Abstract
The period from late gestation to weaning in neonatal mammals is a critical window when the adaptive immune system develops and replaces the protection temporarily provided by passive immunity and pre-adaptive antibodies. It is also when oral tolerance to dietary antigen and the distinction between commensal and pathogenic gut bacteria becomes established resulting in immune homeostasis. The reproductive biology of swine provides a unique model for distinguishing the effects of different factors on immune development during this critical period because all extrinsic factors are controlled by the experimenter. This chapter reviews this early stage of development and the usefulness of the piglet model for understanding events during this transitional stage. The review also describes the major features of the porcine immune system and the immune stimulatory and dysregulatory factors that act during this period. The value of the model to medical science in such areas as food allergy, organ transplantation, cystic fibrosis and the production of humanized antibodies for immuno-therapy is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The critical window of immunological development
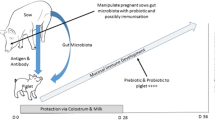
Offspring of eukaryotic organisms experience an especially critical period, i.e. “a window” in development which for mammals extends from late gestation to weaning (Fig. 1). All eukaryotic offspring suffer their highest rate of mortality and morbidity during this transitional period while simultaneously undergoing major physical and molecular changes as exemplified by complete metamorphosis in insects [1]. The major immunological events within this window for mammals are summarized in Fig. 1. These include the progressive development of the adaptive immune system that becomes superimposed on the innate immune system that developed during fetal life. The transition is “cushioned” by passive immunity and germline-encoded pre-adaptive or “natural” antibodies that provide protection by recognizing many ubiquitous epitopes on bacterial pathogens [2, 3]. Development of adaptive immunity during this period in truly naïve neonates is initiated through stimulation of TLRs and NOD receptors of the innate system by MAMPs (microbial associated molecular patterns) provided by colonizing bacteria which perhaps may do so by causing maturation of APCs [4–6]. Also during this time, the broadly-specific and highly connective pre-adaptive antibodies that also recognize autoantigens [7–9] are down-regulated and/or supplanted by a diversified repertoire of more specific adaptive antibodies. These are equally protective but their refined specificity diminishes the potential for autoimmunity. During the “critical window” neonatal infants and piglets: (a) develop tolerance to the one ton per year of foreign food antigens they will encounter as adults and (b) establish an equilibrium with commensal gut bacteria while retaining the ability to distinguish pathogenic forms, i.e. the “no harm, no foul” or so-called “danger concept”[10]. The desired outcome of these transitional events is the establishment of immune homeostasis. However, infectious agents, inappropriate colonizers and other external factors such as certain dietary antigens can disturb the development of this homeostatic condition. This results in what we call immune dysregulation and is manifest in autoimmunity, inflammatory bowel disease (IBD) and perhaps an increase in the frequency of allergy [11, 12].
The “critical window”of development for mammals. The heavy dotted line indicates the period in which pre-adaptive antibodies are believed to be important. Converging arrows highlight the major immunological events occurring during the critical window. It should be noted that the passive immunity section labelled Grp I & II is absent in swine since there is no in utero transfer of passive immunity as in rodents and humans
The isolator piglet model
The isolator piglet model is built around the features of mammals that comprise Group III in terms of the method of transferring immunity from mother to young [13–15]. Swine belong to this group and are a species in which the developing fetus receives no maternal antibodies or immune complexes during gestation. This allows a 94 day window from the time of the first VDJ rearrangement on day of gestation (DG) 20 to DG114 in which to study fetal lymphocyte development without the influence of the maternal immune system (Fig. 1). Since piglets, unlike rodents, give birth to precocial offspring, they can be recovered by Caesarian section and placed into germfree isolators separated from their birth mothers. In these units their exposure/supply of passive immunity, commensal bacteria, dietary antigen and pathogens is controlled by the experimenter. The offspring of farm pigs can be maintained in such units for up to 6 weeks and mini-swine for 8 weeks. This is several weeks beyond the normal weaning date for piglets and is inclusive of the critical window (Fig. 1). Since conventional swine give birth to 10–12 offspring, a single litter provides an entire experimental group. Maintenance can be labor intensive and expensive but the system allows questions to be addressed that cannot be addressed in lab rodents. While mostly outbred animals are used, inbred minipigs can also be used. However data show that four outbred animals/group are sufficient to establish statistical significance suggesting that most variation seen in the immune response of neonates is the results of differential exposure to environmental influences acting on genetic polymorphisms [5]. By controlling these factors in isolator piglets, the number of animals needed for study is significantly reduced.
The system is attractive because sequence homology between porcine and human Igs and TCRs is often <70%, higher than between rodents and humans [16]. This high degree of homology is not universal, depending rather on the gene being compared [17–19]. Furthermore, swine are omnivorous like humans, have a similar commensal flora [20] and are infected by closely-related viral and bacterial pathogens, such as rotavirus, influenza, Hemophilus, Salmonella, enterotoxigenic E. coli (ETEC), and pyogenic Streptococci. A number of these are transmissible between the two species. In fact swine are often used to model human intestinal diseases caused by ETEC and rotavirus. Before discussing the application of the isolator model to current immunobiological problems, we first review the swine adaptive immune system (section B) and its ontogeny (section C). This is necessary because the systems characterized for mouse and humans cannot be simply extrapolated to other mammals [14, 21].
The T and B cell repertoire in swine
T and B cell phenotypes in swine
T and B lymphocytes in pigs are discriminated by the same criteria as lymphocytes in other species. Beyond conventional CD4+ T helper and CD8+ T cytotoxic αβ T cells, the peripheral lymphoid system of swine contains so-called “peripheral double positive (DP) αβ T cells” that become CD4+CD8+ by subsequent expression of CD8αα on CD4+ T helper cells after activation [22, 23]. Porcine γδ T cells are as numerous as they are in ruminants and chickens [24]. All peripheral γδ T cell are negative for CD4 and are traditionally subdivided into CD2−CD8−, CD2+CD8− and CD2+CD8αα+ subsets that differ in their homing characteristic: CD2+CD8αα+ and CD2+CD8− γδ T cells preferentially accumulate in the secondary lymphoid organs while CD2−CD8− are enriched in the circulation [22, 23].
All μHC+ B cells in swine are MHC-II+, CD25lo, CD45RC+, and CD5+ ,although CD5 expression can be low or high [22, 25]. However, porcine B cells are heterogeneous in their CD2 and CD21 expression. These markers can be used to describe maturation pathways in porcine peripheral B cells [26; Sinkora et al. unpublished data; see D-3). The question of whether these are two B cell populations in swine, e.g., B-1/B-2 remains an open question.
The TCRαβ and TCRγδ repertoires
The same TCR genetic loci found in mice and humans have been identified in swine. The TCRJα (TRAJ), TCRCα (TRAC), TCRJδ (TRDJ), and TCRCδ (TRDC) regions of the genome have been completely sequenced by Uenishi and colleagues [27]. It should be noted that both the conventional and IMGT nomenclature for the T and B cell repertoire [28] is also used for swine, often interchangeably. The TRDC locus encodes three translated exons and a fourth pseudogene. Similar to mice and humans, nearly all TCRVδ genes are located 5′ of the TDRDδ segments but one (TCRVδ3) lies in inverse orientation between TCRCγ and TCRJα. The order and orientation of the remaining TCRVδ genes has not been resolved. Sequence homology studies suggest a higher similarity of TCRJδ and TCRCγ to human than to mice [27]. Studies by Yang et al. [29] have placed the TCRVδ genes into five groups. TCRVδ3,-4, and -5 consist of single genes whereas TCRVδ1 consists of a large number of related members. Three have high sequence homology to two human TCRVδ gene groups. The expression of the five TCRVδ gene groups during development and especially in the intestinal mucosa has been studied by Holtmeier and colleagues [30, 31]. This allowed identification of a non-polymorphic TCRVδ3 chain similar to that described in mice [32]. Despite the early onset of Tdt activity in fetal piglets (see below) the invariant TCRVδ3 chain shows no junctional diversity or N-region additions [31].
The organization of porcine TCRβ is well established. The 3′ region is organized as a tandem Dβ-Jβ-Cβ repeat as it is in other species (GenBank ABO079894; 33). Analysis of >300 TCRVβ transcripts has allowed the identification of 19 groups (families) of porcine Vβ genes that cluster into seven supergroups [33]. Based on >70 % sequence homology, we named 17 of these families according to their human counterparts. However one Vβ gene sequence (VT100; 34 or gt203; 33) is unrelated to any human Vβ family. We found that TCRVb 4, 5, 7, and 12 accounted for >80% of gene usage by both thymocytes and peripheral T cells. Recent unpublished data suggest that TCRV 12 is a single gene encoding several alleles. Jβ1 usage from both sources was directly correlated with their 5′ position in the locus; Jβ1.1,1.2 and 1.3 alone accounted for >35% of all Jβ usage. This pattern was not true for Jβ2 in which Jβ2.4, 2.5, and 2.7 were most frequently used.
The major observation from studies of TCRα/β and TCRγδ is that they are extremely similar to their human and mouse counterparts which is not true for the Ig heavy chain locus in swine but is the case for the kappa and lambda loci. This pattern indicates that one family of Ig superfamily receptor genes can diverge in a species (Ig heavy chain) while others (TCRαβ, TCRδ, Igλ, and Ig κ) remain conserved. Since all genetic systems do not follow a similar evolutionary pattern of divergence in even the same species, extrapolating from one species to the other using any one system, can be dangerous [17–19].
The CH and VH repertoire
T cell repertoires are highly conserved among vertebrates and the major TCRα/β and TCRγ/δ gene families can be traced from sharks to humans [35, 36]. However, antibody repertoires are often order and even species–specific. While IgM-like antibodies occur in all vertebrates, sharks and some teleosts have isotypes called IgW, IgX, and IgNAR that are not found in mammals. The amphibian Xenopus has CH genes encoding IgM, IgD, IgX, IgY, and IgF. The first two are homologs of their mammalian counterparts and the last three are believed to share a common ancestor with mammalian IgG, IgA, and IgE [37]. Many mammals, the rabbit excluded [14], have diversified their genes for Cγ (IgG) into 2–7 different subclasses after speciation [38, 39]. There has been similar diversification among VH genes whereas kappa and lambda appear highly conserved among mammals [36].
So how do swine that we use as our model for immunological development fit into the phylogenetic scheme? The CH locus of swine encodes IgM, IgD, IgE, IgA and at least six subclasses of IgG, several with multiple allelic variants [40]. It should be cautioned that although the porcine IgG subclasses bear the same designation as in humans (as is the case for other mammals as well) they are not evolutionary homologs since in all major mammalian groups, speciation preceded subclass diversification [38, 39, 41]. A complete map of the CH region is not yet available but preliminary evidence suggests it follows the same order as in other mammals [16]. Unlike mice and humans, both porcine and bovine IgD have a small switch region and can be transcribed either using Cμ1 or Cδ1 as the first heavy chain domain [42]. There are two IgA alleles that differ only because of a 12 nucleotide hinge deletion [43] although pigs homozygous for the “hingeless” variant do not appear to be more prone to disease [44]. In swine, class switch recombination (CSR) occurs in utero in the absence of environmental antigen, IgG1 is expressed as early at DG40 and at the highest frequency among IgG transcripts except for the IPP in late fetal/early neonatal life (section C-5).
The VH repertoire of swine differs markedly from that in mice and humans and there is no other mammal yet described with the exact same system. Like rabbits, but unlike mice and humans, all VH genes in swine are VH3 family genes and 29 variants (genes or alleles) have been identified [45] in contrast to ∼100 in rabbits [46]. As will be discussed in C-3, four VH genes comprise 80% of the pre-immune repertoire, two DH segments account for >98% of expressed diversity segments and swine have only one JH [47, 48]. The various VH genes differ only in their CDR regions so this simple system is very user friendly for studies on repertoire diversification as will be described in Section D-2.
The kappa and lambda repertoire
The organization of the porcine kappa locus is nearly identical to that described in other mammals. Expressed Vκ genes belong to two families (Vκ1 and Vκ2) that are sequence homologs to their namesake in humans. There are five Jκ segments that are homologous in sequence and orientation to those in other mammals and a single Cκ [49]. >90 % of the pre-immune Vκ repertoire is formed from a few Vκ2 genes and one Jκ segment. The lambda locus has not been completely mapped but it is organized into Jλ-Cλ tandem cassettes as in other vertebrates; expressed Vλ genes comprise two groups with homology to human Vλ3 and Vλ8 (Butler, Wells, Wertz unpublished.).
Pre-natal lymphocyte development
Organ system development in fetal piglets
Morphogenesis and organogenesis of pigs can be arbitrarily divided into 3 intervals: initial development of the zygote (DG1–DG12, DG = day of gestation), the embryonic period of embryogenesis (DG12–DG36) and fetal period of embryogenesis (DG36–DG114); the latter being divided into allometric growth phase (DG36–DG55) and isometric growth phase (DG55–DG114/birth). Basic primordial organs like lung buds, liver, stomach, pancreas, mesonephros, uteric bud, primordial intestine, or primordial pouches are easily distinguishable at about DG18–20, at the time when the first precursors of lymphocytes can be found in the yolk sac which is extraembryonic and involutes after DG24. Principal organogenesis is finished at about DG30 with the fetal liver constituting a major tissue over embryo. Lymphoid elements and lymphocytes in different organs are infrequent until the bone marrow starts its hematopoietic activity at about DG45. Lymph nodes including mesenteric lymph nodes are negligible until DG70, which seems temporally associated with the expansion of the peripheral lymphatic pool which occurs between DG60–DG90 (see below). At the birth, all primary and secondary lymphoid organs are present. The next expansion of lymphocytes in secondary lymphoid organs occurs postnatally and is probably related to colonization of gastrointestinal tract and exposure to environmental antigen. At that time, intraepithelial lymphocytes and lamina propria cells appear and mesenteric lymph nodes and jejunal Peyers patches increase in size.
Kinetics of lymphocyte development
The earliest site of lymphopoietic activity in porcine embryo is the yolk sac and the first lymphocytes that can be detected there at DG20 are B cells [50]. The yolk sac in the fetal pig involutes after DG24–27 and lymphopoietic activity and development of the pre-immune B cell repertoire thereafter moves to the fetal liver. This is the major site of B-cell lymphogenesis from DG30 until at about DG45 when the bone marrow becomes active [50]. T-lymphopoietic activity can be first detected in thymus at about DG40 [22, 51], i.e., about 20 days after the first pro-T cell progenitors can be detected in yolk sac and fetal liver. In the same time, the first sIgM+ B cells appear in the periphery and remain the major lymphocyte population until at least DG55, [Fig. 2; 22, 50, 51]. The first T cells appear about 5 days after they are detectable in the thymus, all of which are γδ T cells [Fig. 2; 51]. αβ thymocytes require about 15 days to fully differentiate, while γδ thymocytes do so in less than 3 days. The first αβ T cells appear in the periphery at about DG55 [Fig. 2; 52]. The onset of B-cell lymphogenetic activity in bone marrow starting from DG45 is followed by a rapid expansion of B cells and colonization of thymus with a second wave of progenitors. Lymphopoietic activity in the liver after this period fades and becomes marginal. Lymphopoietic activity in the bone marrow peaks between DG60–DG80 and during this period, the majority of peripheral B and T cells is generated. Although γδ T cells are the first T lymphocyte subset appearing early in ontogeny, the ratio of porcine αβ/γδ lymphocytes gradually increases later in ontogeny, resulting in a predominance of αβ T cells in both the thymus and in the periphery until the end of gestation [Fig. 2; 22, 51].
B cell development
As indicated above, B cell lymphogenesis begins in the yolk sac at DG20 with VDJ rearrangement but without transcription. B cell lymphogenesis then shifts to fetal liver at DG30 and by DG45, begins in bone marrow [50]. Unlike rodents and humans, Tdt is active at the first time of VDJ rearrangement and the degree of junctional diversity does not increase throughout fetal life [50, 53] although this has been observed in porcine TCRδ rearrangements [30]. 80% or more of the fetal pre-immune repertoire uses just four VH genes [48] and by including three additional VH genes, can account for >95% of the repertoire [45]. This is somewhat reminiscent of rabbits that use their most 3′ VH rabbit gene in 90% of rearrangement [54] but differs sharply from what is seen in mice and humans. Given the limited combinatorial diversity in this swine and the nearly identical framework regions of its VH3 genes, >96% of the pre-immune repertoire in swine is determined by junctional diversity in HCDR3 [53]. The light chain repertoire is also combinatorially restricted and shows little or no junctional diversity [49]. Lambda is preferentially used in early B cell development, presumably because of λ5. Otherwise the expressed κ:λ ratio is very similar to humans and therefore very different from the heavily skewed ratios in rodents, rabbits, cattle, and horses [21, 55].
A second feature of B cell development in swine is the early onset of CSR that occurs in utero without contact with environmental antigen. Transcripts for IgG1, IgG2, and IgA and of course IgM are present at midgestation [40, 56]. Furthermore, de novo synthesized IgG, IgM and IgA are present in fetal sera at low levels during the second half of gestation [56]. Remarkably, IgG and IgA plasma cells are present in the thymic medulla during the same time period [56, 57]. In newborn piglets, IgE is also transcribed in thymus [58]. We have speculated that CSR is a programmed event in fetal piglets, independent of antigen and perhaps the result of an intrinsic stochastic phenomenon as suggested by Deenick et al. [59]. The apparently programmed CSR during fetal life takes on special significance with porcine IgG3 in the IPP (see C-5).
Thymus and thymocyte development: thymic α/β versus γ/δ T cells
Differentiating thymocytes belonging to the αβ lineage follow a progression from less-differentiated, large triple-negative precursors through a CD8+CD3ε− immature stage to a small CD3ε− stage and thereafter to a stage of CD3εlo double-positive cells and finally to CD4+ or CD8+ single-positive mature thymocytes expressing CD3εhi [51]. This scenario is consistent with a generally accepted model of intrathymic T-cell differentiation derived from studies in other species. αβ T cells exported from thymus are therefore composed of classical homogenous CD4−CD8αβ+ cytotoxic and CD4+CD8− helper T cells subsets [51].
Ontogenetic studies revealed that γδ T cells require a shorter time period for expression of their TCR receptor than αβ T-cells and are also the earliest detectable T cell subset, developing first in the thymus (DG40) and subsequently (DG45) populating the periphery [Fig. 2; 22]. Because the substantial number of γδ T cell available for study in the lymphoid organs of pigs, we could show that all peripheral γδ T cells appear to be generated in the thymus from a proliferating TCRγδ+CD2+CD8−CD1+CD45RC− common precursor [60]. This stage is followed by diversification into a CD2+CD8αα+, CD2+CD8− and CD2−CD8− γδ thymocyte subsets whose further maturation is accompanied by: (a) loss of CD1 expression and (b) increased expression of CD45RC in a mutually exclusive fashion. Therefore, individual γδ subsets develop from CD1+CD45RC− through CD1−CD45RC− into CD1−CD45RC+ cells [60]. The last two subpopulations can be exported from thymus into the periphery where the final maturation step from CD1−CD45RC− into CD1−CD45RC+ cells may occur. Our data also indicate that there are two developmentally distinct subsets of CD2+CD8αα+ peripheral γδ T-cells. One develops in the thymus while the second acquires CD8αα in the periphery as a result of activation of CD8− thymus-dependent precursors [61]. The CD2+CD8αα+ γδ T-cells acquire MHC class II expression in the periphery while only MHC-II− γδ T-cells occur in the thymus. All of these results indicate that the maturation of γδ thymocytes occurs after successful expression of TCR γδ. Although γδ T cells do go through MHC dependent positive and negative selection like αβ T cell progenitors, αβ and γδ cell lineages require about the same time for full maturation [60, 61]. Apart from CD4− γδ T cells, a small fraction of γδ thymocytes are CD4+CD8αβ+CD1+. These cells have no counterpart in the periphery and have been shown to be a transient and independent population of thymocytes that can further divide and re-activate genes rearrangement to become αβ T cells [60].
Role of the ileal Peyers patches (IPP)
The terminal portion of the ileum, proximal to the ileal-caecal junction in many hoofed mammals, whales and dogs, is comprised of a continuous series of lymphoid follicles referred to as the ileal Peyers patches (IPP; Fig. 3A). These are distinct from the jejunal Peyers patches (JPP) that are common to all mammals and function as part of the mucosal immune system. Since the IPP are absent in mice and humans, understanding their role has been left to studies in sheep and swine. The IPP has to date been best-studied in sheep. The IPP is considered to be important in antibody repertoire diversification, analogous to the bursa of Fabricius [62–64]. The IPP has been less-well studied in swine but some preliminary data are presented in Fig. 3. Spectratypic analysis of follicular debris from individual follicles (Fig. 3B, left) of newborn piglet reveal a restricted clonality suggesting that each follicle may be the site of diversification of a single B cell clone (Fig. 3B, center). Sequence analysis of cloned VDJ from a single follicle allows diversification lineage trees to be drawn (Fig. 3B, right). In older conventional pigs the IPP began to regress (involute) to small-scattered follicles [65]. After colonization there are many Ig-containing cells and IgA+ and IgM+ cells are numerous [5]. These observations have lead to the idea that the function of the IPP changes with age. During the same period the JPP grow in prominence and assume the major role in mucosal immunity in the gut.
(A) Lymphoid anatomy of the porcine intestinal tract. The IPP and JPP are indicated. (B left) histology of the IPP showing individual B cells in the follicle that can be collected using a micromanipulator. (B center) spectratypic analyses of VDJs recovered from the scrapings of individual follicles. These are compared to polyclonal and oligoclonal spectratypes obtained from lymph nodes. (B right) Lineage tree constructed from cloned VDJs from a single follicle
An unusual phenomenon in the IPP is the transcription of IgG3 which starts in late fetal life and continues postnatally to account for >50% of total IgG transcripts [40]. However, its predominance diminishes with age and this appears to be related to colonization of the gut. Elsewhere in the newborn, IgG3 accounts for usually <5% of all IgG transcripts. It will be important to know the role of these IgG3 antibodies and their repertoire. We speculate that porcine IgG3 may represent a type of T independent pre-adaptive anti-bacterial system. This might be similar in function to mouse IgG3 antibodies (no homology) from marginal zone B cells perhaps derived from B-1 cells, that recognize bacterial carbohydrates [8, 9, 66, 67]. The decline in porcine IgG3 transcription may be part of the development of immune homeostasis during the “critical window” (Fig. 1) that suppresses the potential autoreactive pre-immune repertoire.
Post-natal development of adaptive immunity
The role of colonization and MAMPs
The role of normal gut flora in neonatal development has been studied for at least 50 years by comparing GF, gnotobiotic, and conventionally-reared animals [68]. Colonization influences enterocyte and immunological development. GF piglets have long, slender villi compared to their colonized counterparts [69] and colonization is needed for crypt development and Paneth cell differentiation [70, 71]. Enterocyte maturity appears to be determined when bacterial adhesions are expressed [72] so not surprisingly, colonization is associated with major shifts in gene transcription [73] including expression of bacterial adhesions [72]. Microflora are also necessary for development of oral tolerance [74] and therefore attainment of immune homeostasis (75: Fig. 1].
Applying the isolator piglet model to study the role of colonization has shown that a truly naïve neonatal mammal cannot respond to either TD or TI-2 immunogens without the adjuvant effect of bacterial colonization [5]. This differs from newborn rodents perhaps because they are not completely naïve having received maternal antibodies and regulatory factors both pre-and post-natally [13, 15; Fig 1]. Further studies have shown that living or intact bacteria are not required, only their purified MAMPs, especially bacterial DNA as CpG ODN [5]. Surprisingly, E.coli LPS alone has no in vivo effect but rather, synergistically augments the effect of bacterial DNA like MDP, a NOD-2 ligand. Early exposure to MAMPs can also induce tolerance to dietary protein and airways hypersensitivity [76].
Post-natal antibody repertoire development
Postnatal changes in the apparent function of the IPP were previously discussed. Age- and experience-related changes are also seen in the antibody repertoire. For example,∼80% of all VH genes in conventional pigs (labeled “other”in Fig. 4) are no longer identifiable as one of the seven VH genes that comprise >95% of the pre-immune repertoire (Fig. 4). This is because they have undergone SHM and are no longer able to hybridize with gene-specific probes [45]. The piglet has proven to be user-friendly for studies of this type since usage of the seven VH genes can be monitored with only 10 specific probes to generate data like that shown in Fig. 4. Such proportional usage data has allowed a repertoire diversification index (RDI) to be calculated (Fig. 4). This index has allows us to quantity the effect of colonization and neonatal viral infection on repertoire diversification (see below).
VH gene usage by IgM and IgG transcripts in the newborn pre-immune repertoire and the diversified repertoire of conventionally-reared pigs (PIC). Proportional usage of the seven VH genes comprising >95% of the pre-immune repertoire is given. “Other” is comprised of somatically mutated version of these genes. Hyb = hybrid VH genes from the genome.The repertoire diversification index (RDI) equation and resultant values are shown below the graph
Although the limited VH usage of swine resembles that of rabbits [77], we have found no evidence for gene conversion in swine and the VDJs generated in vitro by PCR [52] do not have the mosaic character of gene conversion products [45, 78].
Changes in T and B cell phenotypes during post-natal development
Activation of T cells with various antigens in the periphery leads to expression of MHC class II and CD8αα molecules that play an important role in signal transduction associated with cell effector function. Since MHC class II and CD8αα molecules are not down-regulated after activation in pigs, these molecules selectively mark effector/memory T cells. As a result, CD4+CD8αα+ αβ T cells (peripheral DP αβ T cells), CD2+CD8αα+ γδ T cells or CD8αα+ NK cells are generated and can be found in the periphery [22, 61, 79–81]. In this respect, peripheral DP αβ T cells should not be confused with transitional stages of intrathymic development that express CD8αβ+ [81]. Peripheral DP αβ T cells are CD1− [80], mostly CD29+ [79], MHC-II+ [61, 80] and are generated from CD4+ cells upon stimulation [79]. The DP subset is absent or very rare before birth and in newborns [22, 51]. The CD2+CD8αα+ γδ T cell subset has been postulated to be the progeny of CD2+CD8− γδ T lymphocytes based on the observations that: (a) some TCRγδ+CD2−cells may acquire CD8αα upon activation, (b) CD2+CD8αα+ γδ T cells are potentially cytotoxic while other γδ T cell subsets are not and (c) CD2+CD8+ γδ T cell are scarce in porcine fetuses [22, 61, 81, 82].
Porcine B cells differ in expression of CD2 and CD21 depending on activation [22, 50, 83]. Immature and mature unprimed B cells are CD2+CD21+ but lose CD2 expression following stimulation. However, the resultant CD2− CD21+ B cells can rapidly re-express CD2 and after proliferation, mature into CD21− B cells. Either CD2+ or CD2− B cells lacking CD21 are subsequently able to generate proliferating uHC− plasmablast or non-proliferating uHC− plasma cells that represent the pentultimate stage of B cell maturation (Sinkora et al. unpublished data). These findings are based on in vivo studies in fetal and germ-free animals, in which more than 90% of μHC+ lymphocytes are CD2+CD21+ [22, 83] but after colonization lose expression of CD2− and/or CD21− [22, 83, Sinkora et al. unpublished data].
Immune dysregulation during the critical window of development
Figure 1 emphasizes the many immunological changes that occur during the critical window. Due to this complicated transitional period, it is not altogether surprising that the neonate’s naïve immune system is especially sensitive to environmental pressures before homeostasis is established. This can be demonstrated by comparing the impact of an immune dysregulatory virus on isolator versus conventional piglets. Porcine reproductive and respiratory syndrome virus (PRRSV) is a world-wide pandemic costing the swine industry in the USA $560 million annually [84]. Given to isolator piglets (GF or colonized with E.coli) it causes extraordinary B cell proliferation and lymphoid hyperplasia (adenopathy) and autoimmunity [85]. This proliferation raises IgG, IgM, and IgA levels 10–1,000 fold in 21 days. However when conventional piglets, suckling non-immune mothers, are inoculated with the same dosage of PRRSV, the immune dysregulatory effects are significantly reduced [86]. Thus, non-immune factors transmitted via colostrum/milk or the effect of the complex commensal flora of conventional piglets, provide factors that dampen immune dysregulation presumably through establishment of immune homeostasis.
The mechanism of immune dysregulation caused by PRRSV is not fully understood. We know there is no diversification of the pre-immune repertoire and the expanded B cells display the naïve CD2+ phenotype [86, 87]. Since the pre-immune repertoire is highly cross-reactive and harbors autoantibodies [7, 88, 89] it is not surprising that we found anti-dsDNA and anti-Golgi as well as deposition of immune complexes in the kidney and vasculature. Our recent studies suggest a new form of B cell superantigen may be involved, since the majority of the expanded population is skewed to favor B cells with hydrophobic HCDR3s. A high proportion of the HCDR3s display the hydrophobic AMVLV or similar motifs. At the same time, the normal population of HCDR3s with hydropathicities in the 0.0–0.3 range, fails to develop [87]. Few of the expanded B cells are believe to be virus-specific, since <1% of the IgG binds in diagnostic ELISA tests [85].
PRRSV is but one example of a neonatal virus that can cause immune dysregulation. It is also seen with LCMV and others viruses [88–94]. The effect of PRRSV is not an immune dysregulatory artifact of GF piglets since littermates infected with swine influenza develop normal T and B cell responses, and a HCDR3 hydropathicity profile peaking in the 0.0–0.3 range [87]. Influenza infections are resolved in 2 weeks and sterilizing immunity develops.
The isolator piglet model in medical research
Infectious disease
The value of the model in studies on the effect of neonatal viruses and gut colonization has been discussed. This model has specific application to influenza since swine have been regarded as the “mixing bowel” of influenza genes that could allow H5N1 bird flu to spread to humans [95]. Swine also carry retroviruses that are can replicate in humans [96, 97]. This constitutes a problem in xenotransplantation [98]. However, swine could also provide a model host for studying how organism controls endogenous retroviruses [99]. Swine are used as models for enterotoxigenic E. coli (ETEC) and rotavirus [100]. Given the intense interaction of swine and humans in modern hog confinement facilities (factory farms) and the cross-species transmission of influenza, Strept suis, and ETEC, the piglet may be involved in humans disease research.
Food Allergy
Humans ingest one metric ton of potential allergens annually, yet only 1–6% of the world population suffers from food allergy [101, 102]. However, almost all neonates make vigorous responses to these antigens and most bottle-fed infants and young children have serum antibodies to cows milk proteins, the levels of which wane after 1–2 years [103–105]. However, responses to dietary antigens can persist into adulthood. This is especially true for IgA deficient individuals [106, 107]. A number of these patients also have autoantibodies [107] which can be particularly serious in individuals at risk for type 1 diabetes which can occur by sensitization with bovine milk insulin [108]. The majority of these antibodies are IgG and their presence does not correlate with IgE-mediated hypersentivity and prick test results that characterize true food allergy [102]. Rather they appear to reflect the gradual establishment of immune homeostasis. Classical Type I hypersentivity also targets cows milk protein as well as peanut, wheat, egg, tree nut, seafood, and soy proteins [102]. Isolator piglets provide a model for all types of dietary immune responses since they can be reared on protein-free milk substitutes, including the same ones given to food allergy-prone infants (Alimentum, Ross Lab, Columbus) because the nutritional requirement of swine and humans are similar [109]. Peanut allergy is a serious food and respiratory allergy problem of increasing frequency that effect ∼1% of the U.S. population [110–112] and for which a piglet model is currently under development (Joe Urban, ARS, Beltsville, Md. pers. comm).
Probiotics, normal flora and inflammatory bowel disorder (IBD)
The importance of colonization accepted, the role of each colonizer remains unknown although they appear to differ in importance [73]. Some evidence suggests that Bacteroides, Bacillus, Clostridium, and segmented filamentous bacteria (related to Clostridium) play important roles [73, 113, 114]. The isolator piglet model allows the effect of colonization with individual bacteria or combinations to be studies in terms of: (a) stimulation of immune competence, (b) development of oral tolerance, (c) the effect on immune dysregulatory viruses, and (d) homeostatic interactions with normal gut flora versus pathogens. The last topic can have relevance to IBD which currently suffers from the lack of a good animal model (J. Weinstock, pers. comm). Lay magazines and medical journals increasingly mention probiotics and their value. Many probiotics products are strongly supported by health food advocates and are often centered around bacteria like lactobacilli. The idea is that “re-colonization” of the gut with healthy microbes can reduce intestinal disorders like IBD and systemic diseases like atopic allergy. Since re-colonization is only really possible during the neonatal period [115] the isolator piglet could be a useful model for such studies. In 2005 the American Academy of Microbiology [116] emphasized the need for models to obtain hard scientific data since much support for probiotics relies on testimonials and research supported by suppliers [117]. We think the isolator piglet provides a model to tests the probiotic concept. The weakness of the model is that IBD, many allergies and autoimmune phenomena are not manifest until later in adult life and even mini-swine cannot be logistically maintained in a controlled environment for long periods.
Breast feeding and passive immunity
One of the least understudied areas in neonatal immunology concerns the role of factors other than antibodies that are transmitted from mother to offspring [15]. While there is little question about the importance of passive antibodies, other constituents in colostrum/milk have received less attention. The potential importance of these factors is illustrated by the much lower degree of immune dysregulation in PRRSV-infected conventional piglets versus GF or monoassociated isolator piglets [86]. In these studies, the piglets suckled non-immune dams so the difference is not the results of passive antibodies in colostrum/milk but must reside in non-antibody factors or in the effect of conventional gut flora. An important role for non-immune factors in colostrum/milk might not be surprising considering the number of potential immune regulatory factors transmitted in milk/colostrum [15]. For example IL-6 and TGFβ occur at relatively high concentration; IL-6 levels are 10–20 times higher in milk than serum and >125 μg of active TGFβ reaches the gut of the sucking neonate daily. Swine colostrum contains 1,500 ng/ml of EGT [118] which accelerates intestinal growth [119] and up-regulates TGF expression [120]. Since the experimenter controls the supply (and quality) of colostrum/milk given to isolator piglets, it is possible to use the model to measure the effects of these maternally derived factors. Maternally derived factors can also include narcotics, pharmaceuticals, and infectious agents. Some have long regarded the mammary gland as a second excretory organ. The piglet model may be useful in studying the effect of such negative factors.
Immunotechnology
The technology of producing transgenic or gene-disrupted (knock-out) piglets is well developed [121, 122]. Such technology has implications for using swine as organ donors to offset the shortage of human organs [123]. Swine can also be used for producing humanized antibodies for immunotherapy [124], much like in cattle [125] by transfecting the human chromosome fragment containing the hIGH genes into animals with a disrupted endogenous IGH locus. This is more straightforward in swine since they have a single copy JH gene [47]. Furthermore, the isolator piglet allows the impact and importance of extrinsic influences on these transgenic animals to be addressed. A recent example of porcine genetic engineering is the CFTR knockout pig [126]. CFTR controls ion transport and is heavily correlated with cystic fibrosis (CF). A major question in CF is whether the disease is totally intrinsic or rather the result of an intrinsic gene defect coupled with respiratory infection. This can be tested in the isolator piglet model if CFRT−/− animals develop human-like CF disease.
We hope that this review of the isolator piglet model and its potential advantages in addressing biological and medical issues will lead to a greater awareness of its potential, perhaps even to wean some investigators off “mouse only” immunology.
References
Butler JE, Leone CA. Antigenic changes during the life cycle of the beetle, Tenebrio molitor. Comp Biochem and Physiol 1966;19:699–711.
Ochsenbein AF, Zinkernagel R. Natural antibodies and complement link innate and acquired immunity. Immunol Today 2000;1:624–30.
Marchalonis JJ, Adelman MK, Schluter SF, Ramsland PA. The antibody repertoire in evolution: Chance, selection and continuity. Devel Comp Immunol 2006;30:223–47.
Butler JE, Sun J, Weber P, Francis D. Antibody repertoire development in fetal and neonatal piglets. III. Colonization of the gastrointestinal tracts results in preferential diversification of the pre-immune mucosal B-cell repertoire. Immunology (British) 2000;100:119–30.
Butler JE, Weber P, Sinkora M, Baker D, Schoenherr A, Mayer B, Francis D. Antibody repertoire development in fetal and neonatal piglets. VIII. Colonization is required for newborn piglets to make serum antibodies to T-dependent and type 2 T-independent antigens. J Immunol 2002;169:6822–30.
Butler JE, Francis D, Freeling J, Weber P, Sun J, Krieg AM. Antibody repertoire development in fetal and neonatal piglets. IX. Three PAMPs act synergistically to allow germfree piglets to respond to TI-2 and TD antigens. J Immunol 2005;175:6772–85.
Dighiero GP, Lymberi P, Holmberg D, Lundquist I, Coutinho A, Avrameas S. High frequency of natural autoantibodies in normal mice. J Immunol 1985;134:765–71.
Lopez-Carvalho T, Foote J, Kearney JF. Marginal zone B cells in lymphocyte activation and regulation. Curr Opin Immunol 2005;17:244–50.
Fulton RJ, Nahm MH, Davie JM. Monoclonal antibodies to streptococcal group A carbohydrate. II. The Vk1GAC light chain is preferentially associated with serum IgG3. J Immunol 1983;131:1326–31.
Matzinger P. The danger model: a renewed sense of self. Science 2002;296:301–5.
Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002;347:911–20.
Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF Jr, Weinstock JW. Heligmosomoides polygyrus inhibits established colitis in IL-10 deficient mice. Eur J Immunol 2004;34:2690–98.
Butler JE. Immunoglobulins of the mammary secretions. In: Larson BL, Smith V, editors. Lactation a Comprehensive Treatise, Vol. III, Chapter V. New York: Academic Press; 1974. p. 217–55.
Butler JE. Preface: why I agreed to do this. In: (Butler JE, Guest Ed). Antibody repertoire development. Dev Comp Immunol 2006;30:1–17.
Butler JE, Kehrle ME. Immunocytes and immunoglobulins in milk. In: Mestecky J, Lamm ME, Strober W, McGhee JR, Mayer L, Bienenstock J, editors. Mucosal Immunology. 3rd ed. Academic Press, NY; 2005. p. 1763–93.
Butler JE, Sun J, Wertz N, Sinkora M. Antibody repertoire development in swine. Dev Comp Immunol 2006;30:199–221.
Murphy WJ, Eizirik E, Johnson WE, Zhang YP, Ryder OA, Obrien SJ. Molecular phylogenetics and the origins of placental mammals. Nature 2001;409:614–18.
Waddell PJ, Shelley S. Evaluating inter-ordinal phylogenies with novel sequences including RAG1, g-fibrinogen, NDG and mt-tRNA plus MCMC driven nucleotide, amino acid and codon models. Mol Phylogeny and Evol 2003;28:197–224.
Madsen O, Scally M, Douady CJ. Parallel adaptive radiations in two major clades of placental mammals. Nature 2001;409:610–14.
Lesser TD, Amenuvor JZ, Jensen TK, Lindecrona RH, Boye M, Møller K. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl Environ Microbiol 2002;68(2):673–90.
Butler JE. Immunoglobulin gene organization and the mechanism of repertoire development. Scand J Immunol 1997;45:455–62.
Sinkora M, Sinkora J, Rehakova Z, Splichal I, Yang H, Parkhouse RME, Trebichavsky I. Prenatal ontogeny of lymphocyte supoulations in pigs. Immunology 1998;95:595–603.
Yang H, Parkhouse RM. Phenotypic classification of porcine lymphocyte subpopulations in blood and lymphoid tissues. Immunology 1996;89:76–83.
Hein WR, Mackay CR. Prominence of γδ T cells in the ruminant immune system. Immunol Today 1991;12:30–4.
Appleyard GD, Wilkie BN. Characterization of porcine CD5 and CD5+ B cells. Clin Exp Immunol 1998;111:225–30.
Sinkora J, Rehakova Z, Sinkora M, Cukrowska B, Tlaskalova-Hogenova H, Bianchi AT, De Geus B. Expression of CD2 on porcine B lymphocytes. Immunology 1998;95:443–49.
Uenishi H, Hiraiwa H, Yamamoto R, Yasue H, Takagaki Y, Shiina T, Kikkawa E, Inoko H, Awata T. Genomic structure around joining segments and constant regions of swine T-cell receptor αβ (TRA/TRD) locus. Immunology 2003;109:515–26.
Lefranc M-P, Giudicelli V, Kaas Q, Duprat E, Jabado-Michaloud J, Scaviner D, Ginestoux C, Clément O, Chaume D, Lefranc G. IMGT, the international ImMunoGeneTics information system®. Nucl Acids Res 2005;33:D593–97.
Yang YG, Hota S, Yamada S, Shimizu M, Takagaki Y. Diversity of T cell receptor δ-chain cDNA in the thymus of a one-month-old pig. J Immunol 1995;155:1981–93.
Holtmeier W, Geisel W, Bernert K, Butler JE, Sinkora M, Rehakova Z, Sinkora J, Caspary WF. Prenatal development of the porcine TCRδ repertoire: dominant expression of an invariant T cell receptor Vδ3-Jδ3 chain, Eur. J Immunol 2004;34:1941–9.
Holtmeier W, Käller J, Geisel W, Pabst R, Caspary WF, Rothkötter HJ. Development and compartmentalization of the porcine TCRδ repertoire at mucosal and extraintestinal sites: The pig as a model for analyzing the effect of age and microbial factors. J Immunol 2002;169:1993–2002.
Havran WL, Carbone A, Allison JP. Murine T cells with invariant γδ antigen receptors: origin, repertoire, and specificity. Semin Immunol 1991;3:89–97.
Butler JE, Wertz N, Sun J, Sacco R. Characterization of the porcine Vβ repertoire in thymocytes versus peripheral T-cells. Immunology 2005;114:184–93.
Baron C, Sachs DH, LeGuerin C. A particular TCRβ variable used by T-cells infiltrating kidney transplants. J Immunol 2001;166:2589–96.
Rast JP, Anderson MK, Strong SJ, Luer C, Litman RT, Litman GW. αβγ and δ T-cell antigen receptor genes arose early in vertebrate phylogeny. Immunity 1997;6:1–12.
Marchalonis JJ, Schluter SF, Bernstein RM, Shanxiang S, Edmundson AB. Phylogenetic emergence and molecular evolution of the immunoglobulin family. Adv Immunol 1996;70:417–506.
Zhao Y, Pan-Hammarstrom Q, Yu S, Wertz N, Zhang X, Li N, Butler JE. Hammarstrom L. Identification of IgF, a hinge-region containing Ig class and IgD in Xenopus tropicalis. Proc Natl Acad Sci (USA) 2006;103:12087–92.
Butler JE, Sinkora M, Wertz N, Holtmeier W, Lemke CD. Development of the neonatal B- and T-cell repertoire in swine: Implications for comparative and veterinary immunology. Vet Res 2006;37:417–41.
Nguyen VK. Generation of heavy chain antibodies in camelids, Ph.D., Thesis, Free University of Brussels 2001. p. 109–11.
Butler JE, Wertz N. Antibody repertoire development in fetal and neonatal piglets. XVII. Fetal IgG subclass transcription re-visited. J Immunol 2006;177:5480–9.
Kehoe JM, Capra JD. Nature and significance of immunoglobulin subclasses. N Y State J Med 1974;74:489–91.
Zhao Y, Pan-Hammarstrom Q, Kacskovics I, Hammarstrom L. The porcine Ig δ gene: Unique chimeric splicing of the first constant region domain in its heavy chain transcripts. J Immunol 2003;171:1312–8.
Brown WR, Kacskovics I, Amendt B, Shinde R, Blackmore N, Rothschild M, Butler JE. The hinge deletion variant of porcine IgA results from a mutation at the splice acceptor site in the first Cα intron. J Immunol 1995;154:3836–42.
Navarro P, Christenson R, Ekhardt G, Lunney JK, Rothschild M, Bosworth B, Lemke J, Butler JE. Genetic differences in the frequency of the hinge variants of porcine IgA is breed dependent. Vet Immunol Immunopath 2000;73:287–95.
Butler JE, Weber P, Wertz N. Antibody repertoire development in fetal and neonatal pigs. XIII. “Hybrid VH genes” and the pre-immune repertoire revisited. J Immunol 2006;177:5459–70.
Gallarda JL, Gleason KS, Knight KL. Organization of rabbit immunoglobulin genes. I. Structure and multiplicity of germ-line VH genes. J Immunol 1985 135:4222–8.
Butler JE, Sun J, Navarro P. The swine immunoglobulin heavy chain locus has a single JH, no identifiable IgD. Int Immunol 1996;8:1897–904.
Sun J, Hayward C, Shinde R, Christenson R, Ford SP, Butler JE. Antibody repertoire development in fetal and neonatal piglets. I. Four VH genes account for 80% of VH usage during 84 days of fetal life. J Immunol 1998;161:5070–8.
Butler JE, Wertz N, Wang H, Sun J, Chardon P, Piumi F, Wells K. Antibody repertoire in fetal and neonatal pigs. VII. Characterization of the pre-immune kappa light chain repertoire. J Immunol 2004;173:6794–805.
Sinkora M, Sun J, Sinkorova J, Christenson RK, Ford SP, Butler JE. Antibody repertoire development in fetal and neonatal piglets. VI. B-cell lymphogenesis occurs at multiple sites with differences in the frequency of in-frame rearrangements. J Immunol 2003;170:1781–8.
Sinkora M, Sinkora J, Rehakova Z, Butler JE. Early ontogeny of thymocytes in pigs: sequential colonization of the thymus by T cell progenitors. J Immunol 2000;165:1832–9.
Sinkora M, Sun J, Butler JE. Antibody repertoire development in fetal and neonatal piglets. V. VDJ gene chimeras resembling gene conversion products are generated at high frequency by PCR in vitro. Mol Immunol 2000;37:1025–34.
Butler JE, Weber P, Sinkora M, Sun J, Ford SJ, Christenson R. Antibody repertoire development in fetal and neonatal piglets. II. Characterization of heavy chain CDR3 diversity in the developing fetus. J Immunol 2000;165:6999–7011.
Becker RS, Knight KL. Somatic diversification of immunoglobulin heavy chain VDJ genes: Evidence for somatic gene conversion in rabbits. Cell 1990;63:987–97.
Butler JE, Wertz N, Sun J, Wang H, Lemke C, Chardon P, Puimi F, Wells K. The pre-immune variable kappa repertoire of swine is selectively generated from certain subfamilies of Vκ2 and one Jκ gene. Vet Immunol Immunopath 2005;108:127–37.
Butler JE, Sun J, Weber P, Ford SP, Rehakova Z, Sinkora J, Lager K. Antibody repertoire development in fetal and neonatal piglets. IV. Switch recombination, primarily in fetal thymus occurs independent of environmental antigen and is only weakly associated with repertoire diversification. J Immunol 2001;167:3239–49.
Cukrowska B, Sinkora J, Mandel L, Splichal I, Bianchi ATJ, Kovaru F, Tlaskalova-Hogenova H. Thymic B cells of pig fetuses, germ free pigs spontaneiously produce IgM, IgG and IgA: detection of ELISPOT method. Immunology 1996;87:487–94.
McAleer J, Weber P, Sun J, Butler JE. Antibody repertoire development in fetal and neonatal piglets. XI. The thymic B cell repertoire develops independently from that in blood and mesenteric lymph nodes. Immunology 2005;114:171–83.
Deenick EK, Hasbold J, Hodgkins PD. Switching to IgG3, IgG2b and IgA is division linked and independent revealing a stochastic framework for describing differentiation. J Immunol 1999;163:4707–17.
Sinkora M, Sinkorova J, Cimburek Z, Holtmeier W. Two groups of porcine TCRγδ+ thymocytes behave and diverge differently. J Immunol 2007;178:711–19.
Sinkora M, Sinkorova J, Holtmeier W. Development of γδ thymocyte subsets during prenatal and postnatal ontogeny. Immunology 2005;115:544–55.
Jenne CN, Kennedy LJ, Reynolds JD. Antibody repertoire development in the sheep. Dev Comp Immunol 2006;30:165–74.
Reynaud CA, MacKay CR, Miller RG, Weill JC. Somatic generation of diversity in a mammalian primary lymphoid organ: the sheep ileal Peyers patches. Cell 1991;64:995–1005.
Reynolds JD, Morris B. The evolution and involution of Peyer’s patches in fetal and postnatal sheep, Eur. J Immunol 1983;13:627–35.
Pabst R, Geist M, Rothkötter HJ, Fritz FJ. Postnatal development and lymphocyte production of jejunal and ileal Peyers patches in normal and gnotobiotic pigs. Immunology 1988;64:539–44.
Hardy RR. B-1 B cell development. J Immunol 2006;177:2749–54.
Snapper CM, Marcu KB, Zelazowski P. The immunoglobulin class switch: beyond “accessibility”. Immunity 1997;6:217–23.
Wostmann BS. Germfree and gnotobiotic animal models. Boca Raton: CRC Press; 1996. p. 19–35.
Kenworthy R, Allen WD. Influence of diet and bacteria on small intestinal morphology with special reference to early weaning and Escherichia coli. Studies with germfree and gnotobiotic pigs. J Comp Path 1966;76:291–6.
Bry L, Falk P, Huttner K, Quellette A, Midtvedt T, Gordon JI. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc Natl Acad Sci (USA) 1994;91:10335–9.
Heneghan JB. Enterocyte kinetics, mucosal surface area and mucus in gnotobiotes. In: Fliedner T et al, editors. Clinical and experimental gnotobiotics. Proceedings of VIth International Symposium on Gnotobiology. NY: Gustav. Fisher, 1979. p. 19–31.
Stokes CR, Miller BG, Bourne FJ. Animal models of food sensitivity. In: Brostoff J, Challacombe SJ, editors. Food allergy and intolerance. London: Bailliere Tindall, 1987. p. 286–300.
Hooper LV, Wong MH, Thelin A, Hanson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationship in the intestine. Science 2001;29:881–4.
Sudo N, Sawamura SA, Tanaka K, Alba Y, Kulo C, Koga K. The requirement of intestinal flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol 1997;1549:1739–45.
Christen U, van Herrath MG. Infection and autoimmunity-good or bad? J Immunol 2005;174:7481–6.
Wang Y, McCusker CM. Neonatal exposure with LPS and/or allergen prevents experimental airway disease: development of tolerance using environmental antigen. J Allergy Clin Immunol 2006;118:143–51.
Knight KL, Becker RS. Molecular basis of allelic inheritance of rabbit immunoglobulin VH allotypes: Implications for the generation of antibody diversity. Cell 1990;60:963–70.
Reynaud CA, Dahan A, Anquez V, Weill JC. Somatic hyperconversion diversifies the single VH gene of the chicken with a high incidence in the D region. Cell 1989;59:171–83.
Zuckermann FA, Husmann RJ. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology 1996;87:500–12.
Saalmuller A, Hirt A, Reddehase MJ. Phenotypic discrimination between thymic and extrathymic CD4−CD8− and CD4+CD8+ porcine T lymphocytes. Eur J Immunol 1989;19:2011–6.
Yang H, Parkhouse RME. Differential expression of CD8 epitopes amongst porcine CD8-positive functional lymphocyte subsets. Immunology 1997;92:45–52.
Reddehase MJ, Saalmuller A, Hirt W. γδ T-lymphocyte subsets in swine. Curr Top Microbiol Immunol 1991;173:113–7.
Sinkora J, Rehakova Z, Sinkora M, Cukrowska B, Tlaskalova-Hogenova H. Early development of immune system in pigs. Vet Immunol Immunopathol 2002;87:301–6.
Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc 2005;227:385–92.
Lemke CD, Haynes JS, Spaete R, Adolphson D, Vorwald A, Lager K, Butler JE. Lymphoid hyperplasia resulting in immune dysregulation is caused by PRRSV infection in pigs. J Immunol 2004;172:1916–25.
Lemke CD. PRRSV infection of neonatal piglets induces immune dysregulation and modulation. Ph D. Thesis. University of Iowa; 2006.
Butler JE, Lemke CD, Weber P, Sinkora M, Lager KM. Antibody repertoire development in fetal and neonatal piglets. XIX. Undiversified B cells with hydrophobic HCDR3s preferentially proliferate in PRRS. J Immunol 2007;178:6320–31.
Hunziker L, Recher M, Macpherson AJ, Ciurea A, Freigand S, Hengartner H. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infection. Nat Immunol 2003;4:343–9.
Casali P, Schettino EW. Structure and function of natural antibodies. In: Rose N, Potter M, editors. Immunology of silicones. Springer Verlag, 1996. p. 167–79.
Blutt SE, Crawford SE, Warfield KL, Lewis DE, Estes MK, Conner ME. The VP7 outer capsid protein of rotavirus induces polyclonal B-cell activation. J Virol 2004;78:6974–81.
Kim SH, Shin YK, Lee IS, Bae YM, Shon HW, Suh YH, Ree HJ, Rowe M, Park SH. Viral latent membrane protein 1 (LMP-1) induced CD99 down regulation in B cells leads to the generation of cells with Hodgkin’s and Reed-Sternberg phenotype. Blood 2000;95:294–300.
Coutelier J-P, Coulie G, Wauters P, Heremans H, der Logt JT. In vivo polyclonal B-lymphocyte activation elicted by murine viruses. J Virol 1990;64:5383–8.
Stevenson PG, Doherty PC. Non-speecific B cell activation following murine gammaherpesvirus infection is CD4 indepednet in vitro but CD4 dependent in vivo. J Virol 1999;73:1075–9.
Karupiah G, Sacks TE, Klinman DM, Frederickson TN, Hartley JW, Chen JH, Morse HC. Murine cytomegalovirus infection-induced polyclonal B cell activation is independent of CD4+ T cells and CD40. Virology 1998;240:12–26.
Scholtissek C. Pigs as the “mixing vessel” for the creation of new pandemic influenza A viruses. Med Princ Pract 1990;2:65–71.
Patience C, Takeuchi Y, Weiss RA. Infection of human cells by an endogenous retrovirus of pig. Nat Med 1997;3:282–6.
Wood JC, Quinn G, Suling KM, Oldmixon BA, van Tine BA, Cina R, Arn S, Huang CA, Scobie L, Onions DE, Sachs DH, Schuurman H, Fishman JA, Patience C. Identification of exogenous forms of human-tropic porcine endogenous retrovirus in minature swine. J Virol 2004;78:2494–501.
Blusch JH, Patience C, Martin U. Pig endogenous retroviruses and xenotransplantation. Xenotransplantation 2002;9:242–51.
Jonsson SR, Hache G, Stenglein MD, Fahrenkrug SC, Andresdottir V, Harris RS. Evolutionarily conserved and non-conserved retrovirus restriction activities of artiodactyl APOBEC3F proteins. Nucleic Acid Res 2006;34:5683–94.
Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systemic and intestinal antibody-secreting cell responses and corrletaes of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol 1996;70:3075–83.
Ventor C, Pereira B, Grundy J, Clayton CB, Arshad SH, Dean T. Prevalence of sensitization reported and objectively assessed food hypersensitivity amongst six-year-old children: a population-based study. Pediatr Allergy Immunol 2006;17:356–63.
Allen KJ, Hill DJ, Heine RG. Food allergy in children. MJA Pract Essent 2006;185:394–400.
Kletter B, Gery I, Freier S, Davies AM. Immune responses of normal infants to cow’s milk. I. Antibody type and kinetics of production. Int Archives Allergy Appl Immunol 1971;40:656–66.
Gunther M, Aschaffenburg R, Matthews RH, Parish WE, Coombs RRA. The level of antibodies to the proteins of cow’s milk in the serum of normal human infeants. Immunology 1960;3:296–306.
Anderson AF, Schloss OM. Allergy to cow’s milk in infants with nutritional disorders. Amer J Dis Children 1923;26:451–74.
Cunningham-Rundles C, Brandeis W, Good RA, Day NK. Milk precipitins, circulating immune complexes and IgA deficiency. Proc Natl Acad Sci (USA) 1978;75:3387–89.
Butler JE, Oskig R. Cancer, autoimmunity and IgA-deficiency related by a common antigen-antibody system. Nature (London) 1974;249:830–3.
Paronen J, Knip M, Savilahti E, Virtanen SM, Ilonen J, Akerblom HS, Vaarala O. Effect of cow’s milk exposure and maternal type 1 diabetes on cellular and humoral immunization to dietary insulin in infants at genetic risk for type 1 diabetes. Diabetes 2000;49:1657–65.
Reeds P, Odle J. Pigs as models for nutrient functional interaction. In: Tumbleson ME, Schook LB, editors. Avd. Swine Biomedical Res. Plenum Press, Vol. 2, 1996. p. 709–12.
Sicherer SH, Munoz-Furlong A, Sampon HA. Prevalence of peanut and tree nut allergy in the United States determined by means of randon digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol 2003;112:1203–7.
Fischer R, McGhee JR, Vu HL, Atkinson TP, Jackson RJ, Tome D, Boyaka PN. Oral and nasal sensitization promote distinct immune responses and lung reactivity in a mouse model of peanut allergy. Am J Path 2005;167:1621–30.
Sampson HA, Mendelson L, Rosen JR. Fatal and near-fatl anaphylactic reactions to food in children and adolescents. N Eng J Med 1992;327:380–4.
Rhee KJ, Sethupathi P, Driks A, Lanning DK, Knight KL. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol 2004;172:1118–24.
Talham GL, Jiang H-Q, Bos NA, Cebra JJ. Segmented filamentous bacteria are potent stimuli of a physiological noram state of the murine gut mucosal immune system. Infect Immun 1990;67:1992–2000.
Adlerberth I, Jahil F, Carlsson B, Mellander L, Hanson LA, Larson P, Khahil K, Wold AE. High orunover rate of Escherichia coli strains in the intestinal flora of infants in Pakistan. Epidemiol Infect 1998;121:587–98.
Walker R, Buckley M. Probiotic microbes: the scientific basis. Am. Academy of Sciences Colloquium Report. Am. Academy of Microbiology. 2006; 22.
Blum S, Alvarez S, Haller D, Perez P, Schiffrin EJ. Intestinal microflora and the interaction with immunocompetent cells. Antonei van Leeuwenhock 1999;76:199–205.
Jaeger MA, Lamar CH, Bottoms GD, Cline TR. Growth-stimulating substances in porcine milk. Amer J Vet Res 1987;48:1531–3.
Jin Y, Cox DA, Kenecht R, Raschdorf S, Cerletti N. Separation, purification and sequence identification of TGF-β1 and TGF-β2 from bovine milk. J Prot Chem 1991;10:565–75.
Miettinen PJ. Transforming growth factor β and epithelial growth factor expression in the human fetal gastrointestinal tract. Pediatr Res 1993;33:481–6.
Wheeler MB, Walters EM. Transgenic technology and applications in swine. Theriogenology 2001;56:1345–69.
Niemann H, Rath D, Wrenzycki C. Advances in biotechnology: new tools in future pig production for agriculture and biomedicine. Reprod Domest Anim 2003;38:82–9.
Sachs DH, Sykes M, Robson SC, Cooper DK. Xenotransplantation. Adv Immunol 2001;79:129–223.
Waltz E. Polyclonal antibodies step out of the shadow. Nature Biotechnology 2006;24:1181–81.
Kuriowa Y, Kasinathan P, Choi YJ, Naem R, Tomizuka K, Sullivan EJ, Knott JG, Duteau A, Goldsby RA, Osborne BA, Ishida I, Robl JM. Cloned transchromosomic calves producing human immunoglobulin Nat. Biotechnol 2002;20:889–94.
Rodgers C, Hao Y, Rokhlina T, Yan Z, Engelhardt J, Prather R, Welsh M. Gene targeting of pig CFTR: progress toward a large animal model of cystic fibrosis. Pediatr Pulmonol 2006;Suppl 29. Abstract 231.
Acknowledgments
This work was supported by Grant Agency of the Czech Republic Grant 524/07/0087 and 523/07/0088 and Grant Agency of Academy of Sciences of the Czech Republic Grant A5020303 and by a grant from the National Porkboard and by the Carver Trust of the University of Iowa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Butler, J.E., Šinkora, M. The isolator piglet: a model for studying the development of adaptive immunity. Immunol Res 39, 33–51 (2007). https://doi.org/10.1007/s12026-007-0062-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-007-0062-7