Abstract
Inflammation within the central nervous system (CNS) is strictly controlled and if possible prevented. Such a tight control is necessary due to high sensitivity of nervous tissue to mechanical and biochemical consequences of inflammation. Still, neuroinflammation is a typical feature of a chronic, inflammatory, demyelinating disease multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE). It is assumed that mechanisms that should prevent activation of immune cells at the periphery, in the lymphoid tissues, and/or inflammation within the CNS are inadequately efficient in MS patients. Here, some recent data about the importance of CXCL12 for regulation of neuroinflammation and contribution of its deviant expression within the CNS to EAE and MS pathogenesis are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
CXCL12 in brief
CXCL12 or stromal cell derived factor-1 (SDF-1) is a 68-amino-acid CXC chemokine with important roles in essential biological processes such as vascular and neuronal development and hematopoiesis. The response to CXCL12 occurs at a very early stage of embryonic development and appears to be widely operative whenever cell migration is required [1]. Indeed, mice lacking CXCL12 die prenatally and exhibit defects in vascularization, neuronal development, and hematopoiesis [2–5]. CXCL12 regulates the bone marrow homing and egress of stem and endothelial progenitor cells and their migration into peripheral tissues in both steady-state conditions and injury [6, 7]. Besides these physiological functions, CXCL12 seems to be involved in pathological processes such as neoplasia, tumor progression, and chronic inflammation [8–14]. In immune system, the principal role of this chemokine is to regulate the trafficking and localization of myeloid, lymphoid, and progenitor cells between central and peripheral compartments [15, 16].
CXCL12 is constitutively expressed in a broad range of tissues [17, 18], and the major sources of CXCL12 expression are bone marrow stromal elements and endothelial cells [19, 20]. It has been shown that the effects of CXCL12, such as mobilization of leukocytes from the bone marrow and transendothelial migration of inflammatory cells, are mainly dependent on the interaction of the chemokine with CXC chemokine receptor 4—CXCR4 [19–22]. CXCR4 has been considered as the unique receptor for CXCL12 and as the only mediator of its biological effects for many years. However, recent studies have found that CXCL12 binds not only to CXCR4 but also to CXCR7 (RDC1) [23]. While CXCL12 is exclusive ligand for CXCR4, CXCR7 binds both CXCL12 and CXCL11. This recently discovered receptor for CXCL12 is phylogenetically closely related to chemokine receptors but fails to induce typical chemokine receptor–mediated intracellular responses. Actually, CXCR7 functions as a decoy for CXCL12, thus regulating signaling of CXCL12 through CXCR4 [24, 25]. It seems that crucial activity of CXCR7 is to locally scavenge CXCL12 and thereby form a CXCL12 gradient for CXCR4-dependent migration [24]. However, there are other reports showing that CXCR7 actions are more than scavenging of CXCL12 and modulation of CXCR4 signaling. For instance, CXCR7 is critical for CXCL12-mediated survival of renal progenitor cells [5]. It is also essential for CXCL12-induced mitogen-induced protein kinases signaling and proliferation of astrocytes [26]. Finally, CXCR7-mediated signaling is involved in tumor cell growth, survival, and metastasis [27]. Similar to their ligand, CXCR4 and CXCR7 display a wide expression pattern in mammalian tissues. They are coexpressed on the surface of T- and B-cell subsets, monocytes, endothelial cells, dendritic cells, neurons, and in some tumor cells, primary human tumors, and tumor-associated endothelial cells [23, 28–32].
CXCL12 and inflammation
Much attention has been focused on the role of chemokines in inflammatory processes and especially, inflammatory autoimmune diseases. As chemoattractants of inflammatory leukocytes, chemokines are essential for leukocyte homing and the recruitment during inflammation, thereby directing the development of inflammatory responses, including inflammatory autoimmune diseases. CXCL12 is essential for attracting activated, CXCR4+ T cells to the sites of inflammation, and it also acts as a costimulator in T cell activation [33]. Moreover, it promotes the adhesion of T cells to ICAM-1 and VCAM-1 by upregulating the binding activity of LFA-1 and VLA-4 on T cells, respectively, and modulates the α4β7 integrin-mediated lymphocyte adhesion to mucosal addressin cell adhesion molecule-1 (MAdcam1) and fibronectin [34–36]. CXCL12 enhances the inflammatory infiltration of lymphocytes in diverse models and settings involving acute inflammation or fulminant infection [37]. In addition, upregulation of CXCR4 or CXCL12 has been reported in many inflammatory diseases such as rheumatoid arthritis (RA), multiple sclerosis (MS), inflammatory bowel disease (IBD), uveitis, nephritis, and lupus erythematosus [38–41]. Therefore, it has been suggested that CXCL12 plays a proinflammatory role in various autoimmune diseases and that it could be a valid target for neutralization in these diseases.
One of the basic agents in the research of CXCL12 functions is an antagonist of CXCR4, AMD3100. The rationale for using a chemokine receptor antagonist in inflammatory diseases is to selectively alter the recruitment of particular subsets of cells that initiate and maintain the inflammatory responses. AMD3100, a bicyclam molecule, has been identified as a specific inhibitor of CXCR4 [42, 43]. As CXCR4 serves as a coreceptor for human immunodeficiency virus (HIV) type 1 to infect T cells [44], it was shown that AMD3100 effectively and specifically blocked HIV entry into CXCR4-expressing cells [45]. In more recent studies, AMD3100 was shown to specifically inhibit CXCL12-mediated responses and to have beneficial effects in various animal models of inflammatory diseases, including asthma [46], RA [47], uveitis [48], and diabetes [49]. AMD3100 attenuated allergic lung inflammation and airway hyperreactivity in mice [46]. Treatment of allergic mice with AMD3100 significantly reduced number of pathological parameters related to asthmatic-type inflammation such as airway hyperreactivity, peribronchial eosinophilia, and the overall inflammatory responses. In addition, there was a shift from the pathogenic T helper (Th)-2 cytokine profile to the counteracting Th1 profile in the AMD3100-treated animals. Specifically, there was a significant reduction in interleukin (IL)-4 and IL-5 and a marked increase in IL-12 and interferon (IFN)-γ levels in the lungs of treated allergic mice [46]. CXCL12 expression has been demonstrated in rheumatoid synovium, and increased levels of this chemokine are detected in RA synovial fluid [50–52]. RA synovial tissues had higher levels of CXCL12 on high endothelial venules, where this chemokine was colocalized with heparan sulfate proteoglycans [53]. Importantly, interaction of CXCL12 with glycosaminoglycans is supposed to be important for leukocyte diapedesis and neovascularization during inflammation [53]. AMD3100 was also shown to be efficient in RA model, autoimmune collagen-induced arthritis (CIA) as it was demonstrated that exogenous CXCL12 injected in periarthritic tissue of mice elicited an inflammatory response that could be inhibited by AMD3100 [47]. Further, intraperitoneal injection of AMD3100 attenuated ovalbumine (OVA)-induced uveitis, and it significantly ameliorated ocular inflammation in vivo, thus suggesting that CXCL12 has pathogenic role in this inflammatory ocular disease characterized by the infiltration of T lymphocytes and other leukocytes into the eye [48]. Further, AMD3100 exerts therapeutic effects on an IBD model, experimental colitis. There, it inhibited colonic inflammation, decreased epithelial apoptosis and gut permeability, and consequently enhanced the epithelial barrier integrity [54]. In non-obese diabetic (NOD) mice, which are predisposed to develop type 1 diabetes (T1D) and serve as a model for studying diabetes in humans, AMD3100 mobilized T cells from the bone marrow to peripheral lymphoid tissues and simultaneously inhibited disease development [55]. It was suggested that elevated CXCL12 expression promotes T1D in NOD mice by altering T-cell and hematopoietic stem cell trafficking. On the other hand, there are findings that CXCL12 exerts beneficial regulatory functions in the NOD mouse model as it protects NOD mice from autoimmune diabetes. It was reported that when T splenocytes from NOD mice treated with AMD3100 were mixed with diabetogenic T cells during adoptive cell cotransfer experiments, prevalence of diabetes in the recipients significantly rose [49]. Also, AMD3100 reduced the number of CXCR4+ and CXCL12+ cells in the inflamed islets in mice, while CXCL12 attenuated diabetes and promoted pancreatic β-cell survival by the activation of the prosurvival kinase Akt [56]. Thus, AMD3100 application has helped us to understand that besides proinflammatory role in experimental models and inflammatory autoimmune diseases, CXCL12 has also anti-inflammatory properties. This observation challenges the concept of strict pathogenic role of CXCL12 in chronic inflammatory and autoimmune disorders. Such an assumption is further substantiated with data obtained in MS and its animal models, which are presented in the following paragraphs.
Experimental autoimmune encephalomyelitis (EAE) and multiple sclerosis (MS)
Experimental autoimmune encephalomyelitis (EAE) is an autoimmune disease of the central nervous system (CNS). EAE can be induced in susceptible experimental animals, including rodents and primates, by immunization with CNS-specific antigens, such as myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG), proteolipid protein (PLP), CNS tissue homogenate, etc. (active EAE). Alternatively, the disease can be evoked by the transfer of encephalitogenic CD4+ T cells, obtained from draining lymph nodes of animals immunized for active EAE induction, into syngeneic animals (transfer or passive EAE) [57]. Current concepts suggest two steps in the autoimmune pathogenesis of active EAE. First, dendritic cells (DC) located at the site of injection ingest the inoculated CNS antigens and migrate into the lymph nodes draining the sites of immunization. CNS antigens are also transported via the soluble routes into the lymph nodes where they are ingested by resident DCs. Within the lymph nodes, DCs present the antigenic peptides in the context of major histocompatibility complex (MHC) class II molecules to naive autoreactive CD4+ T cells. These cells then differentiate into IFN-γ- and/or IL-17-producing effector T cells, namely T helper (Th) cells, and even more specifically Th1 and Th17 cells, respectively [58]. Second, Th cells migrate from the secondary lymphatic organs into the CNS where they are reactivated after interacting with MHC class II+ CNS resident phagocytes [59, 60]. The stimulated effector T cells locally release proinflammatory cytokines and thus initiate an inflammatory reaction, which may finally result in demyelination and axonal degeneration [61, 62]. Inflammation, demyelination, and axonal loss result in various neurological deficits, usually an ascending progressive paralysis in a caudal to rostral direction starting with tail paralysis and eventually leading to forelimb paralysis and/or a moribund state and death in severe cases.
Since EAE shares many clinical, histological, immunologic, and genetic features with human demyelinating diseases, including MS, it has been widely used to gain important insight into MS pathogenesis and to validate new targets for MS therapy [63, 64]. MS is an inflammatory disease of the CNS. The most prominent feature of the disease is demyelination, which is the major cause for neurological symptoms expressed in the patients. Axonal loss and neurodegeneration are becoming increasingly appreciated as pathological hallmarks of MS as well [65]. There are at least four subtypes of MS regarding clinical expression: relapsing–remitting, primary progressive, secondary progressive, and relapsing progressive. Also, there are four defined pathological subtypes of the disease: macrophage-mediated, antibody-mediated, distal oligodendrogliopathy, and primary oligodendrocyte damage with secondary demyelination [66]. We can say that MS is not a single ailment, but rather a common name for a group of diseases that, although different in specific inductive and effector pathogenic mechanisms, converge at the point of inflammation and demyelination. Thus, it is relevant to have variants of the EAE model, which provide information of importance for understanding pathogenic specificities of MS forms. Importantly, depending on the species and strain used in experiments, on encephalitogen and adjuvants applied to these animals, as well as on the way the immunization is performed, there is a divergence in clinical manifestation of EAE, roughly corresponding to the major clinical subtypes of MS [63, 67]. Actually, among numerous strains of mice and rats in which EAE can be induced, every strain or even substrain has specificities regarding immunization protocol that leads to EAE induction. These specificities include myelin proteins and/or peptides, intensity of costimulation with adjuvants, number of injections, etc. Besides that, every strain has some pathogenic and clinical feature that makes it unique to other strains and species. Therefore, investigation of one particular EAE variant will give us useful information regarding some aspects of pathogenesis of one clinical subtype of MS. As EAE is mostly studied in inbred strains of animals, it is possible to consider one animal strain as of one human being. Unlike humans and natural populations of animals where every individual is genetically unique, with an exception of identical twins, all animals of an inbred strain are genetically identical to each other. Of paramount interest are those strains of mice and rats that show exceptional susceptibility to EAE induction, as well as those strains that are resistant to the disease induction. Having in mind correlation with humans, an extremely susceptible strain corresponds to a MS patient and a persistently resistant strain corresponds to a healthy person. Thus, through comparative investigation of such strains, it is possible to identify genes, molecules, mechanisms, etc. that define susceptibility and/or resistance to the autoimmune CNS disorder. In our laboratory, we are investigating such strains of rats, namely Dark Agouti (DA) rats and Albino Oxford (AO) rats.
Our way to CXCL12
AO and DA rats are at the opposite poles of susceptibility to EAE induction. We can claim this as we have shown that AO rats do not develop EAE in conditions under which other relatively resistant rat strains such as Fischer 344, Brown Norway (BN), or PVG rats do [68–71]. Also, we have demonstrated that DA rats develop the clinical disease after immunization with spinal cord homogenate (SCH) even in the absence of any adjuvant [72], which is a rare exception from the rule that EAE induction requires application of an adjuvant. Initial paper of Mostarica Stojkovic et al. [73] in which it was shown that rats of inbred AO strain are highly resistant to EAE induction was published some 30 years ago. This work was the beginning of the series of investigations aiming to elucidate mechanisms of AO rats’ resistance and DA rats’ susceptibility to EAE induction. During the course of the research, various cells and molecules relevant for EAE pathogenesis have been investigated in the peripheral blood, draining lymph node (DLN), and spinal cord (SC) lesions of AO and DA rats after the induction of EAE [74–76]. We have recently shown that upon immunization, the number of cells increased more vigorously in DLN of DA rats than in AO rats, suggesting a higher proliferation rate and/or a more efficient recruitment of immune cells, accompanied by a larger production of proinflammatory cytokines, including IFN-γ, IL-17, IL-6, IL-12, and IL-23 [71, 77]. Accordingly, number of cells infiltrating SC was markedly higher in DA rats than in AO rats at the time of peak of EAE in DA rats [78]. Thus, lower numbers of cells infiltrating the CNS as a consequence of a limited cell proliferation at the periphery could well explain the observed resistance of AO rats toward EAE. However, more than 20 years ago, Sedgwick et al. [79] showed that a small number of encephalitogenic cells were sufficient to induce severe EAE in Lewis rats. Therefore, the relatively low numbers of the cells infiltrating the SC of AO rats might not necessarily be the limiting factor for the induction of the disease. Moreover, histological lesions in the CNS tissue during the early phases of EAE (7–9 days after immunization) were similar in clinically diseased DA and symptom-free AO rats [74]. Further, SC infiltrations of similar degree and distribution were observed in AO and DA recipients of highly encephalitogenic (DA × AO) F1 T cells. Importantly, equal numbers of F1 cells did not induce any clinical symptoms in AO recipients while provoking a severe paralytic disease in F1 hybrids and DA rats [80]. All above mentioned suggests that mere number of cells infiltrating the CNS might not be the crucial factor for encephalitogenicity and that CNS intrinsic regulatory mechanism might contribute to the resistance of AO rats to the induction of EAE.
Therefore, we focused our research on the influence of the CNS on the encephalitogenic cells and CD4+ T cells in particular, as they are well known to represent the major culprits in the pathogenesis of EAE [58, 64]. We observed a substantial accumulation of CD4+ T cells in both rat strains at the peak of the disease in DA rats, but this increase was markedly pronounced in DA rats. Even in the early EAE, there were more CD4+ cells in the mononuclear cell infiltrates of DA rats [74]. Moreover, CD4+ T cells isolated from the SC of AO rats were less activated, and there were more naïve T cells. Also, there was a clear difference in the ratio of naïve and activated CD4+ T cells in the CNS versus peripheral blood between two strains [78]. Further, the expression of IFN-γ and IL-17 was also significantly lower in AO rats when determined in T cells immediately after isolation from the SC. However, if cells infiltrating the SC were removed from the CNS milieu and propagated in vitro, there was no difference in the percentage of cells expressing these cytokines, thus indicating that the CNS tissue of AO rats limits the expression of these cytokines in the infiltrating cells [78]. Finally, we observed that cells infiltrating SC of AO rats are more prone to apoptosis, which confirmed previous findings in passively transferred encephalitogenic CD4+ T cells in AO and DA rats [81]. These results further corroborated the assumption that factors in the CNS contribute to the limited autoimmune response and therefore to the resistance of AO rats to EAE induction.
CNS tissue may influence composition and activation state of the infiltrates by regulation of cell egression from circulation at blood–brain barrier (BBB) and by regulatory mechanisms working within the CNS parenchyma. Entrance of immune cells into the CNS is highly regulated at the level of BBB through various interactions of infiltrating cells and CNS-resident cells, including interactions between chemokines and their receptors [82]. While investigating gene expression of various neuroinflammation-related molecules in SC homogenates, we observed an interesting phenomenon regarding one of the chemokines, CXCL12. Its expression was remarkably downregulated in DA rats at the peak of EAE and strongly upregulated in AO rats analyzed at the same time point [78]. This result implied that CXCL12 might be of importance for the regulation of CNS inflammation or at least that this molecule’s abrogated expression is a marker of EAE pathogenesis.
CXCL12 in neuroinflammation
We have been aware of the potential importance of the interaction of CXCL12 and its receptor CXCR4 for neuroinflammation, as it has been previously shown that CXCL12 could be a key chemokine regulating the entrance of lymphocytes into the CNS [82]. Also, CXCL12 expression was detected in MS patients, both in cerebrospinal fluid and in MS plaques [83–86]. Therefore, we investigated CXCL12 expression in our system in details [78]. We found out that CXCL12 is expressed in similar amounts in the SC of non-immunized AO and DA rats. After immunization, it was increased in AO rats but decreased in DA at the time of peak of EAE in DA rats. As endothelial cells are among the major producers of CXCL12, we checked the expression of CXCL12 in these cells as well. Expression of CXCL12 in isolated SC microvessels of immunized animals was significantly higher than in total SC homogenates in AO, thus implying that the blood–brain barrier was the major source of CXCL12 expression. This notion was supported by the finding that by day 12–14 post-immunization, there was high CXCL12 expression on blood vessel cells in SC tissue sections of symptom-free AO rats and low, almost undetectable, expression of CXCL12 in the sections of paralyzed DA rats [78]. Importantly, when we examined expression of CXCR4, the principal receptor of CXCL12 in immune cells infiltrating SC, there was no significant difference in the expression of CXCR4 at the peak of EAE in DA rats and immunized AO rats killed at the same time. Thus, we concluded that it was not CXCR4 expression in immune cells that determined the difference in infiltration of CNS in AO and DA rats. Further, we have also detected downregulation of CXCL12 expression in SC of Lew rats at the peak of transfer EAE (unpublished observation), which showed that inhibition of CXCL12 was not limited to DA rats or active EAE, but that it was rather a general phenomenon linked to neuroinflammatory processes. In order to elucidate whether upregulated expression of CXCL12 has a protective role in EAE, we applied an antagonist of CXCL12, AMD3100, to AO rats. As a result, treatment with AMD3100 exacerbated otherwise mild EAE in AO rats [78], thus suggesting that CXCL12 could indeed be a factor that largely contributes to the limitation of encephalitogenicity.
Our results implying that CXCL12 has regulatory and not inflammatory role in neuroinflammation are not solitary. On the contrary, CXCL12 has been reportedly associated with down-regulation of EAE [82, 87, 88]. It was shown that CXCL12 prevented egression of infiltrating cells from perivascular space through the glia limitans into CNS parenchyma [87, 89]. Furthermore, CXCL12 was reported to redirect the polarization of antigen-specific effector Th1 cells into IL-10-producing T cells [88]. Also, it was found to induce CD4+ T-cell apoptosis via upregulation of the Fas–Fas ligand pathway [90]. Interestingly, while in rat EAE it was the level of expression that determined susceptibility to neuroinflammation, in a mouse EAE and in MS patients localization of CXCL12 was the most important parameter (Fig. 1). Namely, CXCL12 is normally expressed on basolateral side of endothelial cells, while in mice suffering from EAE and in active lesions of MS patients, its expression is shifted to luminal side [87, 89]. As suggested by the authors of the reports, it seems that basolateral expression of CXCL12 is necessary to keep infiltrating cells within perivascular cuffs and that the shift of CXCL12 expression to luminal side allows infiltrating cells to penetrate into CNS parenchyma. This change in localization of CXCL12 is induced by inflammatory cells, and it was shown that IL-1β had a major role in the shift [91]. Still, there is also a report which shows that the localization of CXCL12 expression in MS plaques blood vessels is strictly limited to the outer layer of blood vessels, consisted of pericytes and extracellular matrix, and not inner layer made of endothelial cells [84]. Also, there is a study that relates CXCL12 expression in astrocytes with activity of MS lesions [86]. Moreover, a truncated form of CXCL12 (P2G2) that antagonized CXCR4 also inhibited EAE in mice and reduced the accumulation of CD4+ T cells in the CNS [92]. This result seems to be in discrepancy with results of McCandless et al., as well as with our results. The discrepancy might be due to different EAE models used, that is, species and strains (SJL or C57BL/6 mice vs. DA rats) and/or encephalitogenic emulsions (PLP or MOG vs. SCH). This would imply that CXCL12 is not universally protective in EAE, but that its action is specific for some EAE variants and consequently for some variants of MS as well. While this possibility has to be seriously accounted in future research of the role of CXCL12 in neuroinflammation, especially regarding potential therapeutic approach aiming at neutralization of CXCL12, there are still more options for explanation of the diverse functions of CXCL12 in the reports. Namely, P2G2 inhibited the sensitization phase of the immune response and had no effect on EAE if applied to mice that received encephalitogenic cells [92]. Thus, we could say that the role of CXCL12 in propagating EAE is restricted to peripheral development of an immune response, while it does not have such effect within the CNS. The other important difference is the way in which CXCL12 effects were neutralized. While Kohler et al. used truncated form of CXCL12, AMD3100 was used by McCandless et al. and us. AMD3100 is an antagonist of CXCL12 signaling through CXCR4, but not through CXCR7, while P2G2 might affect both receptors. It has recently been reported that CXCR7 actually serves as a scavenger of CXCL12 in neuroinflammation and that it removes CXCL12 from basolateral side of blood vessel endothelial cells and thus allows for leukocyte to entry into the CNS parenchyma [93]. Therefore, if P2G2 affects both CXCR4 and CXCR7, its net effect on CXCL12-dependent route of leukocyte entry into the CNS might be neutral. This would then explain the lack of effect of P2G2 on transfer EAE course. Finally, the fact that CXCL12 level is important for EAE regulation in rats and CXCL12 localization is essential for the regulation of neuroinflammation in mice and humans implies that CXCL12 availability might be a conserved mechanism of inhibition of immune cell infiltration into the CNS, while ways in which this availability is regulated diverged among species in evolution.
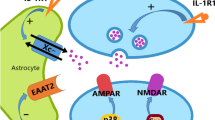
Proposed roles of CXCL12 in neuroinflammation. a Basic elements of the CNS that interact with infiltrating immune cells at the level of a postcapillary venule: vessel lumen (VL), endothelial cells (EC), endothelial basal lamina (EBL), Virchow-Robin space (VRS), parenchymal basal lamina (PBL), astrocytic end feet (AEF), parenchyma (P). b CXCL12 (black dots) expressed at basolateral side of endothelial cells of the blood vessels arrests T cells (T) within VRS and prevents infiltrating cells to progress into the CNS parenchyma. Delocalization of CXCL12 from the basolateral side, induced by IL-1β and mediated by CXCR7, allows for the infiltration into the CNS parenchyma. Alternatively, high expression of CXCL12 in EC prevents infiltration of immune cells into the CNS. Reduction in CXCL12 expression under the influence of NO allows the infiltration of T cells. Also, CXCL12 provokes apoptosis in T cells and induces generation of regulatory T cells (Treg)
Besides endothelial cells, astrocytes are important producers of CXCL12 in the CNS. Actually, they were identified as the most potent source of CXCL12 in active MS lesions [86, 94, 95]. CXCL12 expression in astrocytes was induced by neuroinflammation-related molecules IL-1β and MBP in vitro [86]. Astrocytes are considered to be major players in neuroinflammation, where their role might be both pro- and anti-inflammatory [96]. Therefore, their capability to express CXCL12 both at the area of glia limitans and within the CNS parenchyma is important for understanding EAE and MS pathogenesis and should be addressed in detail in future studies. Particularly interesting is the report that astrocytes attract immature DCs into the CNS through engagement of CXCL12/CXCR4 axis [94]. Immature DCs have been supposed to control inflammatory environment in MS if stimulated with IL-10 [97]. As stated previously, CXCL12 shifts Th1 cells toward IL-10-producing regulatory T cells [88]. Thus, we can speculate that high CXCL12 generation within the CNS will simultaneously induce IL-10 generation and immigration of immature DCs and that this will provide favorable conditions for limitation of neuroinflammation.
CXCL12 is also expressed in the healthy CNS where it serves as an important factor for survival and migration of neuronal and oligodendrocyte precursors [98, 99]. Thus, it is rational to assume that this chemokine might also contribute to the process of remyelination and neuroprotection. Indeed, CXCL12 expression on astrocytes was detected in cuprizone model of demyelination in mice, while CXCR4 expression on oligodendrocyte precursor cells (OPC) was shown essential for OPC migration and remyelination [100]. Similar results were obtained in virus-mediated demyelinating disease provoked by infection of the mouse CNS with the neurotropic JHM strain of mouse hepatitis virus (JHMV). Neural stem cells (NSCs) were introduced into spinal cords of JHMV-infected mice with established demyelination. This engraftment resulted in migration, proliferation, and differentiation of the cells into OPCs and mature oligodendrocytes and subsequent axonal remyelination [101]. However, if the NSC transplanted animals were treated with anti-CXCL12 blocking serum or with an antagonist of CXCR4, but not CXCR7, migration and proliferation of the engrafted stem cells were markedly impaired [101]. Finally, it was shown that CXCL12 is important for migration of human mesenchymal stem cells (MSC) in vitro [102, 103]. MSC have increasingly been appreciated as candidates for MS therapy, as they were shown to ameliorate clinical course and decrease demyelination, immune infiltrates, and axonal loss in EAE, and they were reported to be safe for application in humans [104].
It is clear that the observed differences in CXCL12 expression emerge during neuroinflammation, and it seems plausible to assume that immune factors, possibly extrinsic to the CNS, might influence CXCL12 expression. Cytokines previously related to the resistance/susceptibility of rat and mice strains to EAE induction, such as TNF, IL-4, TGF-β, and IL-10/IL-12 [105–108], must be taken into consideration. Precise dissection of the regulation of CXCL12 expression in AO and DA rats, as well as of the influence of CXCL12 on encephalitogenic cells is the focus of ongoing work in our laboratory. We are particularly interested in the influence of NO on CXCL12 expression. NO and its secondary metabolite peroxynitrite have been reportedly associated with inflammatory demyelination and neurodegeneration [109–111]. The overproduction of NO is related to the generation of peroxynitrite and consequently to extensive tissue damage in the CNS autoimmunity [109, 112, 113]. Although we showed that there was no disparity in NO generation in DLN of AO and DA rats [71] and that NO does not contribute to the resistance of AO rats to EAE induction, we also observed clear difference in inducible NO synthase (iNOS) gene expression within the CNS in AO and DA rats. Namely, opposite to CXCL12, iNOS gene expression was high in DA rats and low in AO rats SC during EAE course, and we observed negative correlation in the expression of iNOS gene and CXCL12 gene in SC of immunized rats (unpublished observation). Our preliminary data show that in vitro NO inhibits CXCL12 expression, both in astrocytes and in micro-blood vessels isolated from the CNS. Therefore, we are currently investigating influence of NO on CXCL12 expression and possibility that NO determines susceptibility of DA rats to EAE.
Concluding remarks
Taken together, the observed pattern of expression of CXCL12 in the SC of AO, DA, and Lewis rats along with data from the studies in mice and humans suggests that CXCL12 expression at the blood–brain barrier could be an important component in the net effect of the target tissue on encephalitogenic cells. Moreover, it appears that CXCL12 may largely contribute to the resistance of animals to EAE induction, and it is tempting to speculate that it might be protective in MS pathogenesis as well. Ongoing and future research should provide details about the regulation of CXCL12 expression during neuroinflammation.
References
Nagasawa T, Nakajima T, Tachibana K, Iizasa H, Bleul CC, Yoshie O, Matsushima K, Yoshida N, Springer TA, Kishimoto T. Molecular cloning and characterization of a murine pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 receptor, a murine homolog of the human immunodeficiency virus 1 entry coreceptor fusin. Proc Natl Acad Sci U S A. 1996;93:14726–9.
Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman D. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9.
Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, Ben-Hur H, Lapidot T, Alon R. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–211.
Grabovsky V, Feigelson S, Chen C, Bleijs DA, Peled A, Cinamon G, Baleux F, Arenzana-Seisdedos F, Lapidot T, van Kooyk Y, Lobb RR, Alon R. Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J Exp Med. 2000;192:495–506.
Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, Parente E, Mancina R, Netti GS, Becherucci F, Gacci M, Carini M, Gesualdo L, Rotondi M, Maggi E, Lasagni L, Serio M, Romagnani S, Romagnani P. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205:479–90.
Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53.
Chen CN, Chang SF, Lee PL, Chang K, Chen LJ, Usami S, Chien S, Chiu JJ. Neutrophils, lymphocytes, and monocytes exhibit diverse behaviors in transendothelial and subendothelial migrations under coculture with smooth muscle cells in disturbed flow. Blood. 2006;107:1933–42.
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6.
Gonzalo JA, Lloyd CM, Peled A, Delaney T, Coyle AJ, Gutierrez-Ramos JC. Critical involvement of the chemotactic axis CXCR4/stromal cell-derived factor-1 alpha in the inflammatory component of allergic airway disease. J Immunol. 2000;165:499–508.
Pablos JL, Santiago B, Galindo M, Torres C, Brehmer MT, Blanco FJ, García-Lázaro FJ. Synoviocyte-derived CXCL12 is displayed on endothelium and induces angiogenesis in rheumatoid arthritis. J Immunol. 2003;170:2147–52.
Petty JM, Sueblinvong V, Lenox CC, Jones CC, Cosgrove GP, Cool CD, Rai PR, Brown KK, Weiss DJ, Poynter ME, Suratt BT. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol. 2007;178:8148–57.
Pablos JL, Amara A, Bouloc A, Santiago B, Caruz A, Galindo M, Delaunay T, Virelizier JL, Arenzana-Seisdedos F. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am J Pathol. 1999;155:1577–86.
Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48.
Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–7.
Nagasawa T, Tachibana K, Kishimoto T. A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Semin Immunol. 1998;10:179–85.
Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–10.
Tashiro KH, Tada R, Heilker M, Shirozu T, Nakano T, Honio T. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science. 1993;261:600–3.
Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A. 1994;91:2305–9.
Möhle R, Rafii S, Moore MA. The role of endothelium in the regulation of hematopoietic stem cell migration. Stem Cell. 1998;16:159–65.
Möhle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. Regulation of transendothelial migration of hematopoietic progenitor cells. Ann N Y Acad Sci. 1999;872:176–85.
Juarez J, Bendall L, Bradstock K. Chemokines and their receptors as therapeutic targets: the role of the SDF-1/CXCR4 axis. Curr Pharm Des. 2004;10:1245–59.
Lataillade JJ, Domenech J, Le Bousse-Kerdilès MC. Stromal cell-derived factor-1 (SDF-1)\CXCR4 couple plays multiple roles on haematopoietic progenitors at the border between the old cytokine and new chemokine worlds: survival, cell cycling and trafficking. Eur Cytokine Netw. 2004;15:177–88.
Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–13.
Naumann U, Cameroni E, Pruenster M, Mahabaleshwar H, Raz E, Zerwes HG, Rot A, Thelen M. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS ONE. 2010;5:e9175.
Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113:6085–93.
Odemis V, Boosmann K, Heinen A, Küry P, Engele J. CXCR7 is an active component of SDF-1 signalling in astrocytes and Schwann cells. J Cell Sci. 2010;123:1081–8.
Maksym RB, Tarnowski M, Grymula K, Tarnowska J, Wysoczynski M, Liu R, Czerny B, Ratajczak J, Kucia M, Ratajczak MZ. The role of stromal-derived factor-1-CXCR7 axis in development and cancer. Eur J Pharmacol. 2009;625:31–40.
Schonemeier B, Kolodziej A, Schulz S, Jacobs S, Hoellt V, Stumm R. Regional and cellular localization of the CXCL12/SDF-1 chemokine receptor CXCR7 in the developing and adult rat brain. J Comp Neurol. 2008;510:207–20.
Infantino S, Moepps B, Thelen M. Expression and regulation of the orphan receptor RDC1 and its putative ligand in human dendritic and B cells. J Immunol. 2006;176:2197–207.
Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, Kleer CG, Essner JJ, Nasevicius A, Luker GD, Howard MC, Schall TJ. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci U S A. 2007;104:15735–40.
Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–6.
Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M, Harvey RP, Martínez-A C, Mackay CR, Mackay F. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104:14759–64.
Nanki T, Lipsky PE. Stromal cell-derived factor-1 is a costimulator for CD4 + T cell activation. J Immunol. 2000;164:5010–4.
Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–4.
Wright N, Hidalgo A, Rodríguez-Frade JM, Soriano SF, Mellado M, Parmo-Cabañas M, Briskin MJ, Teixidó J. The chemokine stromal cell-derived factor-1 alpha modulates alpha 4 beta 7 integrin-mediated lymphocyte adhesion to mucosal addressin cell adhesion molecule-1 and fibronectin. J Immunol. 2002;168:5268–77.
DiVietro JA, Brown DC, Sklar LA, Larson RS, Lawrence MB. Immobilized stromal cell-derived factor-1alpha triggers rapid VLA-4 affinity increases to stabilize lymphocyte tethers on VCAM-1 and subsequently initiate firm adhesion. J Immunol. 2007;178:3903–11.
Ding Z, Jia SH, Marshall JC, Downey GP, Waddell TK. Up-regulation of functional CXCR4 expression on human lymphocytes in sepsis. Crit Care Med. 2006;34:3011–7.
Balabanian K, Couderc J, Bouchet-Delbos L, Amara A, Berrebi D, Foussat A, Baleux F, Portier A, Durand-Gasselin I, Coffman RL. Role of the chemokine stromal cell-derived factor 1 in autoantibody production and nephritis in murine lupus. J Immunol. 2003;170:3392–400.
Bajetto A, Bonavia R, Barbero S, Florio T, Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol. 2001;22:147–84.
Haringman JJ, Smeets TJ, Reinders-Blankert P, Tak PP. Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis, and reactive arthritis. Ann Rheum Dis. 2006;65:294–300.
Mikami S, Nakase H, Yamamoto S, Takeda Y, Yoshino T, Kasahara K, Ueno S, Uza N, Oishi S, Fujii N, Nagasawa T, Chiba T. Blockade of CXCL12/CXCR4 axis ameliorates murine experimental colitis. J Pharmacol Exp Ther. 2008;327:383–92.
Schols D, Struyf S, Van Damme J, Este JA, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–8.
Donzella GA, Schols D, Lin SW, Este JA, Nagashima KA, Maddon PJ, Allaway GP, Sakmar TP, Henson G, De Clercq E, Moore JP. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–7.
Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7.
Hendrix CW, Collier AC, Lederman MM, Schols D, Pollard RB, Brown S, Jackson JB, Coombs RW, Glesby MJ, Flexner CW, Bridger GJ, Badel K, MacFarland RT, Henson GW, Calandra G. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir Immune Defic Syndr. 2004;37:1253–62.
Lukacs NW, Berlin A, Schols D, Skerlj RT, Bridger G. AMD3100, a CXCR4 antagonist, attenuates allergic lung inflammation and airway hyperactivity. Am J Pathol. 2002;160:1353–60.
Matthys P, Hatse S, Vermeire K, Wuyts A, Bridger G, Henson GW, De Clercq E, Billiau A, Schols D. AMD3100, a potent and specific antagonist of the stromal cell-derived factor-1 chemokine receptor CXCR4, inhibits autoimmune joint inflammation in IFN-gamma receptor-deficient mice. J Immunol. 2001;167:4686–92.
Zhang Z, Zhong W, Hall MJ, Kurre P, Spencer D, Skinner A, O’Neill S, Xia Z, Rosenbaum JT. CXCR4 but not CXCR7 is mainly implicated in ocular leukocyte trafficking during ovalbumin-induced acute uveitis. Exp Eye Res. 2009;89:522–31.
Aboumrad E, Madec AM, Thivolet C. The CXCR4/CXCL12 (SDF-1) signalling pathway protects non-obese diabetic mouse from autoimmune diabetes. Clin Exp Immunol. 2007;148:432–9.
Kanbe K, Takagishi K, Chen Q. Stimulation of matrix metalloprotease 3 release from human chondrocytes by the interaction of stromal cell-derived factor 1 and CXC chemokine receptor 4. Arthritis Rheum. 2002;46:130–7.
Nanki T, Imai T, Nagasaka K, Urasaki Y, Nonomura Y, Taniguchi K, Hayashida K, Hasegawa J, Yoshie O, Miyasaka N. Migration of CX3CR1-positive T cells producing type 1 cytokines and cytotoxic molecules into the synovium of patients with rheumatoid arthritis. Arthritis Rheum. 2002;46:2878–83.
Blades MC, Ingegnoli F, Wheller SK, Manzo A, Wahid S, Panayi GS, Perretti M, Pitzalis C. Stromal cell-derived factor 1 (CXCL12) induces monocyte migration into human synovium transplanted onto SCID mice. Arthritis Rheum. 2002;46:824–36.
Santiago B, Baleux F, Palao G, Gutiérrez-Cañas I, Ramírez JC, Arenzana-Seisdedos F, Pablos JL. CXCL12 is displayed by rheumatoid endothelial cells through its basic amino-terminal motif on heparan sulfate proteoglycans. Arthritis Res Ther. 2006;8:R43.
Xia XM, Wang FY, Xu WA, Wang ZK, Liu J, Lu YK, Jin XX, Lu H, Shen YZ. CXCR4 antagonist AMD3100 attenuates colonic damage in mice with experimental colitis. World J Gastroenterol. 2010;16:2873–80.
Leng Q, Nie Y, Zou Y, Chen J. Elevated CXCL12 expression in the bone marrow of NOD mice is associated with altered T cell and stem cell trafficking and diabetes development. BMC Immunol. 2008;9:51.
Yano T, Liu Z, Donovan J, Thomas MK, Habener JF. Stromal cell derived factor-1 (SDF-1)/CXCL12 attenuates diabetes in mice and promotes pancreatic beta-cell survival by activation of the prosurvival kinase Akt. Diabetes. 2007;56:2946–57.
Ben-Nun A, Wekerle H, Cohen IR. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981;11:195–9.
El-behi M, Rostami A, Ciric B. Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2010;5:189–97.
Kawakami N, Lassmann S, Li Z, Odoardi F, Ritter T, Ziemssen T, Klinkert WE, Ellwart JW, Bradl M, Krivacic K, Lassmann H, Ransohoff RM, Volk HD, Wekerle H, Linington C, Flügel A. The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis. J Exp Med. 2004;199:185–97.
Bartholomäus I, Kawakami N, Odoardi F, Schläger C, Miljkovic D, Ellwart JW, Klinkert WE, Flügel-Koch C, Issekutz TB, Wekerle H, Flügel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–8.
Becher B, Bechmann I, Greter M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med. 2006;84:532–43.
Wujek JR, Bjartmar C, Richer E, Ransohoff RM, Yu M, Tuohy VK, Trapp BD. Axon loss in the spinal cord determines permanent neurological disability in an animal model of multiple sclerosis. J Neuropathol Exp Neurol. 2002;61:23–32.
Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–71.
Krishnamoorthy G, Wekerle H. EAE: an immunologist’s magic eye. Eur J Immunol. 2009;39:2031–5.
Lassmann H. Axonal and neuronal pathology in multiple sclerosis: what have we learnt from animal models. Exp Neurol. 2010;225:2–8.
Lassmann H, Brück W, Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol Med. 2001;7:115–21.
Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. 2011;164:1079–106.
Gold DP, Schroder K, Powell HC, Kelly CJ. Nitric oxide and the immunomodulation of experimental allergic encephalomyelitis. Eur J Immunol. 1997;27:2863–9.
Cowden WB, Cullen FA, Staykova MA, Willenborg DO. Nitric oxide is a potential down-regulating molecule in autoimmune disease: inhibition of nitric oxide production renders PVG rats highly susceptible to EAE. J Neuroimmunol. 1998;88:1–8.
Staykova MA, Cowden W, Willenborg DO. Macrophages and nitric oxide as the possible cellular and molecular basis for strain and gender differences in susceptibility to autoimmune central nervous system inflammation. Immunol Cell Biol. 2002;80:188–97.
Miljkovic D, Stosic-Grujicic S, Markovic M, Momcilovic M, Ramic Z, Maksimovic-Ivanic D, Mijatovic S, Popadic D, Cvetkovic I, Mostarica-Stojkovic M. Strain difference in susceptibility to experimental autoimmune encephalomyelitis between Albino Oxford and Dark Agouti rats correlates with disparity in production of IL-17, but not nitric oxide. J Neurosci Res. 2006;84:379–88.
Stosic-Grujicic S, Ramic Z, Bumbasirevic V, Harhaji L, Mostarica-Stojkovic M. Induction of experimental autoimmune encephalomyelitis in Dark Agouti rats without adjuvant. Clin Exp Immunol. 2004;136:49–55.
Mostarica-Stojkovic M, Petrovic M, Lukic ML. Cellular and genetic basis of the relative resistance to the induction of experimental allergic encephalomyelitis (EAE) in albino oxford (AO) rats. Adv Exp Med Biol. 1982;149:699–702.
Vukmanovic S. Mostarica Stojkovic M, Lukic ML: Experimental autoimmune encephalomyelitis in “low” and “high” interleukin 2 producer rats. I. Cellular basis of induction. Cell Immunol. 1989;121:237–46.
Vukmanovic S, Mostarica-Stojkovic M, Zalud I, Ramic Z, Lukic ML. Analysis of T cell subsets after induction of experimental autoimmune encephalomyelitis in susceptible and resistant strains of rats. J Neuroimmunol. 1990;27:63–9.
Arsov I, Pravica V, Ejdus L, Badovinac V, Mostarica M, Lukic ML. Selection for susceptibility to experimental allergic encephalomyelitis also selects for high IFN-gamma production. Transplant Proc. 1995;27:1537–8.
Markovic M, Miljkovic D, Momcilovic M, Popadic D, Miljkovic Z, Savic E, Ramic Z, Mostarica Stojkovic M. Strain difference in susceptibility to experimental autoimmune encephalomyelitis in rats correlates with T(H)1 and T(H)17-inducing cytokine profiles. Mol Immunol. 2009;47:141–6.
Miljkovic D, Stanojevic Z, Momcilovic M, Odoardi F, Flügel A, Mostarica-Stojkovic M. CXCL12 expression within the CNS contributes to the resistance against experimental autoimmune encephalomyelitis in Albino Oxford rats. Immunobiology. 2011;216:979–87.
Sedgwick J, Brostoff S, Mason D. Experimental allergic encephalomyelitis in the absence of a classical delayed-type hypersensitivity reaction. Severe paralytic disease correlates with the presence of interleukin 2 receptor-positive cells infiltrating the central nervous system. J Exp Med. 1987;165:1058–75.
Mostarica-Stojkovic M, Vukmanovic S, Ramic Z, Lukic ML. Evidence for target tissue regulation of resistance to the induction of experimental allergic encephalomyelitis in AO rats. J Neuroimmunol. 1992;41:97–104.
Lukic ML, Mensah-Brown E, Galadari S, Shahin A. Lack of apoptosis of infiltrating cells as the mechanism of high susceptibility to EAE in DA rats. Dev Immunol. 2001;8:193–200.
Prendergast CT, Anderton SM. Immune cell entry to central nervous system—current understanding and prospective therapeutic targets. Endocr Metab Immune Disord Drug Targets. 2009;9:315–27.
Pashenkov M, Söderström M, Link H. Secondary lymphoid organ chemokines are elevated in the cerebrospinal fluid during central nervous system inflammation. J Neuroimmunol. 2003;135:154–60.
Corcione A, Casazza S, Ferretti E, Giunti D, Zappia E, Pistorio A, Gambini C, Mancardi GL, Uccelli A, Pistoia V. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101:11064–9.
Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisäkk P, Ransohoff RM, Hofbauer M, Farina C, Derfuss T, Hartle C, Newcombe J, Hohlfeld R, Meinl E. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129:200–11.
Calderon TM, Eugenin EA, Lopez L, Kumar SS, Hesselgesser J, Raine CS, Berman JW. A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol. 2006;177:27–39.
McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:8053–64.
Meiron M, Zohar Y, Anunu R, Wildbaum G, Karin N. CXCL12 (SDF-1alpha) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J Exp Med. 2008;205:2643–55.
McCandless EE, Piccio L, Woerner BM, Schmidt RE, Rubin JB, Cross AH, Klein RS. Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis. Am J Pathol. 2008;172:799–808.
Colamussi ML, Secchiero P, Gonelli A, Marchisio M, Zauli G, Capitani S. Stromal derived factor-1 alpha (SDF-1 alpha) induces CD4+ T cell apoptosis via the functional up-regulation of the Fas (CD95)/Fas ligand (CD95L) pathway. J Leukoc Biol. 2001;69:263–70.
McCandless EE, Budde M, Lees JR, Dorsey D, Lyng E, Klein RS. IL-1R signaling within the central nervous system regulates CXCL12 expression at the blood-brain barrier and disease severity during experimental autoimmune encephalomyelitis. J Immunol. 2009;183:613–20.
Kohler RE, Comerford I, Townley S, Haylock-Jacobs S, Clark-Lewis I, McColl SR. Antagonism of the chemokine receptors CXCR3 and CXCR4 reduces the pathology of experimental autoimmune encephalomyelitis. Brain Pathol. 2008;18:504–16.
Cruz-Orengo L, Holman DW, Dorsey D, Zhou L, Zhang P, Wright M, McCandless EE, Patel JR, Luker GD, Littman DR, Russell JH, Klein RS. CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J Exp Med. 2011;208:327–39.
Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, Lande R, Aloisi F, Coccia EM. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2005;64:706–15.
Moll NM, Cossoy MB, Fisher E, Staugaitis SM, Tucky BH, Rietsch AM, Chang A, Fox RJ, Trapp BD, Ransohoff RM. Imaging correlates of leukocyte accumulation and CXCR4/CXCL12 in multiple sclerosis. Arch Neurol. 2009;66:44–53.
Miljkovic D, Timotijevic G, Stojkovic MM. Astrocytes in the tempest of multiple sclerosis. FEBS Lett. 2011;585:3781–8.
Adikari SB, Pettersson A, Soderstrom M, Huang YM, Link H. Interleukin-10-modulated immature dendritic cells control the proinflammatory environment in multiple sclerosis. Scand J Immunol. 2004;59:600–6.
Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–48.
Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol. 2008;84:116–31.
Patel JR, McCandless EE, Dorsey D, Klein RS. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc Natl Acad Sci U S A. 2010;107:11062–7.
Carbajal KS, Schaumburg C, Strieter R, Kane J, Lane TE. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc Natl Acad Sci U S A. 2010;107:11068–73.
Rice CM, Scolding NJ. Adult human mesenchymal cells proliferate and migrate in response to chemokines expressed in demyelination. Cell Adh Migr. 2010;4:235–40.
Croitoru-Lamoury J, Lamoury FM, Zaunders JJ, Veas LA, Brew BJ. Human mesenchymal stem cells constitutively express chemokines and chemokine receptors that can be upregulated by cytokines, IFN-beta, and Copaxone. J Interferon Cytokine Res. 2007;27:53–64.
Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10:649–56.
Chung IY, Norris JG, Benveniste EN. Differential tumor necrosis factor alpha expression by astrocytes from experimental allergic encephalomyelitis-susceptible and -resistant rat strains. J Exp Med. 1991;173:801–11.
Constantinescu CS, Hilliard B, Ventura E, Wysocka M, Showe L, Lavi E, Fujioka T, Scott P, Trinchieri G, Rostami A. Modulation of susceptibility and resistance to an autoimmune model of multiple sclerosis in prototypically susceptible and resistant strains by neutralization of interleukin-12 and interleukin-4, respectively. Clin Immunol. 2001;98:23–30.
Cautain B, Damoiseaux J, Bernard I, van Straaten H, van Breda Vriesman P, Boneu B, Druet P, Saoudi A. Essential role of TGF-beta in the natural resistance to experimental allergic encephalomyelitis in rats. Eur J Immunol. 2001;31:1132–40.
Segal BM, Dwyer BK, Shevach EM. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J Exp Med. 1998;187:537–46.
Cross AH, Manning PT, Stern MK, Misko TP. Evidence for the production of peroxynitrite in inflammatory CNS demyelination. J Neuroimmunol. 1997;80:121–30.
Aboul-Enein F, Weiser P, Höftberger R, Lassmann H, Bradl M. Transient axonal injury in the absence of demyelination: a correlate of clinical disease in acute experimental autoimmune encephalomyelitis. Acta Neuropathol. 2006;111:539–47.
Farias AS, de la Hoz C, Castro FR, Oliveira EC, Ribeiro dos Reis JR, Silva JS, Langone F, Santos LM. Nitric oxide and TNFalpha effects in experimental autoimmune encephalomyelitis demyelination. Neuroimmunomodulation. 2007;14:32–8.
Ahn M, Kang J, Lee Y, Riu K, Kim Y, Jee Y, Matsumoto Y, Shin T. Pertussis toxin-induced hyperacute autoimmune encephalomyelitis in Lewis rats is correlated with increased expression of inducible nitric oxide synthase and tumor necrosis factor alpha. Neurosci Lett. 2001;308:41–4.
Calabrese V, Scapagnini G, Ravagna A, Bella R, Foresti R, Bates TE, Giuffrida Stella AM, Pennisi G. Nitric oxide synthase is present in the cerebrospinal fluid of patients with active multiple sclerosis and is associated with increases in cerebrospinal fluid protein nitrotyrosine and S-nitrosothiols and with changes in glutathione levels. J Neurosci Res. 2002;70:580–7.
Acknowledgments
The authors of the paper are supported by the funds of the Serbian Ministry of Education and Science (Grants No: 173035, 175038, 173013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Momcilović, M., Mostarica-Stojković, M. & Miljković, D. CXCL12 in control of neuroinflammation. Immunol Res 52, 53–63 (2012). https://doi.org/10.1007/s12026-012-8282-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-012-8282-x