Abstract
In the gastro-entero-pancreatic (GEP) tract, neuroendocrine neoplasms (NENs) include well differentiated neuroendocrine tumors (NETs) and high-grade NE carcinomas (NECs), which are thought to make up separate and mutually exclusive tumor entities. Little is known, however, as to whether there may be any pathogenetic link between them. Clustering analysis of a 10-gene panel generated from a previously reported next-generation sequencing analysis on 48 GEP-NENs with clinical annotations was used in the study. Unsupervised cluster analysis showed three histology-independent clusters, namely, C1, C2, and C3, which accounted for 44% of patients but the entire array of mutations. All but two NECs fell into the clusters, yet with different prevalence rates (p < 0.0001). A model was devised according to which NETs were likely to evolve into NECs upon progression of C3 into C1 and C2, despite different morphology. The median Ki-67 labeling index was 5% in C3 showing better prognosis and 50% in C1 and C2 experiencing worse prognosis, with an impressive intra-tumor heterogeneity of diversely proliferating tumor areas. This study suggests that a subset of large cell NECs in the gastroenteropancreatic tract may evolve from pre-existing well-differentiated NETs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the gastro-entero-pancreatic (GEP) tract, neuroendocrine neoplasms (NENs) include well-differentiated NE tumors (WD-NETs) and NE carcinomas (NECs) [1, 2]. These tumors are graded according to a three-tier scheme based on morphology, mitotic count, and Ki-67 labeling index (henceforth, simply Ki-67), whereby NET-G1 and NET-G2 correspond to WD-NETs and NECs to G3 [3, 4]. However, a new category of NET-G3 has recently been devised in the GEP tract inside the category of NECs, by integrating WD morphology, criteria for G3 (> 20 mitoses per 2 mm2 and Ki-67 ≥ 20%), upregulation of neuroendocrine markers [5, 6], somatostatin receptors (STTRs) [7, 8] and retinoblastoma [8] along with p53 downregulation [7, 8], and occurrence of Death Domain Associated Protein (DAXX) and/or ATRX Chromatin Remodeler (ATRX) mutations (at least in the pancreas) along with the lack of the relevant proteins [8,9,10,11,12,13,14,15,16,17]. This additional category is deemed to intermediately behave between NET-G2 and NECs [5, 6, 17,18,19,20]. Although NETs and NECs are actually considered separate and distinct tumor entities [1, 2, 21], we have recently proposed a new pathogenetic hypothesis according to which NECs in resection specimens of lung and thymus NENs of either small or large cells could reflect secondary development from pre-existing carcinoids [22,23,24,25]. Whether such a challenging hypothesis can be supported even in the GEP tract according to the natural history of disease, this is still an unclarified issue.
We herein describe a reappraisal of the mutational profile of a published cohort of 48 GEP-NEN patients [26] aimed to evaluate whether G3 NENs and NETs in the GEP tract may have any developmental relationship. Our findings preliminarily support the hypothesis that a subset of large cell NECs in the gastroenteropancreatic tract may evolve from pre-existing well-differentiated NETs after crucial gene alterations have been acquired for tumor progression.
Materials and Methods
Patients and Tumors
This study deals with a clinically well-annotated cohort of 48 GEP-NENs (25 males and 23 females) from 13 pancreatic and 35 extra-pancreatic digestive sites, which had previously been investigated by means of targeted next-generation sequencing (NGS) analysis [26]. Out of 63 originally reported NE malignancies, 15 tumors developing outside the GEP tract (breast, lung, and head and neck) or presenting as unknown tumor primaries were excluded from the analysis. Anatomical sites other than the pancreas comprised the esophagus (one case), stomach (two cases), small intestine (18 cases), colon-rectum (12 cases), and gallbladder (two cases). Ethnicity was distributed according to 42 Caucasian, five African American, and one Asian patient, whereas smoking habit was present in 22/48 (46%) patients (this information was missing in one patient). Alcohol consumption was documented in 46/48 (96%) patients, with 20 (42%) of whom being active consumers. The patient cohort comprised 12 (25%) G1, 20 (42%) G2, and 16 (33%) G3 tumors, which were classified according to World Health Organization (WHO) classifications [1, 2] and ENETS guidelines (at https://www.enets.org/enets_guidelines.html). In particular, no typical case of NET-G3 could be documented upon morphology in the subgroup of NEC patients, who belonged to the histologic subtype with large cell according to 2017 WHO and 2019 WHO classifications (large cell neuroendocrine carcinoma, LCNEC) [1, 2]. No cases of small cell NECs were present. Twenty-nine out of 48 (60%) tumors had been surgically removed (11 NETs G1, 11 NETs G2, and seven NECs), with no neoadjuvant treatment being administered. Thirty-four (71%) patients were staged IV, with the remaining 14 (29%) being staged I–III according to the current TNM staging system, 8th edition [1, 2]. Eastern Cooperative Oncology Group (ECOG) performance status ranked score 0 in 28 (58%) patients, score 1 in 18 (38%) patients, and score 2 in the remaining two (4%). Paraffin material had been selected for immunohistochemistry (IHC) assessment of Ki-67 and molecular investigations.
Study Design
This is a cancer mutational profile analysis to explore an innovative concept of secondary NECs in the GEP tract evolving from pre-existing WD-NETs through sequential gene mutations, as previously hypothesized in the lung [23, 25, 27] and the thymus [22, 24]. To this purpose, our previous NGS study on GEP-NENs, either primary or metastasis, conducted at Fox Chase Cancer Centre (Philadelphia, USA), was reappraised by clustering analysis [26]. All NENs were sporadic, affected adult patients (≥ 18 years), and included NET-G1, NET-G2, and NEC. All the ten recurrently altered genes from a panel of 50 oncogenes and tumor suppressor genes frequently involved in human cancers by targeted NGS were used as an investigative signature in the study [26]. This 10-gene panel included APC (APC regulator of WNT signaling pathway), ATM (ATM serine/threonine kinase), BRAF (B-Raf proto-oncogene, serine/threonine kinase), CTNNB1 (catenin beta 1), IDH1 (isocitrate dehydrogenase [NADP(+)] 1), KRAS (KRAS proto-oncogene, GTPase), PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha), PTEN (phosphatase and tensin homolog), RB1 (RB transcriptional corepressor 1), and TP53 (tumor protein p53), which underwent clustering analysis.
Statistical Analysis
Hierarchical clustering analysis was performed as elsewhere detailed [23, 28]. Briefly, we used Spearman rank correlation as similarity metric and Centroid linkage as clustering method in Cluster 3.0 and Java TreeView software environment (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm). The three main branches (N = 3) of hierarchical clustering were selected to construct clusters. Bar and radar plots were prepared using Excel 2020 (Microsoft Office). Continuous and categorical variables were compared by Kruskal-Wallis test and Fisher’s exact test, respectively, using the SAS software, version 9.4 (SAS Institute, Inc., Cary, NC). Kaplan-Meier plots and log-rank test for overall survival were performed using JMP 12 (SAS). Cox univariate and multivariable analyses were performed using the SAS software, version 9.4 (SAS Institute, Inc., Cary, NC). All p values were two-sided, and p < 0.05 were considered as significant.
Results
Hierarchical Clustering Analysis Reveals Distinct Groups of Tumors
Supervised cluster analysis of the 10 gene panel in 21 out of 48 (44%) tumor samples with mutations ordered according to tumor grade revealed that mutations in TP53/KRAS/RB1/PTEN genes co-occurred more frequently in G2 and G3 tumors (Fig. 1a). Overall, NET-G1 had the lowest mutation burden (one out of 12 cases, 8.3%), NET-G2 an intermediate value (six out of 20 cases, 30%), and NECs the highest one (15 out of 16 cases, 93.7%) (Supplemental Table 1). All NECs featured large cell neuroendocrine carcinoma. Expectedly, grade-related survival curves showed the best and the worst prognosis for NET-G1 and NECs, respectively, while G2 run an intermediate clinical course (Fig. 1c). There were no differences in survival between resected and unresected NECs. The whole set of molecular and clinicopathologic data of our cohort of 48 patients is presented in Supplemental Table 1.
a–d Unsupervised and supervised cluster analyses. a One-way hierarchical cluster analysis of the 10-gene mutations profile in 21 NEN (with mutations). Samples were ordered based on tumor grade. Colors are as per the legend. b Unsupervised two-way hierarchical cluster analysis of the 10-gene mutations profile in 21 NEN. Data on Ki-67, gender, and smoking are presented as well. Colors are as per the legend. In light gray four NECs (three in C1 and one in C2) are reported, where RB1 assessment was not available. c Kaplan-Meier survival plots of the entire cohort of NAN (48 samples) stratified based on cluster identity (on the left) or on tumor grading (on the right). P values were calculated by the log-rank test. d Radar plot of the prevalence of genetic alterations in the clusters identified: numbers identify the percentages of the relevant gene mutations
Unsupervised cluster analysis by means of the same 10 gene panel pushed three distinct clusters to emerge, namely, C1, C2, and C3 (Fig. 1b). The remaining unclustered tumors (i.e., with no panel-related mutations) were descriptively labeled as “others” (Table 1). The clusters C1, C2, and C3 comprised eight, nine, and four patients, respectively, and showed different distribution of mutations: the cluster C1 included mutations in TP53 (six cases), PIK3CA (three cases), and BRAF (two cases); the cluster C2 mutations in KRAS (nine cases), TP53 (seven cases), RB1 (three cases), and APC/CTNNB1/PTEN (one case for each); and the cluster C3 mutations in CTNNB1 (three cases) and ATM/IDH1 (one case for each) (Fig. 1b and d).
To further investigate the relationship existing among C1, C2, and C3, we considered the prevalence of mutations in each cluster (Fig. 2). While no cluster shared all the same mutations, TP53 were observed in C1 and C2; CTNNB1 in C2 and C3; and APC, ATM, BRAF, IDH1, KRAS, PIK3CA, PTEN, and RB1 across clusters with different prevalence rates among them (Figs. 2 and 3). Interestingly, the median Ki-67 value ranked 5% (range 1–70%), 50% (range 3.2–95%), and 50% (range 3.2–95%) in clusters C3, C2, and C1, respectively (Fig. 3; Supplemental Table 1).
Distribution of genetic alterations according to clusters (C1 → C3). Bar plots indicate frequency (Y-axis) of alterations found in each cluster (shown on X-axis) highlighted by different colors. Simple asterisks indicate significant p values; double asterisks indicate marginal p values (calculated by Fisher test)
Relationship between clusters and tumor composition (C1 → C3). Pie charts indicate the distribution of different tumor types in the clusters, which are positioned according to the clustering tree shown on the left of picture with the corresponding gene alterations (the numbers in brackets correspond to mutation burden). APC (APC regulator of WNT signaling pathway), ATM (ATM serine/threonine kinase), BRAF (B-Raf proto-oncogene, serine/threonine kinase), CTNNB1 (catenin beta 1), IDH1 (isocitrate dehydrogenase [NADP(+)] 1), KRAS (KRAS proto-oncogene, GTPase), PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha), PTEN (phosphatase and tensin homolog), RB1 (RB transcriptional corepressor 1), and TP53 (tumor protein p53)
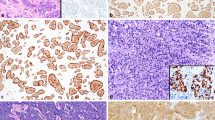
All but one C2-associated NEC had Ki-67 ranging from 50 to 95% and none of them showed well-differentiated morphology (Supplemental Table 1). In all NEC samples, which belonged to the LCNEC subtype, Ki-67 was heterogeneously distributed inside tumors, showing diversely proliferating tumor areas intermingled with each other (Fig. 4).
a–h Intra-tumor heterogeneity of diversely graded neoplastic components in two different instances of neuroendocrine carcinomas. Trabecular to solid aggregates of cancer cells with poor differentiation featuring LCNEC a show high levels of Ki-67 activity b, whereas concurrent well differentiated tumor areas in the same lesion c exhibit correspondingly lower labeling of Ki-67 consistent with NET G2 d. In another case of LCNEC, a high-grade component e with an elevated value of Ki-67 f contrasts with a well differentiated tumor g showing lower activity of Ki-67 consistent with NET G2 h. LCNEC stands for large cell neuroendocrine carcinoma; NET stands for neuroendocrine tumor. All pictures were taken at 200X
Univariate and Multivariable Survival Analyses
Survival curves showed that C3 had the best prognosis, whereas C1 and C2 exhibited the lowest probability of survival with no significant difference between them, in keeping with their tumor composition (p = 0.69) (Fig. 1c). As a matter of fact, each cluster remarkably exhibited an unexpected admixture of NETs and NECs, with all but two NECs being distributed among cluster C1 (six out of eight cases, with two NET-G2), cluster C2 (seven out of nine cases, with two NET-G2), and cluster C3 (one out of four cases, with one NET-G1 and two NET-G2) (p < 0.0001) (Table 1; Fig. 3). Unmutated tumors according to this 10-gene panel (i.e., the group “others”) totaled 27 cases, mostly NETs (25/27 cases, 93%), particularly NET-G2 (14 cases), with only two NECs being on record (Fig. 3). No differences were documented across population as far as age at diagnosis, sex, ethnicity, smoking status, alcohol consumption, tumor stage, and performance status were concerned, although a preferential localization of cluster C2 and the “others” group was seen in the colon-rectum and small intestine, respectively (p = 0.0018; Table 1).
Cox univariate analysis showed that tumor site (stomach vs. small intestine), grade, stage IV, and clusters C1 and C2 impaired survival, while cluster C3 did not present any event (Table 2). Multivariable analysis confirmed that only clusters affected survival independently (Table 2).
Discussion
We herein challenge the current pathogenesis of GEP-NENs by favoring the hypothesis that a subset of large cell NECs in the gastroenteropancreatic tract is likely to evolve from pre-existing well-differentiated NETs. In this context, an intra-tumor heterogeneous distribution of Ki-67 as depicted in Fig. 4 could reflect the existence of diversely proliferating cell clones in these secondary NECs as previously indicated in the lung and the thymus [22, 23, 25]. Our study showed that 14 out of 16 (87%) NECs tightly joined NETs (NET-G2 but also NET-G1) to realize three distinct and separate clusters, namely, C1, C2, and C3 (Fig. 1; Table 1), which accounted for 44% of patients according to the relevant 10-gene signature. These clusters revealed genetic lesions which were shared by NETs and NECs (Fig. 3), thus supporting a compelling hypothesis on an evolution of NETs to some NECs. Remarkably, the group of “other” tumors devoid of any mutation with our gene panel mostly included NET-G1 (11 cases) and NET-G2 (14 cases) and predominantly grew in small intestine (15 cases) over the pancreas and colon (Table 1; Fig. 3; Supplemental Table 1). Of note, small intestine is known to harbor a minor fraction of mutational driver events (putative drivers in CDKN1 and APC would be documentable in only 10% of instances or less) [29, 30].
We would like to speculate that according to our hypothesis, NETs clustered in C3, which proliferated by 5% Ki-67, would have a potential to transform into NECs (Table 1). As a matter of fact, cluster C3 harbored CTNNB1 mutation as dominant alteration, which has been linked to epithelial-mesenchymal transition upon beta-catenin nuclearization and tumor progression in GEP tract [31] NENs. Of the other two genes in the cluster, ATM (a master controller of cell cycle checkpoint signaling in response to DNA damage and genome stability) is an independent risk factors for recurrence in pancreatic NENs [32], and IDH1 (involved in metabolic cytoplasmic NADPH production) has been associated with higher histologic grade, lymphovascular invasion, and recurrence rate in rectal and gastric GEP-NENs [33]. Of note, ATM and IDH1 mutations, which were found in two long surviving metastatic NETs of the pancreas (NET G1 and NET G2, respectively; Supplemental Table 1), could be associated with the occurrence and development of these tumors as documented in low-grade glioma and secondary glioblastoma [34, 35]. Cluster C2, beyond CTNNB1 mutation, presented with additional master genes repeatedly altered in high-grade NENs at different anatomical sites (including the GEP tract), such as APC, KRAS, PTEN, RB1, and TP53. In particular, cluster C2 was repository of all KRAS mutations [36, 37], which are common to NECs of the lung [23, 27, 38,39,40] and the uterine cervix [41]. Even cluster C1 shared with cluster C2 TP53 mutations in most instances (75%), along with PIK3CA (37%) and BRAF (25%) mutations, both of which are frequently documented in gastrointestinal NECs as driver mechanisms [42,43,44]. Of note, repetitive pathogenic/likely pathogenic TP53 mutations were found in more aggressive rectal NETs, supporting the hypothesis of a risk evolution [33].
The relevance of clustering to clinics (and hence of gene alterations) even more emerged from the analysis of survival curves, with the best prognosis in C3 and the worst in C1 and C2 (the median Ki-67 ranked 5% in C3 and 50% in C1 and C2) (Fig. 1). These survival trends of clustered NECs were likely to mirror the close relationship among cell differentiation, proliferative activity, and biological aggressiveness in NENs of the GEP tract [16, 17, 45,46,47,48]. Noteworthy, cluster C1 (and marginally C2) was also an independent factor of survival on multivariable analysis, thus suggesting a role for gene alteration burden in dictating tumor morphology and clinical behavior.
The heterogeneity in GEP NEC distribution across clusters could reflect major differences in their natural history [15, 23, 25], which in turn might reflect endogenous and/or exogenous risk factors as a function of the primary anatomical sites (e.g., prior/familial history of non-NE cancer or smoking in the small intestine [49] or urinary bladder [50], smoking in the colon and uterine cervix [51, 52], and non-recent onset diabetes [53] in the pancreas). Indeed, in our hand, KRAS-mutated C2 cases developed in the colon and the pancreas. Therefore, endogenous and/or exogenous risk factors might give rise to different genetic/epigenetic alterations at the onset of tumor development according to the different anatomical site (so important for the clinical behavior of GEP NENs), whereby imprinting preclinical phase, clinical outcome, and morphologic appearance [23, 25, 54].
In our series, we did not encounter morphologic NET-G3 and the range of Ki67 in NECs clustering C1 (50–95%) and C2 (30–95%), the survival curves and the prevalence of TP53 mutations (TP53 is inactivated in most NECs anywhere [1, 2]) were all in keeping with the diagnosis of NECs. However, less proliferating tumor areas as depicted in Fig. 4 could meet some morphological traits of NET-G3, whose diagnostic recognition may have been blurred at the level of an individual patient’s cancer by using morphology or immunohistochemistry as supervised defining criteria [8, 55,56,57]. Moreover, one case clustering C2, mutated in TP53 and featuring NEC, fell into the same proliferation category as NET-G3 (mitoses > 20; 20% < Ki-67 < 50%), but was joined two other NETs bearing TP53 or RB1 mutation (RB1 is inactivated in most NECs anywhere) [1, 2] (Supplemental Table 1). In our study, unsupervised clustering analysis (Figs. 1 and 3) revealed a clear admixture of NETs and NECs, which in turn showed admixture of well differentiated NETs with high-grade components (Fig. 4), thus letting us hypothesize an evolution of some NECs with large cell morphology from pre-existing NETs (Fig. 3). This admixture of diversely escalating tumor components in cell proliferation was paralleled by a heterogeneous intra-tumor distribution of Ki-67 (Fig. 4), which could help to recognize these secondarily evolving NECs, as previously observed [6, 22, 23].
Despite limitations of our study principally due to its retrospective character, limited number of tumors, and the small number of genes under evaluation, which precluded an independent validation, a strength point was however represented by unsupervised clustering analysis centered on molecular alterations rather than the more traditional supervised analysis based on tumor categorization that may be challenging especially at the level of an individual patient’s cancer.
Conclusions
We herein introduce the concept of secondary large cell NEC in the GEP-NENs which is likely to incorporate an underrecognized perspective mirroring risk factors and the natural history of disease. This is in keeping with other models of NE neoplasms arising elsewhere, in which gene alterations are the best modelers of tumor fate.
Data Availability
All data and materials being used and presented in this study are available as Supplemental Table 1.
Abbreviations
- APC :
-
APC regulator of WNT signaling pathway
- ATM :
-
ATM serine/threonine kinase
- ATRX :
-
ATRX Chromatin Remodeler
- BRAF :
-
B-Raf proto-oncogene, serine/threonine kinase
- CTNNB1 :
-
Catenin beta 1
- DAXX:
-
Death Domain Associated Protein
- IDH1 :
-
Isocitrate dehydrogenase [NADP(+)] 1
- KRAS :
-
KRAS proto-oncogene, GTPase
- PIK3CA :
-
Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha
- PTEN :
-
Phosphatase and tensin homolog
- RB1 :
-
RB transcriptional corepressor 1
- TP53 :
-
Tumor protein p53
- DNA:
-
Deoxyribonucleic acid
- GEP:
-
Gastroenteropancreatic
- IHC:
-
Immunohistochemistry
- LCNEC:
-
Large cell neuroendocrine carcinoma
- NE:
-
Neuroendocrine
- NEC:
-
Neuroendocrine carcinoma
- NEN:
-
Neuroendocrine neoplasm
- NET:
-
Neuroendocrine tumor
- NGS:
-
Next-generation sequencing
- TNM:
-
Tumor node metastasis
- WD:
-
Well differentiated
- WHO:
-
World Health Organization
References
Board WCoTE (2019) Digestive system tumours, vol 1. WHO Classification of Tumours, 5th edn. IARC
Lloyd R, Osamura R, Klöppel G, Rosai J (2017) WHO Classification of Tumours of Endocrine Organs. World Heatlth Organization Classification of Tumours. IARC, Lyon
Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, Fernandez-Cuesta L, Kloppel G, McCluggage WG, Moch H, Ohgaki H, Rakha EA, Reed NS, Rous BA, Sasano H, Scarpa A, Scoazec JY, Travis WD, Tallini G, Trouillas J, van Krieken JH, Cree IA (2018) A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 31 (12):1770-1786. https://doi.org/10.1038/s41379-018-0110-y
Rindi G, Kloppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, Korner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B (2006) TNM staging of foregut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch 449 (4):395-401. https://doi.org/10.1007/s00428-006-0250-1
Tang LH, Basturk O, Sue JJ, Klimstra DS (2016) A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am J Surg Pathol 40 (9):1192-1202. https://doi.org/10.1097/PAS.0000000000000662
Tang LH, Untch BR, Reidy DL, O'Reilly E, Dhall D, Jih L, Basturk O, Allen PJ, Klimstra DS (2016) Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin Cancer Res 22 (4):1011-1017. https://doi.org/10.1158/1078-0432.CCR-15-0548
Bellizzi AM (2020) Immunohistochemistry in the diagnosis and classification of neuroendocrine neoplasms: what can brown do for you? Hum Pathol 96:8-33. https://doi.org/10.1016/j.humpath.2019.12.002
Konukiewitz B, Schlitter AM, Jesinghaus M, Pfister D, Steiger K, Segler A, Agaimy A, Sipos B, Zamboni G, Weichert W, Esposito I, Pfarr N, Kloppel G (2017) Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20. Mod Pathol 30 (4):587-598. https://doi.org/10.1038/modpathol.2016.217
Nielsen K, Binderup T, Langer SW, Kjaer A, Knigge P, Grondahl V, Melchior L, Federspiel B, Knigge U (2020) P53, Somatostatin receptor 2a and Chromogranin A immunostaining as prognostic markers in high grade gastroenteropancreatic neuroendocrine neoplasms. BMC Cancer 20 (1):27. https://doi.org/10.1186/s12885-019-6498-z
Sorbye H, Baudin E, Borbath I, Caplin M, Chen J, Cwikla JB, Frilling A, Grossman A, Kaltsas G, Scarpa A, Welin S, Garcia-Carbonero R, Participants EMAB (2019) Unmet Needs in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms (WHO G3). Neuroendocrinology 108 (1):54-62. https://doi.org/10.1159/000493318
11. Rindi G, Klersy C, Albarello L, Baudin E, Bianchi A, Buchler MW, Caplin M, Couvelard A, Cros J, de Herder WW, Delle Fave G, Doglioni C, Federspiel B, Fischer L, Fusai G, Gavazzi F, Hansen CP, Inzani F, Jann H, Komminoth P, Knigge UP, Landoni L, La Rosa S, Lawlor RT, Luong TV, Marinoni I, Panzuto F, Pape UF, Partelli S, Perren A, Rinzivillo M, Rubini C, Ruszniewski P, Scarpa A, Schmitt A, Schinzari G, Scoazec JY, Sessa F, Solcia E, Spaggiari P, Toumpanakis C, Vanoli A, Wiedenmann B, Zamboni G, Zandee WT, Zerbi A, Falconi M (2018) Competitive Testing of the WHO 2010 versus the WHO 2017 Grading of Pancreatic Neuroendocrine Neoplasms: Data from a Large International Cohort Study. Neuroendocrinology 107 (4):375-386. https://doi.org/10.1159/000494355
Pellat A, Coriat R (2020) Well Differentiated Grade 3 Neuroendocrine Tumors of the Digestive Tract: A Narrative Review. J Clin Med 9 (6). https://doi.org/10.3390/jcm9061677
Mafficini A, Scarpa A (2019) Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr Rev 40 (2):506-536. https://doi.org/10.1210/er.2018-00160
Hackeng WM, Brosens LA, Poruk KE, Noe M, Hosoda W, Poling JS, Rizzo A, Campbell-Thompson M, Atkinson MA, Konukiewitz B, Kloppel G, Heaphy CM, Meeker AK, Wood LD (2016) Aberrant Menin expression is an early event in pancreatic neuroendocrine tumorigenesis. Hum Pathol 56:93-100. https://doi.org/10.1016/j.humpath.2016.06.006
Uccella S, La Rosa S, Volante M, Papotti M (2018) Immunohistochemical Biomarkers of Gastrointestinal, Pancreatic, Pulmonary, and Thymic Neuroendocrine Neoplasms. Endocr Pathol 29 (2):150-168. https://doi.org/10.1007/s12022-018-9522-y
Basturk O, Tang L, Hruban RH, Adsay V, Yang Z, Krasinskas AM, Vakiani E, La Rosa S, Jang KT, Frankel WL, Liu X, Zhang L, Giordano TJ, Bellizzi AM, Chen JH, Shi C, Allen P, Reidy DL, Wolfgang CL, Saka B, Rezaee N, Deshpande V, Klimstra DS (2014) Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol 38 (4):437-447. https://doi.org/10.1097/PAS.0000000000000169
Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, Krasinskas AM, Jang KT, Frankel WL, Balci S, Sigel C, Klimstra DS (2015) The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol 39 (5):683-690. https://doi.org/10.1097/PAS.0000000000000408
Nunez-Valdovinos B, Carmona-Bayonas A, Jimenez-Fonseca P, Capdevila J, Castano-Pascual A, Benavent M, Pi Barrio JJ, Teule A, Alonso V, Custodio A, Marazuela M, Segura A, Beguiristain A, Llanos M, Martinez Del Prado MP, Diaz-Perez JA, Castellano D, Sevilla I, Lopez C, Alonso T, Garcia-Carbonero R (2018) Neuroendocrine Tumor Heterogeneity Adds Uncertainty to the World Health Organization 2010 Classification: Real-World Data from the Spanish Tumor Registry (R-GETNE). Oncologist 23 (4):422-432. https://doi.org/10.1634/theoncologist.2017-0364
Crippa S, Partelli S, Bassi C, Berardi R, Capelli P, Scarpa A, Zamboni G, Falconi M (2016) Long-term outcomes and prognostic factors in neuroendocrine carcinomas of the pancreas: Morphology matters. Surgery 159 (3):862-871. https://doi.org/10.1016/j.surg.2015.09.012
Crippa S, Partelli S, Belfiori G, Palucci M, Muffatti F, Adamenko O, Cardinali L, Doglioni C, Zamboni G, Falconi M (2016) Management of neuroendocrine carcinomas of the pancreas (WHO G3): A tailored approach between proliferation and morphology. World J Gastroenterol 22 (45):9944-9953. https://doi.org/10.3748/wjg.v22.i45.9944
Travis W, Brambilla E, Burke A, Marx A, Nicholson A (2015) WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, vol 7. World Health Organization Classification of Tumours, Fourth Ed. edn. IARC Press, Lyon
Fabbri A, Cossa M, Sonzogni A, Bidoli P, Canova S, Cortinovis D, Abbate MI, Calabrese F, Nannini N, Lunardi F, Rossi G, La Rosa S, Capella C, Tamborini E, Perrone F, Busico A, Capone I, Valeri B, Pastorino U, Albini A, Pelosi G (2017) Thymus neuroendocrine tumors with CTNNB1 gene mutations, disarrayed ss-catenin expression, and dual intra-tumor Ki-67 labeling index compartmentalization challenge the concept of secondary high-grade neuroendocrine tumor: a paradigm shift. Virchows Arch 471 (1):31-47. https://doi.org/10.1007/s00428-017-2130-2
Pelosi G, Bianchi F, Dama E, Simbolo M, Mafficini A, Sonzogni A, Pilotto S, Harari S, Papotti M, Volante M, Fontanini G, Mastracci L, Albini A, Bria E, Calabrese F, Scarpa A (2018) Most high-grade neuroendocrine tumours of the lung are likely to secondarily develop from pre-existing carcinoids: innovative findings skipping the current pathogenesis paradigm. Virchows Arch 472 (4):567-577. https://doi.org/10.1007/s00428-018-2307-3
Dinter H, Bohnenberger H, Beck J, Bornemann-Kolatzki K, Schutz E, Kuffer S, Klein L, Franks TJ, Roden A, Emmert A, Hinterthaner M, Marino M, Brcic L, Popper H, Weis CA, Pelosi G, Marx A, Strobel P (2019) Molecular Classification of Neuroendocrine Tumors of the Thymus. J Thorac Oncol 14 (8):1472-1483. https://doi.org/10.1016/j.jtho.2019.04.015
Pelosi G, Bianchi F, Hofman P, Pattini L, Strobel P, Calabrese F, Naheed S, Holden C, Cave J, Bohnenberger H, Dinter H, Harari S, Albini A, Sonzogni A, Papotti M, Volante M, Ottensmeier CH (2019) Recent advances in the molecular landscape of lung neuroendocrine tumors. Expert Rev Mol Diagn 19 (4):281-297. https://doi.org/10.1080/14737159.2019.1595593
Vijayvergia N, Boland PM, Handorf E, Gustafson KS, Gong Y, Cooper HS, Sheriff F, Astsaturov I, Cohen SJ, Engstrom PF (2016) Molecular profiling of neuroendocrine malignancies to identify prognostic and therapeutic markers: a Fox Chase Cancer Center Pilot Study. Br J Cancer 115 (5):564-570. https://doi.org/10.1038/bjc.2016.229
Alcala N, Leblay N, Gabriel AAG, Mangiante L, Hervas D, Giffon T, Sertier AS, Ferrari A, Derks J, Ghantous A, Delhomme TM, Chabrier A, Cuenin C, Abedi-Ardekani B, Boland A, Olaso R, Meyer V, Altmuller J, Le Calvez-Kelm F, Durand G, Voegele C, Boyault S, Moonen L, Lemaitre N, Lorimier P, Toffart AC, Soltermann A, Clement JH, Saenger J, Field JK, Brevet M, Blanc-Fournier C, Galateau-Salle F, Le Stang N, Russell PA, Wright G, Sozzi G, Pastorino U, Lacomme S, Vignaud JM, Hofman V, Hofman P, Brustugun OT, Lund-Iversen M, Thomas de Montpreville V, Muscarella LA, Graziano P, Popper H, Stojsic J, Deleuze JF, Herceg Z, Viari A, Nuernberg P, Pelosi G, Dingemans AMC, Milione M, Roz L, Brcic L, Volante M, Papotti MG, Caux C, Sandoval J, Hernandez-Vargas H, Brambilla E, Speel EJM, Girard N, Lantuejoul S, McKay JD, Foll M, Fernandez-Cuesta L (2019) Integrative and comparative genomic analyses identify clinically relevant pulmonary carcinoid groups and unveil the supra-carcinoids. Nat Commun 10 (1):3407. https://doi.org/10.1038/s41467-019-11276-9
Pelosi G, Rossi G, Bianchi F, Maisonneuve P, Galetta D, Sonzogni A, Veronesi G, Spaggiari L, Papotti M, Barbareschi M, Graziano P, Decensi A, Cavazza A, Viale G (2011) Immunhistochemistry by Means of Widely Agreed-Upon Markers (Cytokeratins 5/6 and 7, p63, Thyroid Transcription Factor-1, and Vimentin) on Small Biopsies of Non-small Cell Lung Cancer Effectively Parallels the Corresponding Profiling and Eventual Diagnoses on Surgical Specimens. J Thorac Oncol 6:1039-1049. https://doi.org/10.1097/JTO.0b013e318211dd16
Scarpa A (2019) The landscape of molecular alterations in pancreatic and small intestinal neuroendocrine tumours. Ann Endocrinol (Paris) 80 (3):153-158. https://doi.org/10.1016/j.ando.2019.04.010
Simbolo M, Vicentini C, Mafficini A, Fassan M, Pedron S, Corbo V, Mastracci L, Rusev B, Pedrazzani C, Landoni L, Grillo F, Cingarlini S, Rindi G, Luchini C, Scarpa A, Lawlor RT (2018) Mutational and copy number asset of primary sporadic neuroendocrine tumors of the small intestine. Virchows Arch 473 (6):709-717. https://doi.org/10.1007/s00428-018-2450-x
Galvan JA, Astudillo A, Vallina A, Fonseca PJ, Gomez-Izquierdo L, Garcia-Carbonero R, Gonzalez MV (2013) Epithelial-mesenchymal transition markers in the differential diagnosis of gastroenteropancreatic neuroendocrine tumors. Am J Clin Pathol 140 (1):61-72. https://doi.org/10.1309/AJCPIV40ISTBXRAX
Hua J, Shi S, Xu J, Wei M, Zhang Y, Liu J, Zhang B, Yu X (2020) Expression Patterns and Prognostic Value of DNA Damage Repair Proteins in Resected Pancreatic Neuroendocrine Neoplasms. Ann Surg. https://doi.org/10.1097/SLA.0000000000003884
Park HY, Kwon MJ, Kang HS, Kim YJ, Kim NY, Kim MJ, Min KW, Choi KC, Nam ES, Cho SJ, Park HR, Min SK, Seo J, Choe JY, Lee HK (2019) Targeted next-generation sequencing of well-differentiated rectal, gastric, and appendiceal neuroendocrine tumors to identify potential targets. Hum Pathol 87:83-94. https://doi.org/10.1016/j.humpath.2019.02.007
Huang J, Yu J, Tu L, Huang N, Li H, Luo Y (2019) Isocitrate Dehydrogenase Mutations in Glioma: From Basic Discovery to Therapeutics Development. Front Oncol 9:506. https://doi.org/10.3389/fonc.2019.00506
Estiar MA, Mehdipour P (2018) ATM in breast and brain tumors: a comprehensive review. Cancer Biol Med 15 (3):210-227. https://doi.org/10.20892/j.issn.2095-3941.2018.0022
Girardi DM, Silva ACB, Rego JFM, Coudry RA, Riechelmann RP (2017) Unraveling molecular pathways of poorly differentiated neuroendocrine carcinomas of the gastroenteropancreatic system: A systematic review. Cancer Treat Rev 56:28-35. https://doi.org/10.1016/j.ctrv.2017.04.002
Sahnane N, Furlan D, Monti M, Romualdi C, Vanoli A, Vicari E, Solcia E, Capella C, Sessa F, La Rosa S (2015) Microsatellite unstable gastrointestinal neuroendocrine carcinomas: a new clinicopathologic entity. Endocr Relat Cancer 22 (1):35-45. https://doi.org/10.1530/ERC-14-0410
Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, Halpenny DF, Wang H, Tian SK, Litvak AM, Paik PK, Drilon A, Socci N, Poirier JT, Shen R, Berger MF, Moreira AL, Travis WD, Rudin CM, Ladanyi M (2016) Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-like and Non-Small Cell Carcinoma-like Subsets. Clin Cancer Res 22 (14):3618-3629. https://doi.org/10.1158/1078-0432.CCR-15-2946
Simbolo M, Barbi S, Fassan M, Mafficini A, Ali G, Vicentini C, Sperandio N, Corbo V, Rusev B, Mastracci L, Grillo F, Pilotto S, Pelosi G, Pelliccioni S, Lawlor RT, Tortora G, Fontanini G, Volante M, Scarpa A, Bria E (2019) Gene Expression Profiling of Lung Atypical Carcinoids and Large Cell Neuroendocrine Carcinomas Identifies Three Transcriptomic Subtypes with Specific Genomic Alterations. J Thorac Oncol. https://doi.org/10.1016/j.jtho.2019.05.003
Simbolo M, Mafficini A, Sikora KO, Fassan M, Barbi S, Corbo V, Mastracci L, Rusev B, Grillo F, Vicentini C, Ferrara R, Pilotto S, Davini F, Pelosi G, Lawlor RT, Chilosi M, Tortora G, Bria E, Fontanini G, Volante M, Scarpa A (2017) Lung neuroendocrine tumours: deep sequencing of the four World Health Organization histotypes reveals chromatin-remodelling genes as major players and a prognostic role for TERT, RB1, MEN1 and KMT2D. J Pathol 241 (4):488-500. https://doi.org/10.1002/path.4853
Xing D, Zheng G, Schoolmeester JK, Li Z, Pallavajjala A, Haley L, Conner MG, Vang R, Hung CF, Wu TC, Ronnett BM (2018) Next-generation Sequencing Reveals Recurrent Somatic Mutations in Small Cell Neuroendocrine Carcinoma of the Uterine Cervix. Am J Surg Pathol 42 (6):750-760. https://doi.org/10.1097/PAS.0000000000001042
McNamara MG, Scoazec JY, Walter T (2020) Extrapulmonary poorly differentiated NECs, including molecular and immune aspects. Endocr Relat Cancer 27 (7):R219-R238. https://doi.org/10.1530/ERC-19-0483
Idrees K, Padmanabhan C, Liu E, Guo Y, Gonzalez RS, Berlin J, Dahlman KB, Beauchamp RD, Shi C (2018) Frequent BRAF mutations suggest a novel oncogenic driver in colonic neuroendocrine carcinoma. J Surg Oncol 117 (2):284-289. https://doi.org/10.1002/jso.24834
Zakka K, Nagy R, Drusbosky L, Akce M, Wu C, Alese OB, El-Rayes BF, Kasi PM, Mody K, Starr J, Shaib WL (2020) Blood-based next-generation sequencing analysis of neuroendocrine neoplasms. Oncotarget 11 (19):1749-1757. https://doi.org/10.18632/oncotarget.27588
Klimstra DS (2016) Pathologic Classification of Neuroendocrine Neoplasms. Hematol Oncol Clin North Am 30 (1):1-19. https://doi.org/10.1016/j.hoc.2015.08.005
Klimstra DS, Beltran H, Lilenbaum R, Bergsland E (2015) The spectrum of neuroendocrine tumors: histologic classification, unique features and areas of overlap. Am Soc Clin Oncol Educ Book:92–103. https://doi.org/10.14694/EdBook_AM.2015.35.92
Yang Z, Tang LH, Klimstra DS (2013) Gastroenteropancreatic neuroendocrine neoplasms: historical context and current issues. Semin Diagn Pathol 30 (3):186-196. https://doi.org/10.1053/j.semdp.2013.06.005
Pelosi G, Bresaola E, Bogina G, Pasini F, Rodella S, Castelli P, Iacono C, Serio G, Zamboni G (1996) Endocrine tumors of the pancreas: Ki-67 immunoreactivity on paraffin sections is an independent predictor for malignancy: a comparative study with proliferating-cell nuclear antigen and progesterone receptor protein immunostaining, mitotic index, and other clinicopathologic variables. Hum Pathol 27 (11):1124-1134
Rinzivillo M, Capurso G, Campana D, Fazio N, Panzuto F, Spada F, Cicchese N, Partelli S, Tomassetti P, Falconi M, Delle Fave G (2016) Risk and Protective Factors for Small Intestine Neuroendocrine Tumors: A Prospective Case-Control Study. Neuroendocrinology 103 (5):531-537. https://doi.org/10.1159/000440884
Bhatt VR, Loberiza FR, Jr., Tandra P, Krishnamurthy J, Shrestha R, Wang J (2014) Risk factors, therapy and survival outcomes of small cell and large cell neuroendocrine carcinoma of urinary bladder. Rare Tumors 6 (1):5043. https://doi.org/10.4081/rt.2014.5043
Buron Pust A, Alison R, Blanks R, Pirie K, Gaitskell K, Barnes I, Gathani T, Reeves G, Beral V, Green J, Million Women Study C (2017) Heterogeneity of colorectal cancer risk by tumour characteristics: Large prospective study of UK women. Int J Cancer 140 (5):1082-1090. https://doi.org/10.1002/ijc.30527
Chan JK, Loizzi V, Burger RA, Rutgers J, Monk BJ (2003) Prognostic factors in neuroendocrine small cell cervical carcinoma: a multivariate analysis. Cancer 97 (3):568-574. https://doi.org/10.1002/cncr.11086
Valente R, Hayes AJ, Haugvik SP, Hedenstrom P, Siuka D, Korsaeth E, Kaemmerer D, Robinson SM, Maisonneuve P, Delle Fave G, Lindkvist B, Capurso G (2017) Risk and Protective Factors for the Occurrence of Sporadic Pancreatic Endocrine Neoplasms. Endocr Relat Cancer. https://doi.org/10.1530/ERC-17-0040
Meder L, Konig K, Ozretic L, Schultheis AM, Ueckeroth F, Ade CP, Albus K, Boehm D, Rommerscheidt-Fuss U, Florin A, Buhl T, Hartmann W, Wolf J, Merkelbach-Bruse S, Eilers M, Perner S, Heukamp LC, Buettner R (2016) NOTCH, ASCL1, p53 and RB alterations define an alternative pathway driving neuroendocrine and small cell lung carcinomas. Int J Cancer 138 (4):927-938. https://doi.org/10.1002/ijc.29835
Sigel CS, Krauss Silva VW, Reid MD, Chhieng D, Basturk O, Sigel KM, Daniel TD, Klimstra DS, Tang LH (2018) Assessment of cytologic differentiation in high-grade pancreatic neuroendocrine neoplasms: A multi-institutional study. Cancer Cytopathol 126 (1):44-53. https://doi.org/10.1002/cncy.21934
Sigel CS, Krauss Silva VW, Reid MD, Chhieng D, Basturk O, Sigel KM, Daniel TD, Klimstra DS, Tang LH (2018) Well differentiated grade 3 pancreatic neuroendocrine tumors compared with related neoplasms: A morphologic study. Cancer Cytopathol 126 (5):326-335. https://doi.org/10.1002/cncy.21982
Konukiewitz B, Jesinghaus M, Steiger K, Schlitter AM, Kasajima A, Sipos B, Zamboni G, Weichert W, Pfarr N, Kloppel G (2018) Pancreatic neuroendocrine carcinomas reveal a closer relationship to ductal adenocarcinomas than to neuroendocrine tumors G3. Hum Pathol 77:70-79. https://doi.org/10.1016/j.humpath.2018.03.018
Acknowledgments
This work is dedicated to the memory of Carlotta, an extraordinarily lively girl who died an untimely death due to cancer in the prime of her life. The authors are indebted with Ms. Cecilia Pelosi for accurate proofreading of the manuscript.
Author information
Authors and Affiliations
Contributions
GP conceived and designed the study, drafted and finalized the manuscript, and shared all statistical analyses; FB carried out clustering analysis, supervised all statistical procedures, shared the study design, and contributed to draft and finalize the manuscript; ED performed all statistical analyses; JM, AS, AA, MP, YG, and NV critically revised the manuscript and finalized the manuscript. All authors approved the submitted version.
Corresponding author
Ethics declarations
Disclosures and Declarations
F.B is a recipient of grants from the Associazione Italiana Ricerca sul Cancro (AIRC) [IG-22827], the Italian Ministry of Health [GR-2016-02363975 and CLEARLY]. N.V. was supported by Cancer Center Support Grant (CCSG) P30 CA006927 to Fox Chase Cancer Center. All the other authors declare that there are no sources of funding. All authors declare that there are no financial or non-financial interests and that the study does not involve humans and/or animals for which specific approval by the ethics committee and/or informed consent are required. Moreover, the study does not have implications for public health or general welfare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval and Consent to Participate
As this study dealt with reappraisal of previously generated and authorized molecular data by the Fox Chase Cancer Centre’s ethics committee, no further release was necessary. In particular, no new information about patients was collected and no protected health information was used in the current study. The study was performed in keeping with the Declaration of Helsinki and, remarkably, does not contain any individual person’s data, but only aggregated information.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pelosi, G., Bianchi, F., Dama, E. et al. A Subset of Large Cell Neuroendocrine Carcinomas in the Gastroenteropancreatic Tract May Evolve from Pre-existing Well-Differentiated Neuroendocrine Tumors. Endocr Pathol 32, 396–407 (2021). https://doi.org/10.1007/s12022-020-09659-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-020-09659-6