Abstract
Purpose
Endovascular treatment of unruptured intracranial aneurysms with stent-assisted coiling or flow diverter stents requires a prophylactic antiplatelet premedication to avoid thrombo-embolic complications. Guidelines for optimal antiplatelet regimens are poorly defined. The aim of this study is to report our experience using a high dosage antiplatelet premedication regimen for patients with unruptured intracranial aneurysms undergoing endovascular treatment by stent-assisted coiling or flow diverter stents.
Methods
From a retrospective analysis of a prospectively maintained database, we collected clinical and angiographic data of 400 procedures in 362 patients treated by stent-assisted coiling or flow diverter stents for 419 unruptured intracranial aneurysms. Descriptive and analytic statistics were performed to report morbidity, mortality, and complication rates and to demonstrate associations between variables and outcomes. Logistic multivariable regression was performed to rule out confounding factors between subgroups.
Results
Thrombo-embolic complications occurred in 23/400 procedures (5.75%) and hemorrhagic complications in 19/400 procedures (4.75%). The majority of complications were minor and transient with overall procedure-related morbidity and mortality rates of 1.75% (n = 7/400) and 1.25% (n = 5/400) respectively. The co-existence of multiple cardiovascular risk factors among smoking, hypertension, dyslipidemia, and age > 65 years old was significantly associated with permanent procedure-related morbidity (p = 0.006) and thrombo-embolic complications occurrence (p = 0.034). Age alone was associated with higher permanent morbidity (p = 0.029) and was the only variable associated with higher hemorrhagic complication (p = 0.024).
Conclusion
In this study, the use of a high dosage antiplatelet premedication was safe and effective for the treatment of unruptured intracranial aneurysms with stent-assisted coiling or flow diverter stents. Mortality and morbidity rates compare favorably with the current literature. The thrombo-embolic complications rate is low and most of them were clinically silent. However, the hemorrhagic complications rate was substantial and a significant proportion of them were associated with mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last 15 years, stenting techniques have been developed to allow the endovascular treatment (EVT) of unruptured intracranial aneurysms (UIA) that were not amenable to treatment by simple coiling. Stenting techniques are now accepted as reliable therapeutic options particularly for complex UIA. Stents are used in conjunction with coils or as stand-alone devices. Classic stents (braided or laser cut) are mostly used for stent-assisted coiling (SAC) whereas flow diverter (FD) stents are used as stand-alone devices. Flow diverter stents are indicated for complex UIA, such as large necked or bifurcation UIA with side branches, in particular those located in the internal carotid artery or the vertebral-basilar arteries. PCONus stents are another option for bifurcation aneurysms [1,2,3].
In order to limit thrombo-embolic (TE) complications, it is necessary to reduce platelet reactivity prior to EVT with SAC or FD stents. The standard strategy is to use a dual antiplatelet (AP) therapy, combining aspirin and a P2Y12 inhibitor, such as clopidogrel, prasugrel, or ticagrelor [4]. In our institution, a specific regimen was implemented in 2011, promoting the use of aspirin and clopidogrel in high dosage only the day before and on the morning of the procedure. This contrasts with the usual prophylactic regimen for the elective EVT of an UIA, classically longer than 3 days and using a lower dosage [5]. The main rationale for this protocol was to increase the patients’ compliance. Indeed, as the patient was always admitted on the day before the procedure, the medical team could ensure that the premedication was taken.
To the best of our knowledge, there is currently no guideline about which drug, dosage, and protocol should be used in neurovascular treatments with stents. Only a few papers on the subject have been published and no high-evidence-level study demonstrated whether one premedication should be used rather than another [4, 6]. Moreover, there is a high variability of patterns regarding AP premedication among neurovascular teams [5]. In that context, it is crucial to explore that area by reporting experiences using different protocols and to determine guidelines, in order to provide the best care to patients.
The aim of this study is to report our monocentric experience using this specific and unusual AP premedication and to discuss the management of platelet inhibition prior to neurovascular procedures using SAC or FD stents for the EVT of UIA.
Patients and methods
Study design
This study was approved by our institutional ethical committee. We retrospectively analyzed our prospectively maintained database to identify all patients treated in our institution with SAC or FD for one or multiple UIA between January 2011 and September 2016. This period was chosen because at this time a new specific AP regimen was implemented. During this period, patients with a weight ≤ 80 kg received a loading dose of 320 mg of aspirin and 300 mg of clopidogrel the day before and on the morning of the procedure. For patients > 80 kg, the same regimen was empirically administered with higher dosages: 450 mg of clopidogrel and 480 mg of aspirin. No platelet function monitoring was used.
From the retrospective analysis of medical files, we gathered all variables available from the time of the procedure up to the 3-month follow-up. We analyzed clinical and demographic data along with procedural protocols and angiographic results. The final procedural outcome and the complications were also reported based on the 3-month follow-up medical reports.
Permanent morbidity, also referred as morbidity, was defined by the association of three criteria: (1) a mRS (modified Rankin Score) superior or equal to 2 but different to 6 (death) at the 3-month follow-up, (2) an increase of at least 1 of the mRS at 3-month follow-up compared with the baseline mRS, and (3) the occurrence of a peri-procedural complication associated with that increase.
The inclusion criteria were the use of the prophylactic regimen described in the last paragraph and the elective treatment of an UIA using a stenting technique (SAC or stent as stand-alone device, including FD stents). The exclusion criteria were the use of a stenting technique for another indication (ruptured aneurysms, dissecting aneurysms, dissections, stenosis) and the use of a different AP protocol. All consecutive patients matching those criteria were included.
Complications were assessed in two different ways. First, according to the chronology of the complication (intraprocedural vs post-procedural). Second, according to the type of complication (TE complication vs hemorrhagic (HH) complication). A major HH complication was defined as a type 2 bleeding or more according to the Bleeding Academic Research Consortium Definition for Bleeding [7].
Treatment technique
The decision regarding the most appropriate treatment was made for each patient by a multidisciplinary neurovascular team. All procedures were performed by a senior interventional neuroradiologist. Each patient underwent a complete neurological examination by neurologists before and after EVT. Patients were monitored 24 h in our intensive care unit after EVT. Every patient received a dual AP therapy for three months (80 mg of aspirin and 75 mg of clopidogrel) and was then seen in consultation by a senior neurointerventionalist. All patients received aspirin 80 to 100 mg for life after the 3-month appointment. In selected cases, clopidogrel 75 mg was also prescribed for a duration of 3 extra months.
Endovascular procedures were performed under general anesthesia and systemic heparinization that was monitored by frequent measurements of the activated clotting time (ACT). A baseline ACT was obtained prior to the bolus infusion of a high-loading dose of heparin (5000 IU), and then every 30 min. The heparin bolus was followed by a continuous drip (2000 to 2500 IU/h), with the purpose of doubling the baseline ACT. Systemic heparinization was prolonged for 12–24 h in all patients. The procedures were performed through 6F or 8F common femoral arterial access. In tortuous anatomies and in procedures with flow diverter (FD) stents, a coaxial system was used including a long sheath placed in the common carotid artery (Neuron max, Penumbra Inc., Alameda, CA, USA or IVA 6F, Balt, Montmorency, France) and a 5 or 6F intermediate catheter placed as high as required to obtain stability up to the cavernous segment of the ICA. In other cases, a single 6F guiding catheter (Envoy or Envoy DA XB, Codman Neurovascular, Raynham, MA, USA) was placed up to the upper cervical portion of the ICA. Dedicated microcatheters were used to deliver the stents and were navigated with Synchro 14 (Stryker NeuroVascular, Kalamazoo, MI, USA), Terumo 12 (Microvention, Aliso Viejo, CA, USA), and Traxcess (Microvention, Aliso Viejo, CA, USA) guidewires. When additional coiling was performed, Prowler select LP (Codman Neurovascular, Raynham, MA, USA), Headway 17 (MicroVention, Aliso Viejo, CA, USA), and Headway Duo (MicroVention, Aliso Viejo, CA, USA) microcatheters were used using a jailing technique for FD stents and by navigating the microcatheter through the stent mesh for conventional stents. A femoral closure device (Angioseal 6 or 8F (St-Jude Medical, Saint-Paul, MI, USA) and Proglide (Abott, Chicago, IL, USA)) was always placed to obtain hemostasis at the groin.
Statistical analysis
Quantitative data were expressed in mean values ± standard deviation (SD) or medians and 95% confidence intervals (CI), accordingly, after verification of normality of distributions by the Kolmogorov-Smirnov test. Percentages were presented in the categories of qualitative variables. Associations between different outcomes (mortality, morbidity, TE complications, and HH complications) and potential risk factors were analyzed with the χ2 test and Fisher’s exact test. Cochran-Armitage tests were used to study linear tendencies of proportions of outcome in ordinal variables. Student’s t test was used to compare means of quantitative variables if the distribution were normal. Odds ratios with their 95% CI were calculated using univariate and multivariable logistic regression to rule out confounding variables. The level of statistical significance was determined as 0.05. The software used to lead the statistical analysis was Stata/IC 15.1.
In patients with more than one procedure included, only the last one was considered for the univariate and multivariate statistical analysis. The rationale was to avoid a statistical bias because two or more procedures in the same patient are not independent from one another. If more than one aneurysm was treated during one procedure, only the largest was considered in the analysis. If more than one stent was used, only the first one was considered.
Multivariable logistic regression was performed for TE and HH complications only, because there were not enough patients in the permanent morbidity and mortality groups. Moreover, only multivariable analyses with two predictors were possible due to the small number of complications. The potential confounding variables tested were age, cardiovascular (CV) risk factors, aneurysm location, aneurysm size, aneurysm type, use of a pCONus stent, and use of a flow diverter stent. The predictor was considered a confounding variable if the adjusted odd ratio differed by more than 15% from the unadjusted odd ratio.
Results
Patients, aneurysms, and procedures
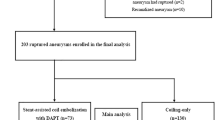
From this analysis, we have included 400 elective procedures for the treatment of 419 UIA in 362 patients.
The mean age was 53.6 ± 12.4 years old with 77.3% of women (n = 280/362) and 22.7% of men (n = 82/362). The current or past smokers represented 55.5% of patients (n = 201/362) and 81.8% (n = 296/362) had at least one CV risk factor among smoking, hypertension, dyslipidemia, and age > 65 years old. The baseline mRS was ≤ 2 in 99.4% (n = 360/362) of them. Full patients’ characteristics are summarized in Table 1.
Aneurysms were mostly saccular (61.9%—n = 259/419) and wide-necked (86.6%—n = 348/402). The median maximal diameter was 5.23 mm (95% CI [4.73–5.75]). The most common location was the internal carotid artery, representing 42.0% of the aneurysms, and the second most common location was the middle cerebral artery representing 26.2%. Full aneurysms characteristics are summarized in Table 2.
Three hundred eighty-five patients (96.2%) received doses of 300 mg/320 mg of clopidogrel/aspirin and 15 patients (3.7%) received 450 mg/480 mg respectively. No other premedication protocol was used. Braided and laser cut stents were used in 309 procedures (68.9%), FD stents in 116 procedures (25.8%), and pCONus stents in 24 procedures (5.3%). Complete obliteration was seen in 52.5% of the procedures. Full procedure details are summarized in Table 3.
Outcomes
Thrombo-embolic complications occurred in 23 procedures (n = 23/400—5.75%) with 6 cases (n = 6/400—1.50%) associated with permanent morbidity or mortality at 3-month follow-up. Hemorrhagic (HH) complications occurred in 19 procedures (n = 19/400—4.75%) with 6 cases (n = 6/400—1.50%) associated with permanent morbidity or mortality at 3-month follow-up (Table 4).
Thrombo-embolic complications were seen during EVT (n = 12/400—3.00%) on DSA controls or were diagnosed as post-procedural stroke or symptomatic ischemic spots on MRI (n = 11/400—2.75%). Intraprocedural TE complications were successfully managed by administration of IV abciximab (n = 11/400—2.75%) or by balloon angioplasty (n = 1/400—0.25%). In 11 of these 12 complications, no permanent morbidity nor mortality were observed; in one of them treated with IV abciximab, a post-procedural hemorrhage occurred resulting in death. Among the post-procedural TE complications, there were 8 cases (n = 8/400—2.00%) of post-procedural strokes: they were all managed conservatively at the ICU and 6 of them were associated with permanent morbidity.
Major hemorrhagic complications occurred in 19 procedures (n = 19/400—4.75%). Retroperitoneal hemorrhages with anemia occurred in 8 cases (n = 8/400—2.00%) and were managed with blood transfusion. Post-procedural intracranial hemorrhages were seen in 9 procedures (n = 9/400—2.25%): 8 were subarachnoid or intraparenchymal hemorrhages from the treated aneurysm and one was an intraparenchymal hemorrhage distant from the aneurysm. Two iatrogenic aneurysmal ruptures occurred (n = 2/400—0.50%) and one of them was associated with morbidity. Three hemorrhagic complications were asymptomatic (n = 3/400—0.75%): two post-procedural intracranial hemorrhages, diagnosed on the control CT, and one aneurysmal perforation, managed during the procedure. All intracranial hemorrhages were managed by a supportive treatment in the ICU.
The procedure-related morbidity and mortality rates were of 1.75% (n = 7/400) and 1.25% (n = 5/400) respectively. Among complications associated with morbidity, 6 were due to TE complications (post-procedural strokes) and only 1 to a HH complication (iatrogenic aneurysmal rupture). All 5 cases of mortality were related to a HH complication, one of them after IV abciximab for the treatment of a TE complication.
Factors associated with outcome
There were no significant differences in terms of TE and HH complications, permanent morbidity, or mortality between the two types of AP regimen. Procedural (stent type, number of stents, aneurysm features) and demographic (cardiovascular risk factors, age) characteristics were not associated with procedure-related mortality, but the number of patients in that group was low (n = 5).
The co-existence of multiple CV risk factors among smoking, hypertension, dyslipidemia, and age > 65 years old was significantly associated with permanent procedure-related morbidity (p = 0.006) and TE complication occurrence (p = 0.034). There is a linear tendency between the number of CV risk factors cumulated and TE complication (p = 0.0025).
Age alone was associated with higher permanent morbidity (p = 0.029) and was the only variable associated with higher HH complication (p = 0.024), with a linear tendency (p = 0.0077).
The type of stent showed a significant association with procedure-related permanent morbidity and TE complications (p = 0.029 and p = 0.004, respectively). Among the stent types, pCONus were shown to have significantly higher rates of morbidity and TE complications, compared with laser cut for morbidity, and compared with either braided stents, laser cut, or FD for TE complications. There was no significant difference between other stents in terms of complications and permanent morbidity/mortality.
The aneurysm type, size range, and location did not influence the occurrence of TE or HH complication nor the procedure-related permanent morbidity/mortality. Neither did the number of stents implanted nor the number of aneurysms treated in one procedure.
Univariate statistical analyses are detailed in Tables 5, 6, 7, and 8.
Multivariable analysis and confounding variables
Risk factors of TE complications
An age superior or equal to 65 years old was in many cases independently significantly associated with TE complications, except we took into account the presence of 2 or more CV risks (p = 0.22) or the use of a pCONus device (p = 0.07).
The presence of 2 or more CV risk factors was a better predictor of TE complications as it was almost always (adjusted for age, p = 0.06) independently associated with TE complications. Adjusted for age, the crude OR (3.77 (1.28–8.87)) decreased from 19%, suggesting age was a confounding factor in the association.
The use of pCONus was an even better predictor and showed a stronger independent association with TE complications, although highly variable (wide confidence intervals of OR). Adjusted for age or for the number of risk factors, the crude OR (5.45 (1.62–18.31)) decreased from 18 or 25%.
Risk factors of HH complications
An age superior or equal to 65 years old and an aneurism size smaller than 5 mm were always independently associated with HH complications.
Saccular aneurysm was no longer associated with HH complications when we adjusted the OR for other predictors.
Crude and adjusted odd ratios are presented in Tables 9, 10, 11, and 12.
Discussion
Findings
In this study, our specific AP regimen was shown to be safe and effective for the premedication of patients undergoing elective EVT of UIA by SAC or FD stents. The thrombo-embolic complications rate is low and most of them were clinically silent. However, the hemorrhagic complications rate was substantial and a significant proportion of them were associated with mortality.
Background
There are 2 types of major complications occurring during or after EVT of UIA: the most frequent is a TE event; the second, a HH complication [8,9,10]. The occurrence of TE complications during or after a stenting procedure for EVT of UIA is estimated between 2 and 21% in the current literature; for HH complications, the rates are estimated between 2.2 and 11.6% [5, 8,9,10,11,12]. These results vary widely between studies for the following reasons: definition of complications, stent type, aneurysm features, populations aimed, practice patterns, and operators’ experience [4, 5].
In this study, the TE complication rate was 5.75% and the overall major HH complication rate was 4.75%. The rate of intracranial hemorrhage was 2.16% (n = 10/400), the other ones being retroperitoneal hematomas requiring blood transfusion. All of the complications leading to mortality were associated with an intracranial hemorrhage. Most of the complications could safely be managed so that the procedure-related morbidity and mortality were of 1.75% and 1.25% respectively. These results are in accordance, or even compare favorably with series published in the literature including meta-analysis on SAC and FD treatments [3, 5, 8,9,10,11,12,13,14]. However, no objective comparison can be made considering the significant disparities between those series.
TE complications
The low occurrence of TE complications may be explained by the following reasons: (1) full control on the patient’s compliance; (2) strict and monitored heparinization protocol, with a high-loading dose bolus and control of the ACT every 30 min; (3) high experience center, with over 200 aneurysms treated by EVT each year, among which 75% with SAC or FD [1, 5]; (4) high-loading dose AP regimen, which might overcome peri-procedural complications in patients with partial clopidogrel resistance. This latter is known to be a major topic in both cardiological and neurovascular procedures [6, 8].
Clopidogrel resistance
Clopidogrel resistance is an important issue associated with an increased risk of TE complications [6, 8]. Whether or not this high dosage premedication might overcome those complications in this cohort is merely speculative: the platelet response was not assessed, and patients were not tested for clopidogrel resistance. However, in the recent literature, some studies have shown the efficacy of using high dosages of aspirin and clopidogrel in preventing TE complications in clopidogrel-resistant patients [15,16,17,18]. Considering the high prevalence of clopidogrel resistance in the population and the results of our study, it seems that using empirically such a high dosage regimen without any platelet function test is a reliable option for neurovascular procedures. In the non-responder patients, a change of strategy could be adopted, for instance a switch towards another AP drug.
The issue of clopidogrel resistance is already well-known in cardiology as it is associated with ischemic complications in patients undergoing percutaneous coronary interventions [8, 9, 19]. In these patients, 4 to 30% do not demonstrate an adequate platelet inhibition with standard doses of clopidogrel [20]. Many solutions were adopted to ensure a predictable and efficient platelet inhibition. The first and most accepted solution was to quantify the platelet reactivity under AP medication using a platelet function test, in order to define the patient as responder, hyporesponder, or non-responder so that the management could be adjusted accordingly [8, 15]. There is however currently no strong evidence about tailoring the AP premedication on the basis of platelet function test in neurointerventional literature. In a recent meta-analysis, Skukalek et al. showed no evidence supporting the use of platelet function test as it did not change the clinical outcome but only the occurrence of asymptomatic ischemic lesions [16]. Moreover, no sufficiently high level of certitude studies have been published yet, and most publications in this area are retrospective, case series, or expert opinions [4, 5, 15, 17, 18, 21]. For all these reasons, we never perform any platelet function test and it does not have a significant impact on our TE rate that is low.
The bodyweight is also known to be a risk factor for subtherapeutic AP therapy, justifying the use of increased doses in patients with body mass index > 25 [22]. In our series, we reported no significant difference, in terms of TE or HH complications, between the 2 regimens.
HH complications
Even if the retrospective non-comparative feature of this study does not allow to demonstrate an increase of hemorrhagic complications with a high-loading dose of aspirin and clopidogrel, it is questionable whether it might promote them. The hemorrhagic complications rate is substantial, and the mortality rate is mainly explained by bleeding. Five patients died in this study, four of them from a post-procedural intracranial hemorrhage and one from an iatrogenic bleeding after administration of abciximab, in the context of intraprocedural stent thrombosis. Moreover, age was shown to be a predictive factor for a HH complication.
In a retrospective comparative study on Pipeline embolization device, Attalah et al. [23] did not observe a significant difference in terms of hemorrhagic complications between standard and high-loading protocol, though they emphasized the higher hemorrhage rate in the loading dose group. In a retrospective study, White et al. [12] suggested platelet overinhibition as a potential mechanism for post-procedural delayed hemorrhage following flow diversion of UIA. Considering the high doses of dual AP therapy used in this study, it is worth considering whether an overinhibition might have played a role in the bleeding that occurred. Since no platelet activity monitoring was used, it is not possible to verify this hypothesis.
Another component that might promote hemorrhage is the administration of unfractionated heparin until 24 h after the procedure. It is still controversial whether or not it should be administered [24]. Nevertheless, the combination of a high-dosing AP premedication with post-procedural heparin probably increase the hemorrhage risk. This should maybe lead to adapt the post-procedural heparin protocol in order to reduce that risk. Further prospective studies are needed to confirm these hypotheses.
Future considerations
A promising change of practice is to use other platelet inhibitors such as prasugrel and ticagrelor, already known in cardiology [25]. Even if there are still no significant studies to recommend the use of these drugs, a few papers have recently been published and have shown encouraging results [4, 26]. When these molecules could be most interesting for clopidogrel-resistant patients in whom there is no bioactivation, they also could replace clopidogrel in the general practice and thus allow to avoid the clopidogrel resistance issue.
Limitations
Our study has several limitations. First, the monocentric retrospective design inherently presents multiple biases, even if our protocol was prospectively implemented. Second, the operators’ technical experience constitutes a possible confounding variable. Indeed, as our daily practice in neurovascular stenting is high, some technical features of stenting, in particular the quality of stents apposition, may not be comparable with other centers. Hence, this may affect our TE complications rate because it has been showed that the stent apposition on the arterial wall is directly correlated with the stenting efficiency [27]. Third, the specific regimen dedicated to patients with a weight > 80 kg remains arbitrary and no evidence supports its use. Moreover, this medication was only used in 15 procedures; thus, no conclusion can be drawn on that. Further prospective studies should be performed, particularly to assess the relationship between the AP regimen and the body mass index. Fourth, in univariate and multivariable tests, the statistical analysis had to leave out 38 procedures. They could not be included in those analyses because only one procedure per patient could be included (the most recent was considered as mentioned before). The rationale was to avoid statistical bias due to dependency between two procedures in a same patient. Among those 38 procedures, three TE complications and one HH complication happened. They were included in the complication rates anyway, in order not to occult them. At last, we acknowledge the lack of generalizability of pre-admitting patients the day before the elective procedures.
Conclusion
Stenting techniques are increasingly used for EVT of UIA. The need of AP premedication remains a major concern for stent placement because no guideline has been published yet and practice patterns are highly heterogeneous. This study shows that the use of high dosages of aspirin and clopidogrel is safe and effective for the premedication of patients undergoing EVT of UIA by ST. Mortality and morbidity rates compare favorably with the current literature. The TE complications rate is low and most of them were clinically silent. However, the HH complications rate was substantial and a significant proportion of them were associated with mortality. Further prospective randomized control trials are needed to confirm those findings with comparing standard vs high dosing of AP premedication and with including new AP drugs such as prasugrel or ticagrelor, considered as new promising therapeutic options for the premedication of patients undergoing EVT for UIA.
Contributorship
Each author made a substantial contribution to this work (procedures, patients follow-up, study design, data analysis, manuscript correction, and submission). Each of them gave their approval before the submission of this paper and they agreed to be accountable for all aspects of the work.
Abbreviations
- EVT:
-
Endovascular treatment
- TE:
-
Thrombo-embolic
- ST:
-
Stenting techniques
- HH:
-
Hemorrhagic
- SAC:
-
Stent-assisted coiling
- FD:
-
Flow diverter
- ACT:
-
Activated clotting time
- AP:
-
Antiplatelet
- INR:
-
Interventional neuroradiology
- UIA:
-
Unruptured intracranial aneurysm
- mRS:
-
Modified Rankin Score
- CV:
-
Cardiovascular
References
Thompson BG, Brown RD, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES, Duckwiler GR, Harris CC, Howard VJ, Johnston SCC, Meyers PM, Molyneux A, Ogilvy CS, Ringer AJ, Torner J, American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention, American Heart Association, American Stroke Association (2015) Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46:2368–2400. https://doi.org/10.1161/STR.0000000000000070
Molyneux AJ, RSC K, Yu L-M, Clarke M, Sneade M, Yarnold JA, Sandercock P, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group (2005) International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 366:809–817. https://doi.org/10.1016/S0140-6736(05)67214-5
Lv X, Yang H, Liu P, Li Y (2016) Flow-diverter devices in the treatment of intracranial aneurysms: a meta-analysis and systematic review. Neuroradiol J 29:66–71. https://doi.org/10.1177/1971400915621321
Kim KS, Fraser JF, Grupke S, Cook AM (2018) Management of antiplatelet therapy in patients undergoing neuroendovascular procedures. J Neurosurg 129:890–905. https://doi.org/10.3171/2017.5.JNS162307
Faught RWF, Satti SR, Hurst RW, Pukenas BA, Smith MJ (2014) Heterogeneous practice patterns regarding antiplatelet medications for neuroendovascular stenting in the USA: a multicenter survey. J Neurointerv Surg 6:774–779. https://doi.org/10.1136/neurintsurg-2013-010954
Shim EJ, Ryu C-W, Park S, Lee HN, Shin HS, Kim S-B (2018) Relationship between adverse events and antiplatelet drug resistance in neurovascular intervention: a meta-analysis. J Neurointerv Surg. https://doi.org/10.1136/neurintsurg-2017-013632
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H (2011) Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 123:2736–2747. https://doi.org/10.1161/CIRCULATIONAHA.110.009449
Fifi JT, Brockington C, Narang J, Leesch W, Ewing SL, Bennet H, Berenstein A, Chong J (2013) Clopidogrel resistance is associated with thromboembolic complications in patients undergoing neurovascular stenting. AJNR Am J Neuroradiol 34:716–720. https://doi.org/10.3174/ajnr.A3405
Yang H, Li Y, Jiang Y (2016) Insufficient platelet inhibition and thromboembolic complications in patients with intracranial aneurysms after stent placement. J Neurosurg 125:247–253. https://doi.org/10.3171/2015.6.JNS1511
Shapiro M, Becske T, Sahlein D, Babb J, Nelson PK (2012) Stent-supported aneurysm coiling: a literature survey of treatment and follow-up. AJNR Am J Neuroradiol 33:159–163. https://doi.org/10.3174/ajnr.A2719
Kim DJ, Suh SH, Kim BM, Kim DI, Huh SK, Lee JW (2010) Hemorrhagic complications related to the stent-remodeled coil embolization of intracranial aneurysms. Neurosurgery 67:73–78; discussion 78-79. https://doi.org/10.1227/01.NEU.0000370937.70207.95
White AC, Kumpe DA, Roark CD, Case DE, Seinfeld J (2018) Patterns, predictors, and outcomes of postprocedure delayed hemorrhage following flow diversion for intracranial aneurysm treatment. World Neurosurg 115:e97–e104. https://doi.org/10.1016/j.wneu.2018.03.190
Phan K, Huo YR, Jia F, Phan S, Rao PJ, Mobbs RJ, Mortimer AM (2016) Meta-analysis of stent-assisted coiling versus coiling-only for the treatment of intracranial aneurysms. J Clin Neurosci 31:15–22. https://doi.org/10.1016/j.jocn.2016.01.035
Wang F, Chen X, Wang Y, Bai P, Wang H-Z, Sun T, Yu H-L (2016) Stent-assisted coiling and balloon-assisted coiling in the management of intracranial aneurysms: a systematic review & meta-analysis. J Neurol Sci 364:160–166. https://doi.org/10.1016/j.jns.2016.03.041
Adeeb N, Griessenauer CJ, Foreman PM, Moore JM, Shallwani H, Motiei-Langroudi R, Alturki A, Siddiqui AH, Levy EI, Harrigan MR, Ogilvy CS, Thomas AJ (2017) Use of platelet function testing before pipeline embolization device placement: a multicenter cohort study. Stroke 48:1322–1330. https://doi.org/10.1161/STROKEAHA.116.015308
Skukalek SL, Winkler AM, Kang J, Dion JE, Cawley CM, Webb A, Dannenbaum MJ, Schuette AJ, Asbury B, Tong FC (2016) Effect of antiplatelet therapy and platelet function testing on hemorrhagic and thrombotic complications in patients with cerebral aneurysms treated with the pipeline embolization device: a review and meta-analysis. J Neurointerv Surg 8:58–65. https://doi.org/10.1136/neurintsurg-2014-011145
Oran I, Cinar C, Bozkaya H, Korkmaz M (2015) Tailoring platelet inhibition according to multiple electrode aggregometry decreases the rate of thrombotic complications after intracranial flow-diverting stent implantation. J Neurointerv Surg 7:357–362. https://doi.org/10.1136/neurintsurg-2013-011023
Hwang G, Huh W, Lee JS, Villavicencio JB, Villamor RBV, Ahn SY, Kim J, Chang JY, Park SJ, Park N-M, Jeong E-A, Kwon O-K (2015) Standard vs modified antiplatelet preparation for preventing thromboembolic events in patients with high on-treatment platelet reactivity undergoing coil embolization for an unruptured intracranial aneurysm: a randomized clinical trial. JAMA Neurol 72:764–772. https://doi.org/10.1001/jamaneurol.2015.0654
Tantry US, Bonello L, Aradi D, Price MJ, Jeong Y-H, Angiolillo DJ, Stone GW, Curzen N, Geisler T, Ten Berg J, Kirtane A, Siller-Matula J, Mahla E, Becker RC, Bhatt DL, Waksman R, Rao SV, Alexopoulos D, Marcucci R, Reny J-L, Trenk D, Sibbing D, Gurbel PA, Working Group on On-Treatment Platelet R (2013) Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 62:2261–2273. https://doi.org/10.1016/j.jacc.2013.07.101
Nguyen TA, Diodati JG, Pharand C (2005) Resistance to clopidogrel: a review of the evidence. J Am Coll Cardiol 45:1157–1164. https://doi.org/10.1016/j.jacc.2005.01.034
Brinjikji W, Lanzino G, Cloft HJ, Siddiqui AH, Hanel RA, Kallmes DF (2015) Platelet testing is associated with worse clinical outcomes for patients treated with the pipeline embolization device. AJNR Am J Neuroradiol 36:2090–2095. https://doi.org/10.3174/ajnr.A4411
Drazin D, Choulakian A, Nuño M, Kornbluth P, Alexander MJ (2011) Body weight: a risk factor for subtherapeutic antithrombotic therapy in neurovascular stenting. J Neurointerv Surg 3:177–181. https://doi.org/10.1136/jnis.2010.004085
Atallah E, Saad H, Bekelis K, Chalouhi N, Tjoumakaris S, Hasan D, Zarzour H, Smith M, Rosenwasser RH, Jabbour P (2017) Safety and efficacy of a 600-mg loading dose of clopidogrel 24 hours before pipeline embolization device treatment. World Neurosurg 106:529–535. https://doi.org/10.1016/j.wneu.2017.07.019
Zaidat OO (2009) Periprocedural management of patients with endovascular treatment of intracranial atherosclerotic disease. J Neuroimaging 19(Suppl 1):35S–38S. https://doi.org/10.1111/j.1552-6569.2009.00421.x
Gurbel PA, Bliden KP, Butler K, Antonino MJ, Wei C, Teng R, Rasmussen L, Storey RF, Nielsen T, Eikelboom JW, Sabe-Affaki G, Husted S, Kereiakes DJ, Henderson D, Patel DV, Tantry US (2010) Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation 121:1188–1199. https://doi.org/10.1161/CIRCULATIONAHA.109.919456
Sedat J, Chau Y, Gaudart J, Sachet M, Beuil S, Lonjon M (2017) Prasugrel versus clopidogrel in stent-assisted coil embolization of unruptured intracranial aneurysms. Interv Neuroradiol 23:52–59. https://doi.org/10.1177/1591019916669090
Aquarius R, de Korte A, Smits D, Gounis M, Verrijp K, Driessen L, Leenders W, de Vries J (2018) The importance of wall apposition in flow diverters. Neurosurgery. https://doi.org/10.1093/neuros/nyy092
Acknowledgements
A special thanks is dedicated to Pr Charles Strother (University of Wisconsin) for his precious assistance and advice.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix. Complications mRS reports
Appendix. Complications mRS reports
Rights and permissions
About this article
Cite this article
Peret, A., Mine, B., Bonnet, T. et al. Safety and efficacy of a pre-treatment antiplatelet regimen of unruptured intracranial aneurysms: a single-center experience. Neuroradiology 62, 1029–1041 (2020). https://doi.org/10.1007/s00234-020-02387-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02387-y