Abstract
After contact shots to the head biological traces can be found inside firearm barrels. So far silicone coated, gelatin filled box models were used to generate such staining according to the triple contrast method (mixture of acrylic paint, barium sulfate and blood sealed in a thin foil bag). This study was conducted to develop a transparent ballistic model allowing contact shots. Gelatin filled polyethylene bottles with and without a silicone coat were tested in comparison to non-covered gelatin blocks. Finally, thin foil bags of 5 cm × 5 cm dimension were glued on a synthetic absorbent kitchen wipe on top of which 1 L 10% gelatin solution was molded to create blocks of 8.5 cm length. A kitchen wipe with a paint pad on its inside formed the front of the cube. Three contact shots each with a 9 mm Luger pistol and a .38 special revolver were performed on all model variations. The staining was documented by endoscopy and swabs gathered from both ends of the barrel were analyzed by quantitative PCR. Reliable staining was achieved using the front covered gelatin block with comparable results to the silicone coated box model used before. For further research using ballistic models to simulate a human head a symmetric form of the gelatin block such as a cube is recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Suicides using firearms are mostly committed with close contact of the weapon with the head or the body. Therefore the resulting gunshot wounds often have, depending on the ammunition and weapon, an atypical aspect because of the muzzle gas pressure. However, backspatter on the hands of a deceased can only be found in some of these cases. The reason for these heterogeneous findings is not currently known. After contact shots, ejected organic material can also be found on and inside the firearm. Systematic endoscopy of firearm barrels after contact shots revealed organic stains which could be confirmed and individualized by DNA analysis [1,2,3,4]. These findings also vary widely. Systematic experimental studies were initiated to reproduce staining inside the barrel. In a first approach, it was found that silicone coated models could generate the required traces [5]. The experimental procedure was refined by introducing the “triple contrast method” [6] first realized with silicone covered small polyethylene (PET) bottles. The liquid mixture of three compounds in a thin foil bag generated detectable staining: acrylic paint for optical inspection of the barrels using endoscopy [4], human blood for detection of invisible traces using PCR, and radiocontrast agent for the radiographic control of material transport within the target models. In further experimental studies this method was successfully applied to silicone covered plastic boxes [7, 8]. The silicone coat facilitated close contact shots, but the non-transparency of the models did not allow the recording of the processes inside the model using high speed video. The objective of the following study was to develop a transparent ballistic model that generated the staining inside the barrels after contact shots.
Material and methods
According to the triple contrast method [6] 2 ml heparinized human blood, 2 ml acrylic paint (CPM, Erkrath, Germany) and 1 ml barium sulfate-based radiocontrast agent Micropaque® (Guerbet, Brussels, Belgium) were mixed and sealed into thin 5 × 5 cm2 foil bags. Blood samples were taken by venipuncture donated by adult, informed, consenting volunteers. The study design was approved by the ethics committee of the University Hospital Bonn.
Five ballistic models, derived step by step from the preceding model, were investigated.
-
A.
A plastic box (12 cm × 10 cm × 9 cm, polypropylene): the foil bag was attached on top of the lid of the box, covered with a 3-4 mm-thick layer of silicone and stored at 4 °C for 16 h. Finally, the boxes were filled with 1 l of 10% gelatin ‘Ballistic III’ (Gelita, Eberbach, Germany) and stored at 4 °C for another 48 h.
-
B.
A 1.5 L PET-bottle of 8 cm diameter: the foil bag was attached on the outer surface, then the bottle was covered with a 3-4 mm-thick layer of silicone and stored at 4 °C for 16 h. After that the bottles were filled with 1 l of 10% gelatin and stored at 4 °C for another 48 h.
-
C.
A 1.5 L PET-bottle of 8 cm diameter: the foil bag was attached inside, then the bottle was covered with a 3–4 mm-thick layer of silicone and stored at 4 °C for 16 h. After that the bottles were filled with 1 l of 10% gelatin and stored at 4 °C for another 48 h.
-
D.
A 1.5 L PET-bottle of 8 cm diameter: the foil bag was attached inside, then the bottles were filled with 1 l of 10% gelatin and stored at 4 °C for 64 h.
-
E.
A gelatin block: the foil bag was flattened by a gauze and glued on a synthetic absorbent kitchen wipe on top of which 10% gelatin solution was molded to create blocks (12 cm wide, 10 cm high, 8.5 cm long). The kitchen wipe with the paint pad on the inside formed the front of the cube (Fig. 1).
Six close contact shots were performed on the models A – E, three each using a semi-automatic pistol Beretta 92FS, a 9 mm calibre Luger, and a Ruger Security-Six, .357 calibre Magnum revolver, respectively. The following non deforming subsonic ammunition was chosen:
-
9 mm Luger full metal jacket bullet (Fiocchi, Lecco, Italy), bullet weight 10.2 g, average velocity (n = 10) 264 m/s, average kinetic energy 355 J.
-
.38 special lead round nose bullet 10.2 g (Magtech, Lino Lakes, USA), bullet weight 10.2 g, average velocity (n = 10) 233 m/s, average kinetic energy 277 J.
In part, the contact shots could be documented using a SA-X2 high-speed camera with 20.000 fps (Photron Europe Ltd.,WestWycombe, UK).
Endoscopy of the barrels was performed using a 21.5 cm long ‘Technoscope’ (Karl Storz, Tuttlingen, Germany) with a 70° side view resp. a 43 cm-long Hawkeye borescope (Gradient Lens Corporation, Rochester, New York) with a 0°-view optic [4]. Two independent investigators were asked to estimate the intensity of staining in the anterior and posterior part of the barrel using four categories:
-
0 no visible trace
-
1 visible colored trace only
-
2 morphologically classified trace
-
3 intensive or extended staining
Using DNA-free cotton swabs moistened with sterile, desalted water the inner surface of the barrel of the firearm was wiped. Samples were collected from the anterior and the posterior part separately [6, 8]. DNA extraction, quantification and detection of PCR was performed according to a previously published procedure [6]. Contamination between individual shots was avoided by cleaning each gun thoroughly with barrel cleaners of woolen felt, oil and DNAExitusPlus™. All guns were endoscopically controlled. This procedure proved to be sufficient to quantitatively remove any traces of typeable DNA from the barrel [6].
The target models were documented by computed tomography [6, 8]. Silicone and /or plastic covers were then removed and the gelatin cores cut in serial slices of 1 cm thickness perpendicularly to the bullet path. Wound ballistic analysis was performed according to the previously published procedure [9].
Results
-
A.
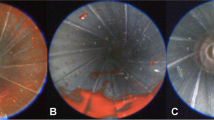
The study started with the previously established target model consisting of a silicone coated, gelatin filled, 12 cm × 10 cm × 9 cm plastic box (model A, Fig. 2). After contact shots with both pistol and revolver, reliable staining inside the barrel was observed (Table 1) as described in previous publications [7, 8].
-
B.
In a first step the container was exchanged for a 1.5 L PET-bottle. The foil bag containing the dye mixture was fixed on the surface of the bottle (Fig. 3) and then covered with silicone in analogous way to model A. Using model B, the staining results yielded in the anterior part of the barrel were comparable to those of model A (Table 1). However, staining in the posterior part was less distinct than with model A, and for the revolver the staining was even invisible and only detected by quantitative PCR.
-
C.
In a second step the foil bag was glued on the inside of a 1.5 L PET-bottle which was subsequently covered with silicone (model C). The modification of placing the foil bag on the inside had already proved successful with model A [7]. However, five of six barrels neither showed any visible traces after contact shots, nor provided typable DNA. The radiological control by computed tomography revealed that the foil bag did not have tight contact to the inner surface of the bottle (Fig. 4). To flatten the foil bag on the curved inside of the bottle a gauze was used to glue and fix it over a greater area. The series with model C was now repeated and showed better optical staining (Table 1), even if the quality did not reach the level of model A or B. Astonishingly, DNA yield was comparable to model B. This was the reason we continued backspatter research based on PET-bottles.
-
D.
For model D we abstained from the sight hampering silicone coating (Fig. 5). The foil bag was placed on the inside of the PET-bottle as performed for model C. The results given in Table 1 demonstrate that it was possible to generate staining comparable to the other bottle models (B and C). Hence, the silicone covering was not necessary to obtain staining results inside the barrels. Because of the transparency of this target model, high speed video could be recorded. However, the rounded surface of the target hampered/obstructed the perpendicular sight on the foil bag to observe the interaction between muzzle gases and medium (Fig. 5).
-
E.
As a result of our experiments we returned to using the ordinary gelatin block. However, the dye foil bag which in previous experiments had been simply embedded in the front of the block [9] had to be covered before contact shots could be performed. The covering material had to form a unit with the gelatin block (Figs. 1 and 6). After a series of trials, synthetic absorbent kitchen wipe (about 1.5 mm thick, 60% viscose, 20% polyester, 20% polypropylene) was found to be simple and reliable for this purpose. The kitchen wipe was glued on the base of a mold. Then, the foil bag was flattened and glued on the kitchen wipe with a gauze. After 24 h of storage at 4 °C the 10% gelatin solution was filled into the mould. In this study the volume of the gelatin block was adapted to the dimension of the other models (12 cm × 10 cm × 8.5 cm depth = approximately 1 L). High speed video documentation showed that muzzle gases entered the block without significant loss. The development of the temporary cavity could be followed without any problem using a high speed camera (Fig. 6). The reliable staining results inside the barrels were comparable to the results obtained with model A, the silicone coated plastic box (Table 1).
The test shots were performed with two different types of handguns which generated similar staining results, demonstrating the reproducibility of the phenomenon independently of the individual firearm. The results of quantitative PCR showed that even in endoscopically poor or negative findings, traces could be detected. Especially in the posterior part of the barrel where staining regularly decreased, a correlation of optical assessment and DNA yield could not be established.
Analysis of the target models
The gelatin slices were analyzed using Fackler’s wound profile (adding the lengths of the two longest cracks). Within each series of three shots to the bottles with the same firearm and ammunition, the profiles of the individual shots were similar, which allowed for them to be averaged. The values measured in the plastic box and in the gelatin block varied more. The results of the different target models showed differences (Fig. 7). The biggest difference was observed between the rectangular models (A and E) and the PET-bottles (B – D). However, the results for the three variations of PET-bottles were not identical either (Fig. 7). The propagation of dye within the bullet track and the cracks was observed for the model types C and D where the paint pad was mounted inside the bottle, while in model B (paint pad on the bottle surface), the cracks were practically not colored by dye. As a consequence of this the contrast in CT imaging was also low. The cracks in the gelatin left by the temporary cavity were longer in the bottle model without a silicone coating (D). By contrast, the gelatin cracks in the unconstrained gelatin block (model E) reached approximately the same length as those in the silicone coated plastic box. Figure 8 shows the cumulated wound profile for each model, averaged over the three shots performed. The values for model C are not shown because the results were practically identical and the curves overlapped fully.
Discussion
Brüning and Wiethold were the first to describe biological material inside suicide weapons [10]. Since 2003, using rigid borescopes, we have systematically inspected gun barrels after suicidal contact shots and have observed biological traces in some cases. With sterile DNA-free swabs samples were taken from both ends of the barrel and analyzed using PCR, which allowed us to identify the victim in most cases [1,2,3, 11]. With the ongoing devlopment of biogenetical analysis it was possible to perform DNA-RNA-coextraction and to detect organ specific RNA from remains inside the barrel. So far, it has been successfully demonstrated for blood and brain residues [12, 13].
Within a research project funded by the Swiss National Scientific Foundation the conditions in which biological material is transferred into the barrel were experimentally investigated. Therefore it was necessary to find a target model generating backspatter and staining inside the barrel. In a first approach acrylic hollow spheres were filled with gelatin. A thin foil bag containing blood was mounted on the surface and covered with silicone [5]. Although the model generated blood traces inside the barrel after a contact shot, it was not possible to perform a morphological analysis by endoscopy. To create endoscopically visible staining blood was mixed with acrylic paint. This method was introduced as the “triple contrast” method, because a third component, barium sulphate, was also added. This radiocontrast agent allowed for non-destructive examination of the target models before and after shooting by computed tomography. The proof of principle was performed using a minimalistic model of a silicone covered 0.5 L polyethylene bottle [6]. Later the volume of the model was extended to a silicone covered plastic box containing about 1 L of gelatin in order to approximate the size of a human brain. This target model was successfully used to generate staining inside barrels of different types of firearms [7, 8]. The present study was initiated to find an improved target model allowing for observation of the processes occurring inside the model during the contact shot.
Step by step, the silicone covered plastic box (A) was modified to a new target model (E). Initially the silicone coating seemed essential for sealing, allowing muzzle gases to be introduced into the model. Therefore the next model, a 1 Liter PET-bottle containing gelatin (B), was designed in analogy to the plastic box (A), the paint pad covered by silicone on the outside of the model. However, this model did not reach the same quality and reliability of staining, especially in the posterior part of the barrel, as the box model. Nevertheless, it was possible to demonstrate that the PET-bottle model worked as well if the paint pad was mounted inside the primarily silicone covered bottle (C). In the next step, a series of six shots documented that the non-silicone covered PET-bottle with a paint pad inside (D) also generated staining inside the barrels. Wound ballistic analysis of the models (B – D) revealed that the silicone coating as well as the polyethylene casing constrained the expansion of the model so that the energy transfer provoked only poor cracking of the gelatin. This could be confirmed by high-speed video footage. Despite the deployed energy, none of the PET-bottles were lacerated. In contrast the plastic boxes (A) used before were broken after the shots. High-speed video documented not only a ballooning of the silicone coat covering the entry and the formation of a powder cavity [7], but also an expansion of the entire model in all directions (Fig. 2). For this reason it was decided to abstain from all constraining elements and to find a way to embed the paint pad in front of the gelatin block. Previous experimental shots on such blocks had been performed from 5 m distance [14], but contact shots would be very messy. After having tested a multitude of cover materials, a simple absorbent kitchen wipe was found to seal reliably the front of the block, forming a unity with the gelatin and covering the paint pad. Using gauze, the thin foil bag was flattened and fixed to the kitchen wipe (Fig. 1). This new target model (E) generated reliable staining inside the gun barrels after contact shots and allowed for high-speed video documentation.
Wound ballistic analysis of the free gelatin block revealed the typical aspect of the cracks left by the temporary cavity. The dye was propagated along the bullet path entering in the gelatin cracks. Astonishingly, the cracks in the box model (A) seemed somewhat longer than in the free block (Fig. 7). This cannot be explained by the dimension of the models, which was identical. Longer cracks indicate a greater energy transfer which could be conceivable because the box, which had a higher resistance, had to be broken. In contact shots to short target models with non-deforming bullets the energy transfer of the bullet is secondary to the influence of the muzzle gas pressure. However, the behavior of the bullet could be different if the plastic of the box model has to be penetrated. In other experiments using Synbone plates this influence has been observed.
The experiences in the present study seemed to indicate an influence of the geometrical form of the target model. Therefore, a gelatin block as a substitute for a head model should be molded as a cube with 11 cm (1.3 kg) or 12 cm (1.7 kg) length to approach a realistic brain size. The greater variation of wound profiles in the rectangular models (A and E) could be caused by the resistance of the underlying material. The target models were simply placed on the table. With respect to the reduced height of these models (10 cm) the bullet path in the middle was relatively close to the bottom. Riva et al. [15] has shown that this distance is uncritical for deflection of the bullet, but nevertheless, the expansion of the temporary cavity could have been constrained. The problem of supporting small ballistic target models should be investigated in future experiments.
Key points
-
1.
All tested models, namely gelatin filled boxes, PET-bottles and gelatin blocks, were prepared according to the triple contrast method and generated staining inside the firearm barrels after contact shots, which decreased from the muzzle to the rear end of the barrel.
-
2.
Silicone coating of the models was not necessary to generate staining.
-
3.
By covering the front of a gelatin block using an absorbent kitchen wipe, muzzle gases in contact shots could penetrate the target model and staining inside the barrel could be reliably generated.
-
4.
The cube form of the transparent gelatin block facilitates observation using high-speed video recording and wound ballistic analysis.
References
Classen U, Makuch D, Wilske J, Schyma C. DNA analysis on material from barrels of firearms. Int Symp Forensic DNA Technol Münster Rechtsmedizin. 2003;13:276.
Regneri W. Diagnostik bei Suizid mit Schusswaffen. Endoskopie von Waffenläufen und DNA-Analyse als komplementäre Methoden. Dissertation: Universität des Saarlandes, Homburg; 2006.
Schyma C, Madea B, Courts C. Persistence of biological traces in gun barrels after fatal contact shots. Forensic Sci Int Genet. 2013;7:22–7.
Schyma C, Brünig J, Madea B, Jackowski C. Die Endoskopie des Waffenlaufes. Rechtsmedizin. 2016;26:224–9.
Courts C, Madea B, Schyma C. Persistence of biological traces in gun barrels – an approach to an experimental model. Int J Legal Med. 2012;126:391–7.
Schyma C, Lux C, Madea B, Courts C. The 'triple contrast' method in experimental wound ballistics and backspatter analysis. Int J Legal Med. 2015;129:1027–33.
Schyma C, Bauer K, Brünig J, Schwendener N, Müller R. Visualization of the powder pocket and its influence on staining in firearm barrels in experimental contact shots. Int J Legal Med. 2017;131(1):167–72.
Schyma C, Bauer K, Brünig J, Courts C, Madea B. Staining in firearm barrels after experimental contact shots. Forensic Sci Int. 2017; doi:10.1016/j.forsciint.2017.01.031.
Schyma C, Madea B. Evaluation of the temporary cavity in ordnance gelatine. Forensic Sci Int. 2012;214(1–3):82–7.
Brüning A, Wiethold F. Die Untersuchung und Beurteilung von Selbstmörderschusswaffen. Dtsch Z Gerichtl Med. 1934;23:71–82.
Courts C, Gahr B, Madea B, Schyma C. Persistence of biological traces at inside parts of a firearm from a case of multiple familial homicide. J Forensic Sci. 2014;59:1129–32.
Lux C, Schyma C, Madea B, Courts C. Identification of gunshots to the head by detection of RNA in backspatter primarily expressed in brain tissue. Forensic Sci Int. 2014;237:62–9.
Grabmüller M, Schyma C, Euteneuer J, Madea B, Courts C. Simultaneous analysis of nuclear and mitochondrial DNA, mRNA and miRNA from backspatter from inside parts of firearms generated by shots at "triple contrast" doped ballistic models. Forensic Sci Med Pathol. 2015;11:365–75.
Schyma C. Colour contrast in ballistic gelatine. Forensic Sci Int. 2010;197(1–3):114–8.
Riva F, Kerkhoff W, Bolck A, Mattijssen EJ. Possible influences on bullet trajectory deflection in ballistic gelatine. Forensic Sci Int. 2016;271:107–12.
Acknowledgements
This research work was funded by the SNF (Swiss National Science Foundation, project 310030E-147628 / 1). The expert technical assistance of Marion Sauer (Bonn) and Nicole Schwendener (Bern) is also gratefully acknowledged. The authors would also like to thank PhD Dr. Eva Brenčičová for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research work was funded by the SNF (Swiss National Science Foundation, project 310030E-147,628 / 1).
Conflict of interests
The authors declare that they have no conflict of interests. Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent: Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Schyma, C., Bauer, K. & Brünig, J. The reference cube: A new ballistic model to generate staining in firearm barrels. Forensic Sci Med Pathol 13, 188–195 (2017). https://doi.org/10.1007/s12024-017-9868-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12024-017-9868-3