Guilbert F, Roy D. Neck-bridge device for endovascular treatment of wide-neck bifurcation aneurysms: initial experience. Radiology 2001;221(2):318–26.
International Study of Unruptured Intracranial Aneurysms (ISUIA) Investigators. Results of the international study of unruptured intracranial aneurysms. The Lancet 2003;362:103–10.
Akiba Y, Murayama Y, Vinuela F, Lofkowitz MA, Duckwiler GR, Gobin YP. Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysm: part I-experimental evaluation. Neurosurgery 1999;45(3):519–27; discussion 527–30.
Lefkowitz MA, Gobin YP, Akiba Y, Duckwoler GR, Murayama T, Guglielmi G, Martin NA, Vinuela F. Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysm: part II-clinical results. Neurosurgery 1999;45(3):531–7; discussion 537–8.
Cottier JP, Pasco A, Gallas S, Gabrillargues J, Cognard C, Drouineau J, Brunereau L, Herbreteau D. Utility of balloon-assisted Guglielmi detachable coiling in the treatment of 49 cerebral aneurysms: a retrospective, multicenter study. AJNR Am J Neuroradiol 2001;22(2):345–51.
Ross IB, Dhillon GS. Complications of endovascular treatment of cerebral aneurysms. Surg Neurol 2005;64(1):12–8; discussion 18–9.
van Rooij WJ, Sluzewski M, Beute GN, Jijssen PC. Procedure complications of coiling of ruptured intracranial aneurysms: incidence and risk factors in consecutive series of 681 patients. AJNR Am J Neuroradial 2006;27(7):1498–501.
Harker LA. Role of platelets and thrombosis in mechanism of acute occlusion and restenosis after angioplasty. Am J Cardiol 1987;60(3):20B–8B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yahia, A.M., Gordon, V., Whapham, J. et al. Complications of Neuroform Stent in Endovascular Treatment of Intracranial Aneurysms. Neurocrit Care 8, 19–30 (2008). https://doi.org/10.1007/s12028-007-9001-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-007-9001-7