Abstract
Aim
Tall cell variant (TCV) of papillary thyroid carcinoma (PTC) shows a poorer prognosis than conventional PTC. The World Health Organization (WHO) classification defines TCV as the tall cell component (TCC) in ≥50% of PTC lesions. We investigated whether and how the proportion of TCC affects the prognosis of patients with PTC with TCC.
Patients and methods
Seventy patients with TCC in ≥30% of their PTC lesions and 210 age- and gender-matched controls with no TCC who underwent locally curative surgery at Kuma Hospital (2006–2014) were enrolled. The 70 PTC patients were divided into two categories: TCC ≥50% (TCC-major, n = 19) and TCC 30–49% (TCC-minor, n = 51). We performed univariate and multivariate analyses of the relationships between disease-free survival (DFS) and variables including the TCC proportion in 276 patients who had no distant metastases at surgery (median follow-up 64 months).
Results
In the univariate analysis, TCC-major, TCC-minor, old age (≥65 years), clinical node metastasis, significant extrathyroid extension (Ex), and high Ki-67 labeling index (≥5%) significantly affected the DFS. In the multivariate analysis, TCC-major and Ex independently affected the DFS, but TCC-minor did not. In an analysis excluding TCC-major patients, TCC-minor was not an independent prognostic factor for DFS.
Conclusions
Studies or larger patient series with longer follow-ups are necessary, but we speculate that in PTC with TCC, TCC-major significantly and independently affects the DFS, whereas TCC-minor does not. Our findings indicate that the WHO definition of TCV is appropriate and that the prognostic impact of TCC-minor is limited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papillary thyroid carcinoma (PTC) is the most common malignancy arising from thyroid follicular cells, and PTC has several variants in addition to conventional PTC. Although PTC generally shows an excellent prognosis, the prognoses of its variants are not uniform. Tall cell variant (TCV) is a representative PTC variant that has a dire prognosis, as shown in many studies [1–7] including an international multicenter study [8]. TCV includes a component of carcinoma cells that have a height that is ≥3 × their width, that is the tall cell component (TCC). The World Health Organization (WHO) provides a definition of TCV: The TCC component is ≥50% of the PTC lesions [9].
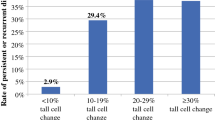
Beninato et al. [10] investigated the relationship between the proportion of TCC and clinicopathological features, and they demonstrated that PTC with 10% TCC is likely to show aggressive characteristics such as extrathyroid extension, lymph node metastasis, and advanced stage. To date, however, no studies have been published regarding whether and how the proportion of the TCC affects the prognosis of PTC patients. In this study, we enrolled patients with PTCs with high and low proportions of the TCC (≥50 and 30–49% of the lesions, respectively) and PTC patients without the TCC as a control group, and we investigated the prognostic impact of TCC.
Patients and methods
Patients in the TCC group
Between 2006 and 2014, 6553 patients underwent initial surgery for PTC at our institution. Of these, 70 cases (1.1%) were diagnosed as TCV based on postoperative pathological reports because the reports included the proportion of tall cells, i.e., cells with a height that was ≥3 × their width, in a considerably large portion of the lesions (≥30%). Other patients were diagnosed as having conventional PTC or variants other than TCV, which did not include TCC. For the present study, tissues of all 70 of these patients were re-checked by one of our coauthors (M.H.) and divided into two categories based on the proportion of the TCC: the TCC-major group, with the TCC in ≥50% of the lesions (19 patients), and the TCC-minor group, with the TCC in 30–49% of the lesions (51 patients). Our definition of ‘TCC-major’ is identical to that of TCV in the WHO classification. Patients with a TCC <30% and those having not only TCC but also components of anaplastic carcinoma or squamous cell carcinoma in primary lesions and/or lymph node metastases were excluded from our study. All 70 of the patients underwent locally curative surgery, but four patients (one TCC-major and three TCC-minor patients) had distant metastasis to the lung at surgery and were classified as M1.
Patients in the age- and gender-matched control group
We randomly selected 210 PTC patients who underwent surgery during the same period as the TCV patients as a control group. The control patients were pathologically diagnosed as having PTC, and their ages and ganders were matched with the patients in the TCC group. They were also matched per year of diagnosis. None of the control patients had the TCC, an anaplastic carcinoma lesion, or a squamous cell carcinoma lesion.
Evaluation of clinicopathological features
We used several important clinicopathological features for analyzing the prognostic impact of TCC. Tumor size and clinical lymph node metastasis (N) were evaluated preoperatively on imaging studies. Extrathyroid extension (Ex) was based on intraoperative findings. When a tumor invaded the trachea requiring the resection of tracheal cartilage or cartilage and mucosa, or it invaded the recurrent laryngeal nerve requiring partial layer resection or complete resection followed by nerve anastomosis, or the tumor invaded the muscular layer of the esophagus, we regarded the cases as having Ex.
In this study, we did not regard tumors invading anterior strap muscles as having Ex. Tracheal invasion was also pathologically confirmed. However, it was impossible to clearly confirm invasion to the recurrent laryngeal nerve or to the muscular layer of the esophagus on pathological examination. In our previous study, we demonstrated that the intraoperative evaluation of Ex significantly affected patients’ prognoses [11] and we also adopted the intraoperative evaluation in the present study.
Ki-67 labeling index (LI) immunostaining
We performed Ki-67 immunostaining using 4-μm-thick formalin-fixed, paraffin-embedded tissues and an antibody against Ki-67 (MIB1, 1:200 dilution: Dako, Carpinteria, CA, USA) for all patients except two requiring decalcification to make paraffin-embedded tissues. The staining was carried out using an Autostainer (Dako Japan, Tokyo) and the Envision Kit (Dako Japan) according to the manufacturer’s recommendation.
To estimate the Ki-67 labeling index (LI), we counted at least 500 carcinoma cells in the hot area observed under 400× magnification and calculated the percentage of nuclei that stained positive. The Ki-67 LI was estimated by a coauthor (M.H.) who did not know the prognoses of the patients. We classified the results into two categories: Ki-67 LI <5% (low) and Ki-67 LI ≥5% (high). Ki-67 staining cannot be applied for cases that have high-grade calcification that requires decalcification. For this reason, two TCC-minor cases and 47 control cases lacked Ki-67 LI data.
Surgical therapy and RAI ablation or RAI therapy
We performed total thyroidectomy for the PTC patients whose tumors were multiple or solitary but >2 cm. Regardless of tumor size, the patients with N1 or M1 PTC or PTC with extrathyroid extension corresponding to T4a in the UICC TNM classification underwent a total thyroidectomy. The other patients underwent a hemithyroidectomy. Level VI dissection was routinely performed even though it is prophylactic. Level II–IV dissection was performed therapeutically for N1b patients. We performed level II–IV dissection prophylactically only when the tumors were >3 cm. Radioactive iodine (RAI) ablation using 13–30 mCi was also performed, mainly for advanced cases based on the physician’s preference and the patient’s acceptance. RAI therapy was performed for M1 cases unless the patient refused it.
Follow-up after surgery
The follow-up periods after surgery ranged from 4 to 131 months (median 64 months) in the entire group of patients plus controls. We performed ultrasound and chest computerized tomography (CT) or roentgenography once or twice per year for each patient. When we detected suspicious lymph nodes on ultrasound, we performed a fine-needle aspiration biopsy for the nodes and measured the thyroglobulin levels in the washout of the needle [12]. We monitored thyroglobulin and its antibody for the patients who underwent a total thyroidectomy, but in this study, we regarded patients as showing carcinoma recurrence only when recurred lesions were confirmed by imaging studies such as ultrasound, CT, or positron emission computerized tomography.
Statistical analyses
Variables were compared by Fisher’s exact test. The Kaplan–Meier method and log-rank tests were used for the analysis of time-dependent variables. The Cox regression model was also used for the multivariate analysis. A p value <0.05 was regarded as significant. We used StatView 5.0 software for these analyses.
Results
Initial treatment
In the TCC group, a total thyroidectomy was performed for 58 patients, including four M1 patients, and the remaining 12 patients underwent a hemithyroidectomy. In the control group, 157 patients underwent a total thyroidectomy and the remaining 53 underwent a more limited thyroidectomy (subtotal thyroidectomy or hemithyroidectomy). Level VI dissection was performed in all patients enrolled in this study. Unilateral or bilateral level II–IV dissection was performed in 47 TCC patients and 84 control patients. Three of the four M1 patients in the TCC group underwent RAI therapy using 100 mCi, and the remaining patient did not undergo RAI therapy because the patient refused it. Twenty-one and 12 patients in the TCC group and control group underwent RAI ablation, respectively.
Recurrence and carcinoma death
In the TCC group, 21 patients showed carcinoma recurrence. The organs in which the carcinoma recurred were regional lymph nodes in 13 patients, subcutaneous tissue in six patients, another local lesion in one patient, lung in eight patients, bone in three patients, liver in one patient, and brain in two patients. Nine patients showed carcinoma recurrence in two or more organs. One TCC-major patient and one TCC-minor patient died due to their PTC.
In the control group, carcinoma recurred in eight patients. Lymph node recurrence was observed in seven patients, and lung and bone recurrence was detected in three patients and one patient, respectively. Two patients showed recurrence in two or more organs. None of the patients in the control group died of PTC during the follow-up.
Clinicopathological features
The results of our assessment of clinicopathological features in the TCC and control groups are summarized in Table 1. The tumor size in the TCC group (consisting of the two subgroups: the TCC-major and TCC-minor patients) (mean 31, 6–100 mm) was significantly larger (p < 0.0001) than that in the control group (mean 21, 7–100 mm). The incidences of positive extrathyroid extension, and high Ki-67 LI were significantly higher in the TCC group than in the control group.
Disease-free survival (DFS)
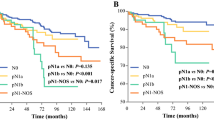
Figure 1 provides the Kaplan–Meier curves for the DFS of the TCC-major and TCC-minor patients and the controls. One TCC-major and three TCC-minor patients were excluded because they were classified as M1, and none of the controls were classified as M1. The 5-year DFS rates of the TCC-major patients, TCC-minor patients, and patients in the control group were 58, 89, and 93%, respectively, which is significantly poorer from the control group to the TCC-major patients (p = 0.0041 for the control group vs. the TCC-minor patients; p = 0.0047 for the TCC-minor patients vs. the TCC-major patients). RAI ablation and the extent of thyroidectomy did not affect the patients’ DFS (data not shown).
Multivariate analysis for DFS
We performed a multivariate analysis for DFS for the high- and low-TCC groups with various clinicopathological features, which were recognized as having prognostic impacts in previous studies [13–16]. As shown in Table 2, TCC-major (p = 0.0037) was recognized as an independent prognostic factor together with significant extrathyroid extension (p = 0.0017), while TCC-minor was not. Table 3 shows the results of a multivariate analysis after excluding TCC-minor from the variables; here too, TCC-major was recognized as an independent prognostic impact for the patients’ DFS (p = 0.0038). We excluded TCC-major patients and conducted another multivariate analysis, and the results indicated that TCC-minor did not independently affect the DFS (data not shown).
We could not evaluate the prognostic impact of the TCC on the cause-specific survival (CSS), because the number of patients who died of PTC was small.
Micro-PTC in the TCC-major, TCC-minor, and control groups
The number of micro-PTC patients was 47 in the control group, two in the TCC-major group, and one in the TCC-minor group. None of the micro-PTC patients in the control group showed recurrence or died of PTC. Of the three micro-PTC patients in the TCC-major and TCC-minor groups, one TCC-major patient and one TCC-minor patient were N- and Ex-negative and are still alive without recurrence. The other TCC-major patient with micro-PTC had large and extensive node metastases, although the primary lesion was small. This patient died of PTC 51 months after surgery because of anaplastic transformation of a recurred node.
Discussion
The results of the present study demonstrated that, in PTC, (1) the cases with TCC (>30%) had more aggressive characteristics than those without TCC; (2) the cases with a high proportion of the TCC (≥50%) harbored a poor DFS, and high TCC was an independent prognostic factor for DFS; and (3) a low proportion of the TCC (30–49%) was not an independent prognostic factor for DFS.
In our series, the patients with TCV >30% more frequently had distant metastasis-positivity, high cell-proliferating activity, large tumor size, and Ex-positivity compared to the other patients. These findings coincide with those of a study reported by Beninato et al. [10], although in the present study, cases with a low proportion of the TCC (≤30%) were excluded.
In the WHO classification, TCV is defined as PTC with the component of tall cells (height/width ratio 3 or more) at 50% or more of the carcinoma lesions [9], which is consistent with TCC-major in the present study. However, the diagnosis varies according to pathologists, probably due to the difference, in the interpretation of the pathology criteria. In our previous study, we showed that the incidence of TCV based on the WHO classification was 3.7% in a series of patients operated on between 1987 and 1995 [13] and that incidence is higher than that observed in the present study. In this study, our thyroid pathologist coauthor applied the diagnostic criteria of TCV much more rigorously than in the previous study, which may be the reason for the difference in the incidences of TCV. A recent international multicenter study analyzed 6,282 PTC cases, including 239 TCV (3.8%) cases [8]. However, since that was a multicenter study, there may have been discrepancies among the pathologists’ interpretation of the diagnosis criteria.
Here we observed that TCC-major consistent with TCV in the WHO classification [9] independently affected the early recurrence of PTC (median follow-up time 63 months). Although our TCC-minor patients showed a poorer prognosis than that of the control group, TCC-minor was not recognized as an independent prognostic factor both in the entire series and in the series excluding TCC-major patients. It is therefore suggested that a small amount of TCC does not affect the biological characteristic or make the prognosis of patients poorer, at least in the early phase after surgery. TCC-major patients require extensive therapies, including a total thyroidectomy (if the initial surgery was not total thyroidectomy, a completion total thyroidectomy should be performed as a second surgery) followed by RAI ablation and TSH suppression.
Our present median follow-up period was short at 63 months, which is a limitation of this study. Although four patients died of PTC during the follow-up period, we could not analyze whether the proportion of TCC and other factors can be predictors of carcinoma death. Another study limitation is that we analyzed patients with local recurrences and those with distant recurrences as a single group, because of the small number of patients, but the predictors of distant and local recurrences may not be the same. Further studies are needed to elucidate these points.
In this study, micro-PTC patients were present in both the TCC group and the control group. Low-risk micro-PTCs lacking Ex, N, and M factors are very indolent, and an active surveillance without immediate surgery is an important therapeutic strategy for these patients [17, 18]. However, micro-PTCs with Ex, N or M are aggressive and have a dire prognosis. Of the three micro-PTC patients in the present TCC group, one patient in the TCC-major group died due to PTC after showing recurrence in the early phase after surgery. However, this patient had multiple node metastases with large sizes (≥3 cm) [14], indicating that although the primary lesion was small, this patient’s PTC had a very aggressive character. The other two patients (one TCC-major and one TCC-minor) were alive without any evidence of recurrence, although they did not undergo a completion total thyroidectomy or RAI ablation. Therefore, we have no evidence that low-risk micro-TCV requires any special and additional therapies compared with low-risk conventional PTC.
Our earlier study demonstrated that a Ki-67 LI greater than 3% was an independent prognostic factor for the CSS of PTC patients [15]. The present study enrolled patients who underwent surgery between 1996 and 1997. Since the sensitivity of Ki-67 immunostaining has increased due to the improvement of paraffin-embedded tissue specimens, we now set the cutoff ratio at 5%. In 2013, we reported the relationships between the Ki-67 LI (cutoffs at 5 and 10%) and persistent disease, the thyroglobulin doubling time, and DFS [16]. In the present study too, Ki-67 had a prognostic impact, although it was not revealed to have independent prognostic significance.
In summary, it is important to accurately evaluate the proportion of TCC in PTCs containing a TCC, because (1) the PTC patients with a TCC ≥50% showed significantly poorer DFS than the PTC patients with a TCC 30–49% and the control group, and (2) a TCC ≥50% independently predicted PTC recurrence, and a TCC 30–49% did not. Studies with larger numbers of patients and longer follow-up periods should be conducted to further clarify the prognostic impact of the proportion of the tall cell component in PTCs.
References
Machens A, Hoizhausen HJ, Lautenschlager C et al (2004) The tall-cell variant of papillary thyroid carcinoma: a multivariate analysis of clinical risk factors. Langenbecks Arch Surg 389:278–282
Michels JJ, Jacques M, Henry-Amar M et al (2007) Prevalence and prognostic significance of tall cell variant of papillary thyroid carcinoma. Hum Pathol 38:212–219
Ghossein RA, Leboeuf R, Patel KN et al (2007) Tall cell variant of papillary thyroid carcinoma without extrathyroid extension: biologic behavior and clinical implications. Thyroid 17:655–661
Morris LG, Shaha AR, Tuttle RM et al (2010) Tall-cell variant of papillary thyroid carcinoma: a matched-pair analysis of survival. Thyroid 20:153–158
Jalisi S, Ainsworth T, Lavalley M (2010) Prognostic outcomes of tall cell variant papillary thyroid cancer: a meta-analysis. J Thyroid Res 2010:325602
Regalbuto C, Malandreino P, Frasca F et al (2013) The tall cell variant of papillary thyroid carcinoma: clinical and pathological features and outcomes. J Endocrinol Investig 36:249–254
Dettmer MS, Schmitt A, Steinert H et al (2015) Tall cell papillary thyroid carcinoma: new diagnostic criteria and mutations in BRAF and TERT. Endocr Relat Cancer 22:419–429
Shi X, Liu R, Basolo F et al (2016) Differential clinicopathological risk and prognosis of major papillary thyroid cancer variants. J Clin Endocrinol Metab 101:264–274
DeLeillis RA, Lloyd RV, Heitz PU (eds) (2004) Pathology of tumours of endocrine organs. IARC Press, Lyon
Beninato T, Scognamiglio T, Rleiman DA et al (2013) Ten percent tall cells confer the aggressive features of the tall cell variant of papillary thyroid carcinoma. Surgery 154:1331–1336
Ito Y, Tomoda C, Uruno T et al (2006) Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: massive but not minimal extension affects the relapse-free survival. World J Surg 30:780–786. doi:10.1007/s00268-005-0270-z
Uruno T, Miyauchi A, Shimizu K et al (2005) Usefulness of thyroglobulin measurement in fine-needle aspiration biopsy specimens for diagnosing cervical lymph node metastasis in patients with papillary thyroid cancer. World J Surg 29:483–485. doi:10.1007/s00268-004-7701-0
Ito Y, Hirokawa M, Fukushima M et al (2008) Prevalence and prognostic significance of poor differentiation and tall cell variant in Japan. World J Surg 32:1535–1543. doi:10.1007/s00268-007-9406-7
Ito Y, Kudo T, Kobayashi K et al (2012) Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: analysis of 5768 patients with average 10-year follow-up. World J Surg 36:1274–1278. doi:10.1007/s00268-012-1423-5
Ito Y, Miyauchi A, Kakudo K et al (2010) Prognostic significance of Ki-67 labeling index in papillary thyroid carcinoma. World J Surg 34:3015–3021. doi:10.1007/s00268-010-0746-3
Miyauchi A, Kudo T, Hirokawa M et al (2013) Ki-67 labeling index is a predictor of postoperative persistent disease and cancer growth and a prognostic indicator in papillary thyroid carcinoma. Eur Thyroid J 2:57–64
Ito Y, Miyauchi A, Kihara M et al (2014) Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 24:27–34
Haugen BR, Alexander EK, Bible KC et al (2016) 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ito, Y., Hirokawa, M., Miyauchi, A. et al. Prognostic Significance of the Proportion of Tall Cell Components in Papillary Thyroid Carcinoma. World J Surg 41, 742–747 (2017). https://doi.org/10.1007/s00268-016-3784-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3784-7