Abstract
Long non-coding RNAs (lncRNAs) may contribute to carcinogenesis and tumor progression by regulating transcription and gene expression. The role of lncRNAs in the regulation of thyroid cancer progression is being extensively examined. Here, we analyzed three lncRNAs that were overexpressed in papillary thyroid carcinomas, long intergenic non-protein coding RNA, regulator of reprogramming (Linc-ROR, ROR) PVT1 oncogene (PVT1), and HOX transcript antisense intergenic RNA (HOTAIR) to determine their roles in thyroid tumor development and progression. ROR expression has not been previously examined in thyroid carcinomas. Tissue microarrays (TMAs) of formalin-fixed paraffin-embedded tissue sections from 129 thyroid cases of benign and malignant tissues were analyzed by in situ hybridization (ISH), automated image analysis, and real-time PCR. All three lncRNAs were most highly expressed in the nuclei of PTCs. SiRNA experiments with a PTC cell line, TPC1, showed inhibition of proliferation with siRNAs for all three lncRNAs while invasion was inhibited with siRNAs for ROR and HOTAIR. SiRNA experiments with ROR also led to increased expression of miR-145, supporting the role of ROR as an endogenous miR-145 sponge. After treatment with TGF-β, there was increased expression of ROR, PVT1, and HOTAIR in the PTC1 cell line compared to control groups, indicating an induction of their expression during epithelial to mesenchymal transition (EMT). These results indicate that ROR, PVT1, and HOTAIR have important regulatory roles during the development of PTCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is the most common malignancy of endocrine organs and papillary thyroid carcinomas (PTCs) constitute approximately 80% of thyroid cancers. PTCs and follicular thyroid carcinomas (FTCs) make up the group of well-differentiated thyroid carcinomas. Most of these PTCs are low-grade lesion with only about 10 to 15% constituting more aggressive carcinomas from these groups. The rare poorly differentiated thyroid carcinomas (PDCs) and undifferentiated or anaplastic thyroid carcinomas (ATCs) are more aggressive thyroid carcinomas that frequently result in the death of patients after the diagnosis is established. Extensive research on morphological subclassification and immunohistochemical analyses have contributed to the precise diagnosis and to predicting the biological behavior of these carcinomas [1], while molecular analyses have advanced our knowledge about the biological behavior and provided new predictive information about these malignancies [1,2,3].
Non-coding RNAs, which are defined as RNA species that do not code for specific proteins, have been studied in thyroid neoplasms in recent years. Most of the studies have been with microRNAs which are composed of approximately 22 nucleotides. However, several recent studies have examined long non-coding RNAs, non-coding RNAs greater than 200 nucleotides, in thyroid neoplasms [4,5,6,7,8,9,10]. Some long non-coding RNAs (lncRNAs) function as oncogenes or tumor suppressor genes and regulate thyroid tumor progression. Most studies of lncRNAs have used real-time quantitative PCR (RT-qPCR) to measure expression levels. Only a few studies have used in situ hybridization (ISH) which allows for the intracellular localization of the lncRNA and distinguishes between nuclear and cytoplasmic localization as well as quantification of expression in individual cells.
Various studies with thyroid neoplasms have shown progression from well-differentiated carcinomas to highly aggressive ATCs usually by epithelial to mesenchymal transition (EMT) [11,12,13,14,15]. Our recent studies with MALAT1 expression in thyroid neoplasms suggested that this lncRNA functions as an oncogene in the progression from normal thyroid to PTCs, but could possibly function as a tumor suppressor in ATCs [9]. The current studies examine long intergenic non-protein coding RNA, regulator of reprogramming (Linc-ROR, ROR) expression in thyroid tissues for the first time. In addition, PVT1 oncogene (PVT1) and HOX transcript antisense intergenic RNA (HOTAIR) are also examined for EMT and we show for the first time that these lncRNAs regulate EMT in thyroid PTCs and also have roles as oncogenes in PTC development during thyroid cancer progression.
Materials and Methods
Tissue Microarrays (TMAs)
TMAs were constructed as described previously [11, 12] from 129 cases of formalin-fixed paraffin-embedded (FFPE) tissues, including normal thyroid (NT, n = 10), Hashimoto thyroiditis (HT, n = 10), nodular goiters (NG, n = 10), follicular adenoma (FA, n = 32), follicular carcinoma (FC, n = 28), conventional papillary thyroid carcinoma (PTC n = 28), follicular variant of papillary thyroid carcinoma (FV, n = 29), and anaplastic thyroid carcinoma (ATC, n = 10). The TMA consisted of triplicate 0.6-mm cores made with a manual tissue microarrayer (Beecher Instruments, Sun Prairie, WI). The NT consisted of tissues from the opposite (histologically normal) thyroid lobe in patients with follicular or papillary carcinomas.
The study was approved by the Institutional Review Board at the University of Wisconsin–Madison.
In Situ Hybridization
TMAs were probed for PVT1 (406951), ROR (402831), and HOTAIR (312341) expression using the RNAscope 2.5 HD-Brown Manual Assay (Advanced Cell Diagnostics, Newark, CA) as per manufacturer’s recommendations [10] with the following modifications to manufacturer’s protocol, including a step in which antigen retrieval was performed in a Decloaker (Biocare Medical, Pacheco, CA) for 3 min, and the probe was incubated overnight at 40 °C with a parafilm cover over sample in a humidified chamber. The probes used included positive control probes hs-PPIB (positive control, 313901), hs-ACTB (actin, positive control, 310141), and negative control probe dapB (negative control, 310043) (Advanced Cell Diagnostics). Probe expression levels were visualized with DAB.
Automated Image Acquisition and Analysis
The stained TMA slides were visualized and analyzed with the Vectra slide scanner and associated software (PerkinElmer, Waltham, MA) as previous described [10]. Briefly, after image acquisition, any core with tissue folding or loss of tissue was excluded for analysis. InForm 1.4.0 software was used to segment tissue compartments (epithelium vs. non-epithelium) and subcellular compartments (nucleus vs. cytoplasm). PVT1, ROR, and HOTAIR expression levels from each sample were quantitated as optical density (OD) per unit area (pixel). Only nuclear signal was used for analysis. Mean lncRNA expression level of each sample from the triplicated cores was used for further analysis.
Real-Time PCR
Total RNA was extracted from samples with TRIzol reagent (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s instructions, and RNA quality and concentrations were assessed with a NanoDrop 1000 spectrophotometer (ThermoFisher Scientific, Waltham, MA). One microgram of total RNA was reverse-transcribed using the All-in-One miRNA RT-qPCR detection kit (GeneCopoeia, Rockville, MD). RT-qPCR was performed on a CFX96 PCR detection system (Bio-Rad Laboratories, Hercules, CA) using Bullseye EvaGreen qPCR master mix (MIDSCI, St. Louis, MO), normalized to 18S rRNA; relative fold change was determined by the ΔΔ CT method as previously reported [14, 15]. The PCR primers used for PVT1, ROR, HOTAIR, SOX2OT, PTCSC1, and miR-145 are as follows: PVT1, forward 5′-TGAGAACTGTCCTTACGTGACC-3′ and reverse 5′-AGAGCACCAAGACTGGCTCT-3′; ROR, forward 5′-CTGGCTTTCTGGTTTGACG-3′ and reverse 5′-CAGGAGGTTACTGGACTTGGAG-3′; HOTAIR, forward 5′-CAGTGGGGAACTCTGACTCG-3′ and reverse 5′-GTGCCTGGTGCTCTCTTACC-3′; SOX2OT, forward 5′-GCTCGTGGCTTAGGAGATTG-3′ and reverse 5′-CTGGCAAAGCATGAGGAACT-3′; PTCSC1, forward 5′-CAGGGCATGGCTGTGTTT-3′ and reverse 5′-GACATTATCAAGTAACTAGTGCTGTCA-3′; miR-145, forward 5′-GCCAGAGGGTTTCCGGTACTTT-3′ and reverse 5′-CCAAGAGTACGGCAGTGCTGAA-3′; and 18S, forward 5′-GTAACCCGTTGAACCCCATT-3′ and reverse 5′-CCATCCAATCGGTAGTAGCG-3′.
Tissue Culture and EMT Induction with TGF-β
The papillary thyroid carcinoma cell line TPC1 was cultured and treated with TGF-β to induce EMT as previously described [14, 15]. TPC-1 cells were treated with serum-free media with and without TGF-β (2 ng/ml) for 7, 14, and 21 days [14, 15].
siRNA Transfection, Proliferation, and Invasion Assays
TPC1 cells were seeded into a 96-well plate at 1 × 104/well (ten replicates each) in 100-ml serum-free/antibiotic-free media. Wells were transfected with 50 nmol PVT1, 30 nmol ROR, and 10 nmol HOTAIR siRNAs or scrambled control Silencer Select siRNAs (ThermoFisher Scientific, Waltham, MA) using X-tremeGENE siRNA Transfection Reagent (Roche, Indianapolis, IN) for 72 h at 37°C, 5% CO2. After 72-h incubation, the Vybrant® MTT Cell Proliferation Assay Kit (Life Technologies, Carlsbad, CA) and the Cultrex BME Cell Invasion Assay (R&D Systems, Minneapolis, MN) were performed according to protocol. The 96-well plate for the invasion assay was coated with 0.1× BME (basement membrane extract) overnight prior to seeding cells. Analysis for both assays used the SpectraMax® M3 Multi-Mode Microplate Reader with SoftMax® Pro (6.2.2) software.
Statistics
Student’s t test was used to analyze lncRNA expression data collected from RNA ISH and RT-qPCR. Two-tailed p values < 0.05 were considered to be statistically significant. Data are expressed as means ± standard error of the mean (SEM).
Results
ISH Analysis
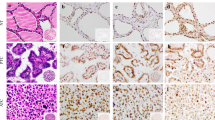
ISH was performed with probes for PVT1, ROR, and HOTAIR on TMAs containing 10 NT, 12 HT, 10 NGs, 32 FAs, 28 FCs, 28 cPTCs, 29 FVs, and 10 ATCs. There was predominantly nuclear localization of the hybridization signals for all three probes with weak cytoplasmic localization. The positive control probe PPIB showed predominantly nuclear localization (Fig. 1) while actin showed nuclear and cytoplasmic localization (data not shown). The negative control probe resulted in no staining of the tissues (data not shown). The highest hybridization signals were observed in PTCs for all three probes, which is consistently higher than NTs (Figs. 1 and 2 and supplementary Fig. 1). In addition, PTCs showed increased hybridization signals compared to FC, FA, NG, and FV as well as ATC for all three lncRNAs, although some of the trends did not meet statistical significance (Fig. 2).
Representative H&E sections and ISH localization of PVT1 lncRNA in NTs (a–c), PTCs (d–f), and ATCs (g–i) using a thyroid TMA. Inset shows a low power section from the TMA. The strongest labeling was present in PTCs, while the expression in NTs and ATCs is similar. The housekeeping gene peptidyl-prolyl cis-trans isomerase B (PPIB) was used to check for preservation of RNA in the TMA samples. Bar = 100 μm
Nuclear expression of PVT1, ROR, and HORTAIR in normal thyroid tissue and thyroid neoplasms. The highest level of expression is observed in PTCs for all three lncRNAs, which is statistically significantly higher than that of NTs. The expression of lncRNAs in ATCs is either similar or less than NTs. FCs in general express higher level of lncRNAs comparing to NTs, although this trend does not reach statistical significance for any of the lncRNAs tested. Error bars expressed as SEM; *p < 0.05
Real-Time PCR Analysis of PVT1, ROR, and HOTAIR in Thyroid Cancers in FFPE Tissue
To support the ISH findings, PVT1, ROR, and HOTAIR expression levels were studied using RT-qPCR in FFPE tissues from a subset of PTCs, FTCs, PTCs, and ATCs used on the TMA. As seen in Fig. 3, PVT1, ROR, and HOTAIR expressions were significantly higher in the PTCs compared to the other groups (p < 0.05), including ATCs (Fig. 3). Analysis of two additional lncRNAs which were not increased in PTCs in some of our preliminary screening experiments, PTCSC1 and SOX2OT, showed that these were significantly decreased in PTCs compared to normal thyroid tissues or ATCs (Supplementary Fig. 2).
Real-Time PCR Analysis of PVT1, ROR, and HOTAIR in Primary Fresh Frozen Tissue
To eliminate potential artifacts from FFPE tissues, RT-qPCR analysis of PVT1, ROR, and HOTAIR was performed in fresh frozen primary PTCs with matching normal thyroid tissues from three different patients. The results showed increased expression of all three lncRNAs in the PTCs compared to the matching normal thyroid tissues (Fig. 4).
The Effect of siRNA Silencing of PVT1, ROR, and HOTAIR on Proliferation and Invasion of PTCs
In order to assess the functions of lncRNAs in PTC progression, silencing was performed on the TPC1, a PTC cell line, using siRNA to PVT1, ROR, and HOTAIR. After testing siRNA concentrations for knockdown efficiency (Supplementary Fig. 3), the TPC1 cells were treated with 50 nmol PVT1, 30 nmol ROR, and 10 nmol HOTAIR siRNAs or scrambled control siRNAs and examined for proliferation and invasion using MTT and invasion assay (Fig. 5). Knockdown of all three lncRNAs showed low level but statistically significant inhibition of proliferation while invasive potential was inhibited by knockdown ROR and HOTAIR, but not PVT1 (Fig. 5).
TPC1 cells were transfected with siRNAs to PVT1, Linc-ROR (ROR), and HOTAIR for 3 days and were analyzed for the effects on proliferation (a) and invasiveness (b). By blocking the lncRNAs HOTAIR and Linc-ROR by specific siRNAs, there was a significant decrease in proliferation and invasion by TPC1 cells. Intriguingly, when PVT1 was blocked, proliferation was highly reduced; however, there was no effect on invasion. Error bars expressed as SEM; *p < 0.05, **p < 0.01, and ***p < 0.001
The Potential Regulatory Role of ROR in PTCs Through Downregulating miR-145
Previously, it was reported that ROR functions as a molecular sponge to downregulate miR-145 [20], a microRNA that was known to inhibit the proliferation of PTCs and TPC1 cell line [21]. To assess the role of ROR on the expression of miR-145, TPC-1 cells were transfected with 30 nmol ROR siRNA or a scramble control probe siRNA and RT-qPCR was performed to measure the expression of miR-145. There was a significant increase in miR-145 expression in the ROR knockdownTPC1 cells, indicating that ROR lncRNA had a negative regulatory role on miR-145 (Fig. 6).
TGF-β Treatment
Our previous studies had shown an increase in the EMT markers including SLUG and OCT4 after TGF-β treatment in the TPC1 cells [5, 11]. To study the effect of EMT on the expression of lncRNAs in PTCs, the expression of PVT1, ROR, and HOTAIR in TPC1 cells were measured after 7, 14, and 21 days of treatment with or without TGF-β. There was increased expression of all three lncRNAs in the TGF-β treated TPC1 cells compared to control groups, indicating an induction of these lncRNAs during EMT (Fig. 7).
TPC1 cells were treated with or without 2 ng of TGF-β1 for 7, 14, and 21 days in serum-free media to induce EMT. Control and treated cells for each time point were analyzed by qPCR for LincRNA expression. HOTAIR (c) and Linc-ROR (b) were consistently upregulated with TGF-β1 treatment. However, only days 14 and 21 were significantly upregulated, respectively. On the other hand, PVT1 (a) was significantly upregulated with TGF-β at all time points. Error bars expressed as SEM. *p < 0.05 and ***p < 0.001
Discussion
It is being increasingly recognized that lncRNAs have a role in the pathogenesis of thyroid carcinomas [2,3,4,5,6,7,8,9]. PVT1, ROR, and HOTAIR have all been reported to have oncogenic roles in the development of various human malignancies [4, 6,7,8,9,10]. However, ROR has not been previously examined in thyroid tissues. These three lncRNAs were shown to be oncogenic in the development of PTCs in the present study. ISH analysis using TMA containing NT and various thyroid neoplasms showed increased expression of all three lncRNAs in the nucleus of PTC cells, and quantitative imaging analysis confirmed the visual ISH observations. In addition, RT-qPCR using RNA extracted from a subset of cases from the TMA supported the ISH findings. This is further supported by analysis using fresh frozen primary PTC tumors with adjacent matched normal thyroid tissues.
Recent studies have increasingly shown that lncRNAs have regulatory roles in thyroid cancers, including PVT1 and HOTAIR. PVT1 was shown to be upregulated in thyroid cancers [16]. Downregulation of PVT1 decreased the recruitment of the transcriptional factor EZH2 in several thyroid cancer cell lines, resulting in decreased expression of cyclin D1 and thyroid-stimulating hormone receptor (TSHR). This inhibited the proliferation of thyroid cancer cells and promoted cell cycle arrest [16]. Similarly, HOTAIR was found to be upregulated in thyroid cancer and promoted proliferation by downregulating the tumor suppressor miR-1 that inhibits the expression of CCND2 (cyclin D2) [17]. Our studies again confirm the upregulation of PVT1 and HOTAIR in PTCs comparing to other thyroid epithelial neoplasms and normal thyroid tissues. In addition, by examining a spectrum of thyroid neoplasm, we demonstrate that despite the lack of statistical significance in some cases, there appear to be a gradual upregulation trend of PVT1 and HOTAIR from NT to benign thyroid neoplasm (FA) then to PTCs.
Our studies are the first reported ones of lncRNA ROR expression in thyroid cancer. Previously, ROR was shown to exert regulatory effect in proliferation, invasion, and stemness of gastric carcinoma stem cells and in breast cancer, pancreatic cancer, hepatocellular cancer, endometrial cancer, and nasopharyngeal carcinomas. In liver cancer cells, it was regulated by hypoxia and was able to promote cell proliferation partially by acting as an endogenous sponge to miR-145, a known microRNA with tumor suppressor functionality [18]. Our studies show upregulation of ROR in PTCs in a similar pattern as PVT1 and HOTAIR. In addition, examining the interaction of ROR with miR-145 showed that there was upregulation of miR-145 after ROR silencing with a specific siRNA.
Previous studies in breast carcinomas [22] and thyroid carcinomas [14] have shown a close relationship between EMT and cancer stem-like cell proliferation [14, 22]. Interestingly, PVT1, ROR, and HOTAIR were all shown to be involved in EMT in the current study. Both PVT1 and HOTAIR were suggested to promote EMT in esophageal carcinoma [19, 20]. Other studies have shown that ROR prevented degradation of the miR-205 target genes including the EMT inducer ZEB2 in breast carcinoma [21], emphasizing its role in EMT [22, 23]. The present study found that downregulation of all three lncRNAs negatively regulated proliferation and migration, and that TGF-β stimulated EMT had increased all three lncRNAs. These findings further support the notion that EMT is an important pathway for lncRNAs to regulate thyroid cancer progression.
Recent large-scale genome sequencing projects suggested that PTCs be divided into BRAFV600E-like and RAS-like groups [24]. The RAS groups included some FVs which were histologically related to PTCs but were recently suggested to behave like very low-grade neoplasms if totally encapsulated [25]. Non-invasive encapsulated neoplasms with papillary-like nuclear features are currently referred to as NIFTP [26]. In the currently study, although it was interesting to note the statistically lower expression of all three lncRNAs in FV compared to PTCs, we were not able to draw further conclusions due to the inability to separate the invasive FV tumors from the NIFTP tumors in our TMAs. This is because the TMA was constructed before these additional histological divisions were formulated and the entire tumor capsule must be examined microscopically before a diagnosis of NIFTP can be made.
Various studies have suggested that there is tumor progression from PTCs to ATC by dedifferentiation [14, 27,28,29]. Mutations of the BRAF V600E gene and the TP53 gene have helped to link tumor progression from PTC to ATC. In light of these findings, our results showing that there was no increased expression of PVT1, ROR, or HOTAIR during progression from PTC to ATC were surprising. LncRNAs can serve as molecular scaffolds, miRNA sponges, protein decoys, and reservoirs of small non-coding RNAs to regulate gene expression and protein function [5, 6]. It is possible that the lncRNA regulatory mechanisms in ATCs are different from those in PTCs and normal thyroid which may explain the differences in the upregulation of these lncRNAs in PTCs compared to ATCs. In addition, the genomic landscape, as well as the microRNA distribution, of ATCs is different from that of PTCs [30,31,32]. All of these differences may contribute to the differences in the regulation of PVL1, ROR and HOTAIR in PTCs and ATCs. Further studies to elucidate these differences are ongoing.
ISH analysis is a powerful method to analyze gene expression in tissues. It can localize the specific intracellular compartment expressing the genes of interest without the need for tissue microdissection by manual methods or by laser capture techniques. The ISH approach as was done in these studies can be used for visualization and quantitation [33] as well as for predicting clinical outcome in specific diseases such as localized prostate cancer [34]. When combined with other methodologies such as RT-qPCR, ISH is a powerful tool in cell biology and pathology.
In summary, our studies showed that there is increased expression of ROR for the first time as well as PVT1 and HOTAIR lncRNAs during progression from NT to PTCs. All three lncRNAs were highly expressed in PTCs with lower levels of expression in normal, benign thyroid tissues as well as in ATCs. SiRNA experiments inhibited cell proliferation with all three lncRNAs, and invasive potential was inhibited with siRNAs for ROR and HOTAIR, but not for PVT1. We also show for the first time that TGF-β-induced EMT increased expression of PVT1, ROR, and HOTAIR in the TPC1 cell line. Collectively, these findings suggest that these three lncRNAs have important regulatory roles in PTC development.
References
Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs, 4th Edition, International Agency for Research on Cancer (IARC) Lyon, France, 2017.
Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7(10):569–580.
Livoisi VA. Papillary thyroid carcinoma: An update. Mod Pathol. 2011;24(Suppl 2):S1–S9.
Li X, Wu Z, Fu X, Han W. Long noncoding RNAs: Insights from biological features and functions to diseases. Med Res Rev. 2013;33(3):517–553.
Zhang R, Hardin H, Chen J, Guo Z, Lloyd RV. Non-coding RNAs in Thyroid Cancer Endocr Pathol. 2016;27:12–20.
Sui F, Ji M, Hou P. Long non-coding RNAs in thyroid cancer: Biological functions and clinical significance. Molecular and Cellular Endocrin. 2017, https://doi.org/10.1016/j.mce.2017.07.020 (In Press).
He H, Nagy R, Liyanarachchi S, Jiao H, Li W, Suster S, Kere J, de la Chapelle A. A susceptibility locus for papillary thyroid carcinoma on chormosome 8q24. Cancer Res. 2009;69(2):625–631.
He H, Li W, Liyanarachchi S, Jendrzejewski J, Srinivas M, Davuluri RV, Nagy R, de la Chapelle A. Genetic predisposition to papillary thryoid carcinoma: Involvement of FOXE1, TSHR, and a Novel lincRNA Gene, PTCSC2. J Clin Endocrinol Metab. 2015;100(1):E164–E172.
Jendrzejewski J, He H, Randomska HS, Li W, Tomsic J, Liyanarachchi S, Davuluri RV, Nagy R, de la Chapelle A. The polymorphism rs944289 predisposes to papillary thryoid carcinoma through a large intergenic noncoding RNA gene of tumor suppressor type. Proc Natl Acad Sci USA. 2012;109(22):8646–8651.
Zhang R, Hardin H, Huang W, Chen J, Asioli S, Righi A, Maletta F, Sapino A, Lloyd RV. MALAT1 Long non-coding RNA expression in thyroid tissues: Analysis by in situ hybridizastion and real-time PCR. Endocr Pathology 2017;28:7–12.
Buehler D, Hardin H, Shan W, Montemayor-Garcia C, Rush PS, Asioli S, Chen H, Lloyd RV. Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Mod Pathol. 2012;26(10):54–61.
Guo Z, Hardin H, Montemayor-Garcia C, Asioli S, Righi A, Maletta F, Sapino A, Llloyd RV. In situ hybridization analysis of miR-146b-5p and miR-21 in thyroid nodules: Diagnostic implications. Endocr Pathol. 2015;26(2):157–163.
Huang W, Eickhoff JC, Mehraein-Ghomi F, Church DR, Wilding G, Basu HS. Expression of spermidine/spermine N1-acetyl transferase (SSAT) in human prostate tissues is related to prostate cancer progression and metastasis. Prostate. 2015;75(11):1150–1159.
Hardin H, Guo Z, Shan W, Montemayor-Garcia C, Asioli S, Yu XM, Harrison AD, Chen H, Lloyd RV. The roles of the epithelial-mesenchymal transition marker PRRX1 and miR-146b-5p in papillary thyroid carcinoma progression. Am J Pathol. 2014;184(8):2342–2354.
Hardin H, Yu X-M, Harrison AD, Larrain C, Zhang R, Chen J, Chen H, Lloyd RV. Generation of novel thyroid cancer stem-like cell clones effects of resveratrol and valproic acid. Am J Pathol. 2016;186(6):1662–1673.
Zhou Q, Chen J, Feng J, Wang J. Long non-coding RNA PVT1 modulates thyroid cancer cell proliferation by recruiting EZH2 and regulating thyroid-stimulation hormone receptor (TSHR). Tumor Biol 2016;37:3105–3113, https://doi.org/10.1007/s13277-015-4149-9
Di W, LiQ, Shen W, Guo H, Zhao S. The long non-coding RNA HOTAIR promotes thyroid cancer cell growth, invasion and migration through the miR-1-CCND2 axis. Am J Cancer Res. 2017;7:1298–1309.
Takahashi K, Yan IK, Haga H, Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-ROR. J Cell Sci. 2014;127:1585–1594.
Zheng X, Hu H, Li S. High expression of lncRNA PVT1 promotes invasion by inducing epithelial-to-mesenchymal transition in esophageal cancer. Oncology Letters. 2016;12(4):2357–2362. https://doi.org/10.3892/ol.2016.5026.
Feng Xu, Jing Zhang, Long non-coding RNA HOTAIR functions as miRNA sponge to promote the epithelial to mesenchymal transition in esophageal cancer, Biomedicine & Pharmacotherapy, 90, 2017, 888–896, https://doi.org/10.1016/j.biopha.2017.03.103.
Pan Y, Li C, Chen J, Zhang K, Chu X, Wang R, Chen L. The emerging roles of long noncoding RNA ROR (lincRNA-ROR) and its possible mechanisms in human cancers. Cell Physiol Biochem. 2016;40:219–229.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipotsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715.
Hou P, Zhao Y, Li Z, Yao R, Ma M, Gao Y, Zhao L, Zhang Y, Huang B, Lu J. LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death and Disease. 2014;5: e1287; https://doi.org/10.1038/cddis.2014.249.
Cancer Genome Atlas Research Network. Integrated genomic characterizatin of papillary thyroid carcinoma. Cell. 2014;159(3):676–690.
Liu J, Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, Tuttle RM, Ghossein RA. Follicular variant of papillary thyroid carcinoma: A clinicopathologtic study of a problematic entity. Cancer. 2006;107:1253–1264.
Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo FR, Thompson LD, Barletta JA, Wenig BM, Al Ghuzlan A, Kakudo K, Giordano TJ, Alves VA, Khanafshar E, Asa SL, El-Naggar AK, Gooding WE, Hodak SP, Lloyd RV, Maytal G, Mete O, Nikiforova MN, Nose V, Papotti M, Poller DN, Sadow PM, Tischler AS, Tuttle AS, Wall KB, LiVolsi VA, Randolph GW, Ghossein RA. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2:1023–1029.
Quiros RM, Ding HG, Gattuso P, Prinz RA, Xu X. Evidence that one subset of anaplastic thryoid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer. 2005;103:2261–2268.
Hardin H, Montemayor-Garcia C, Lloyd RV. Thyroid cancer stem-like cells and epithelial-mesenchymal transition in thyroid cancers. Hum Pathol. 2013;44:1707–1713.
Zane M, Scavo E, Catalano V, Bonanno M, Todaro M, De Maria R, Stassi G. Normal vs cancer thyroid stem cells: The road to transformation. Oncogene. 2016;35:805–815.
Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thryoid cancers. J Clin Invest. 2016;126(3):1052–1066.
Braun J, Hoang-Vu C, Dralle H, Hutelmaier S. Downregulation of microRNAs directs the EMT and invasive potential of anapladstic thyroid carcinomas. Oncogene. 2010;29(29):4237–4244.
Visone R, Pallante P, Vecchione A, Cirombella R, Ferracin M, Ferraro A, Volinia S, Coluzzi S, Leone V, Borbone E, Liu CG, Petrocca F, Troncone G, Calin GA, Scarpa A, Colato C, Tallini G, Santoro M, Croce CM, Fusco A. Specific microRNAs are downregulated inhuman thyroid anaplastic carcinomas. Oncogene. 2007;26(54):7590–7595.
Maqsodi B, Nikoloff C. Non-isotopic method for in situ IncRNA visualization and quantitation. Methods Mol Biol. 2016;1402: 165–176.
Mehra R, Shi Y, Udager AM, Prensner JR, Sahu A, Iyer MK, Siddiqui J, Cao X, Wei J, Jiang H, Feng FY, Chinnaiyan AM. A novel RNA in situ hybridization assay for the long noncoding RNA SChLAP1 predicts poor clinical outcome after radical prostatectomy in clinical outcome after radical Prostatectomy in clinically localized prostate cancer. Neoplasia. 2014;16:1121–1127.
Acknowledgments
We kindly thank Dr. John A. Copland III (Mayo Clinic, Jacksonville, FL) for the THJ-16T and THJ-21T cell lines, Dr. Rebecca E. Schweppe (University of Colorado, Denver, CO) for the BCPAP cell line, Dr. Daniel T. Ruan (Brigham and Women’s Hospital, Boston, MA) for the TPC-1 cell line, the staff of the Translational Research in Pathology (TRIP), Flow Cytometry, 3P, and Experimental Pathology laboratories (University of Wisconsin Carbone Cancer Center Cancer Center Support Grant P30 CA014520).
Funding
This study was supported by a grant from the UW Carbone Cancer Center (RVL) and a resident research grant from the UW Pathology Department to RZ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study received ethical approval from the local Institutional Review Board.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplemental Figure 1
ISH localization of HOTAIR and ROR expression in the thyroid TMA. The strongest labeling was in the PTC while the NT and ATC were similar. Bar = 100 μm. (PDF 820 kb)
Supplemental Figure 2
Three FFPE samples each from the NT, PTC and ATC groups were tested for the lncRNAs PTCSC1 (A) and SOX2OT (B) by qPCR. The PTC group was significantly downregulated for both lncRNAs compared to the NT group. Whereas, both lncRNAs were also downregulated to that of the ATC group, however, this finding was not significant. Error bars expressed as SEM. ** = p<0.01, ***=p<.001 (PDF 43 kb)
Supplemental Figure 3
TPC1 cells were transfected with 10, 30 and 50 nMol of siRNAs for PVT1, ROR and HOTAIR. Represented are optimal concentrations assessed by RT-qPCR. Error bars are expressed as SEM. * = p<0.05, ** = p<0.01. (PDF 43 kb)
Rights and permissions
About this article
Cite this article
Zhang, R., Hardin, H., Huang, W. et al. Long Non-coding RNA Linc-ROR Is Upregulated in Papillary Thyroid Carcinoma. Endocr Pathol 29, 1–8 (2018). https://doi.org/10.1007/s12022-017-9507-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-017-9507-2