Abstract
This study investigated the impact of phosphohistone-H3 (PHH3)-assisted mitotic count by comparing its performance with conventional mitotic count and Ki67 score as well as the status of distant metastasis. A total of 43 surgically resected pancreatic neuroendocrine tumors (panNET) with complete follow-up information has been subjected to a standardized assessment with respect to mitotic count (both conventional and PHH3-assisted) and Ki67 score. Five participants assessed mitotic count and the time spent was recorded in both methods. All tumors were assigned to a G1 category of mitotic rate on conventional mitotic count that failed to identify three tumors with a G2 category of mitotic rate on PHH3. Near-perfect and fair agreements were achieved among observers when using PHH3 and conventional method, respectively. The mean time spent to determine mitotic count on PHH3-stained slides was significantly shorter (p < 0.001). The performance of PHH3-assisted mitotic grade category was significant as the three cases with a G2 mitotic category were associated with distant metastasis (p = 0.01). Despite its performance, the PHH3-assisted mitotic count downgraded 17 cases that were classified as G2 based on Ki67 scores in this series. The Ki67 grade category was either the same or higher than the mitotic grade category. Ten patients developed distant metastasis. Eleven tumors exhibited vascular invasion characterized by intravascular tumor cells admixed with thrombus. Our results indicate that PHH3-assisted mitotic count facilitates an accurate mitotic count with a perfect agreement among observers. The small size of this cohort is an important limitation of the current study, a G2 mitotic grade category based on PHH3 immunohistochemistry was one of the correlates of panNETs with distant metastasis. While the prognostic impact of PHH3-assisted mitotic count needs to be clarified in larger cohorts, Ki67 scores designated higher grade category in all cases; thus, it was the best determinant of the tumor grade. More importantly, the presence of vascular invasion along with the Ki67 grade category was found to be independent predictors of distant metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 2010 World Health Organization (WHO) classification of gastroenteropancreatic neuroendocrine tumors launched a three-tier grading scheme based on the proliferative activity of the tumor [1]. With the rare exception of well-differentiated neuroendocrine tumors with high grade proliferative features [2], pancreatic neuroendocrine tumors (panNETs) are typically clustered into two grades (G): G1 (mitotic count, <2/10 high power field (HPF), and/or Ki-67 proliferation rate, <3 %) and G2 (mitotic count, 2–20/10 HPF, and/or a Ki67 proliferation rate, 3–20 %). While the past years have seen some debate on the cutoff criteria applied to this grading scheme [3], a formal mitotic count based on 50 HPF at sites of high mitotic density and Ki67 assessment by counting at least 500–2000 tumor cells in hot spots are recommended to determine the appropriate tumor grade in these tumors. If the assigned grade is discordant between the mitotic count and Ki67 score, it is suggested that higher grade category should be assumed [1, 4].
Much has been learned on proliferative characteristics of neuroendocrine tumors. Since these tumors often display an intratumoral proliferative heterogeneity, it is important to identify hot spots (regions with high proliferative features) in order to determine the tumor grade. Recent studies highlighted a lack of interobserver agreement in the selection of hot spots as well as discrepancies between implications of mitotic count and Ki67 score in these tumors [5–10]. The computerized digital software algorithms and manual Ki67 assessment by counting tumor cells on printed images have solved a significant amount of queries related to the evaluation of Ki67 [9, 11, 12]. However, performing a formal mitotic count still remains as a time-demanding process in most practices, as the latter challenges pathologists to identify areas with high mitotic density and distinguish mitotic figures from apoptotic and degenerate tumor cells.
To tackle with some of these issues, anti-phosphohistone-H3 immunohistochemistry has been introduced to serve as a reliable tool to detect mitotic figures. Phosphorylation of the histone-H3 is initiated in the late G2 phase of the cell cycle and is gradually dephosphorylated until the end of mitosis [13, 14]. Phosphohistone-H3 (PHH3)-assisted mitotic count has been found to be useful in multiple tumor types including breast cancer, malignant melanoma, meningioma, adrenal cortical carcinoma, and pulmonary neuroendocrine tumors [15–19]. Recently, this method has also been found useful in the accurate grading of panNETs [10]; however, additional observations regarding its application in neuroendocrine tumors are still required.
By taking into consideration these issues, this study investigated the impact of PHH3-assisted mitotic count by comparing its performance with conventional mitotic count and Ki67 in the determination of tumor grade category as well as prediction of distant metastasis in panNETs.
Material and Methods
Patient Selection
A retrospective review of institutional pathology records at the Department of Pathology, Istanbul Faculty of Medicine, Istanbul University, from 2006 to 2014 revealed 50 in-house pancreatectomy specimens (Whipple resection or distal pancreatectomy) with well-differentiated panNETs. Medical records were retrospectively reviewed with respect to demographic features (age and gender), gross (tumor size) and microscopic findings (vascular invasion, lymphatic invasion, perineural invasion, necrosis, status of resection margins, and status of lymph node involvement; pN), and postoperative follow-up including the status of distant metastasis. All tumors were re-examined to ascertain a standardized application of rigid criteria to diagnose vascular invasion as defined earlier in various endocrine tumors [20–23]. Vascular invasion was rendered when tumor cells invading through a vessel wall and/or intravascular tumor cells admixed with thrombus are noted. All tumors were positive for pankeratin, chromogranin-A, and synaptophysin. Five cases that contained less than 50 high power fields (HPF) and two patients who died of postoperative complications within 2 weeks of surgery were excluded from the study. As a result, a total of 43 tumors with complete follow-up information and significant tumor volume enabling a formal grading have been subjected to a standardized assessment with respect to mitotic count (both conventional and PHH3-assisted) and Ki67 score.
Mitotic Count on Hematoxylin and Eosin (H&E)-Stained Slides
Five authors (MG, GY, GU, SO, OT) screened independently all tumor-containing slides to determine regions of high mitotic density by using a light microscope (Olympus BX51, Tokyo, Japan). Detecting hot spots on H&E-stained slides was problematic as in most of the tumors scattered foci of mitotic figures were noted. Mitoses were counted on 50 consecutive HPF including sites that are individually designated as hot spots. The final mitotic count was determined by the number of mitotic figures per 10 HPF, which contained a total of 2 mm2 of tumor area (×400 = ×40 objective × ×10 eyepiece a diameter of 0.55 mm and an area of 0.237 mm2). One of the authors ran a chronometer during the counting process. Interobserver variability was investigated using the data provided by all five participants. The conventional mitotic count of 2–20 per 10 HPF was considered as a mitotic category of G2 (Fig. 1).
Morphological and immunohistochemical features of two G2 pancreatic neuroendocrine tumors (H&E, Ki67, PHH3). Panels a and d illustrate histomorphological features of the tumors (H&E, Ax200, Dx100). Ki67 immunoreactive cells are shown in b and e, respectively (Ki67, Bx400, Ex200). Panels c and f illustrate mitotic figures highlighted by PHH3 immunohistochemistry (PHH3, Cx400, Fx200)
PHH3-Assisted Mitotic Count
Three micron-thick sections of formalin-fixed, paraffin-embedded tissues were subjected to PHH3 immunohistochemistry (polyclonal antibody, 1/400; Cell Marque, Rocklin, CA; Antigen retrieval: EDTA, pH 8.0) that was performed on a Ventana Benchmark XT automated system (Arizona, USA). Signal was visualized with a 3.3′ diaminobenzidine detection kit. All procedures were performed in accordance with the manufacturer’s instructions. Tonsil tissue was used as the positive control. Non-lesional pancreas adjacent to the tumor served as a negative control.
The same five participants were asked to find hot spots by reviewing the PHH3-stained slides at ×100 magnification; however, mitotic figures were counted at ×400 magnification in order to distinguish tumor cells from non-tumorous cells displaying mitotic figures (i.e., endothelial cells).The total numbers of mitotic figures were recorded in 50 consecutive HPF, including high mitotic density regions, and final mitotic count was determined by the number of mitotic figures per 10 HPF or 2 mm2. The PHH3-assisted mitotic count of 2–20 per 10 HPF was considered as a mitotic category of G2. Interobserver agreement among five participants was subjected to the statistical analysis. Moreover, the time spent while counting mitotic figures on both H&E and PHH3-stained slides were also compared.
Assessment of Ki67 Scores
Ki67 immunohistochemistry (clone MIB-1, 1/100; Cell Dako, Carpinteria, CA, Antigen retrieval: EDTA, pH: 8.0) was performed on a Ventana Benchmark XT automated system (Arizona, USA). Signal was visualized with a 3.3′ diaminobenzidine detection kit. All procedures were performed in accordance with the manufacturer’s instructions. Similar to PHH3 assays, tonsil tissue was used as the positive control. Non-lesional pancreas adjacent to the tumor served as a negative control.
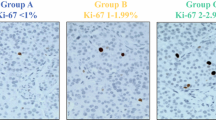
Hot spots were determined by one of the authors (SOS) at ×200 magnification (×20 objective ×10 ocular eyepiece). Subsequently, digital images were captured using an Olympus BX51 microscope and Olympus DP70 camera using the ×40 objective. Three or four photos were captured per tumor, printed in color, and examined by the same author by counting a minimum of 1000 tumor cells. Staining in the tumor cell nuclei and nucleoli were counted as positive, regardless of the intensity of the reactivity. Positive cells were divided by all cells counted and a number as a percentage was calculated. The fractional Ki67 index of 3–20 % was regarded as G2 [4].
Tumor Grade
The higher grade category among Ki67 and mitotic count (both conventional and PHH3-assisted) was assigned as the tumor grade according to the 2010 WHO grading scheme. The data of one of the senior pathologists (MG) were consistently used for all statistical analyses.
Statistical Analyses
All statistical analysis was performed using Statistical Pack for Social Sciences (SPSS) software for Windows version 21.0 (IBM Corp, Armonk, NY, USA). p value less than 0.05 was considered significant in all comparisons. Continuous variables were given as mean ± standard deviation (SD). Comparison between means was performed using non-parametric tests (Mann–Whitney U test or paired- sample t test). The incidences of categorical variables were compared between two groups with Fisher’s exact test and χ 2 test where appropriate. Odds ratio (OR) with 95 % confidence interval (CI) for dichotomous variables were also given. Variables that were found to be significant by univariate tests were included in multivariate forward selection stepwise logistic regression analysis in order to identify the independent variables related to distant metastasis.
The determinants of the tumor grade category (mitotic count and Ki67 score) were subjected for a comparison with respect to the status of distant metastasis. A Kaplan-Meier test was performed for disease-free survival (DFS), comparing survival with the log-rank test. Pairwise Spearman or Pearson correlation test was used to compare conventional mitotic count on H&E-stained slides and PHH3-assisted mitotic count, and also Ki67 scores and the 2010 WHO tumor grades where appropriate. The strength of correlations was defined as follows: weak (r ≥ 0.1 to <0.3), moderate (r ≥ 0.4 to <0.6), strong (r ≥ 0.7 to <0.9), and perfect (0.9–1).
Interobserver agreement between observers was evaluated using intraclass correlation coefficient (ICC) for mitotic counts (both conventional and PHH3-assisted), and Fleiss’ kappa to measure the agreement of the corresponding grades (kappa = 0–0.2, “slight”; kappa = 0.2–0.4, “fair”; kappa = 0.4–0.6, “moderate”; kappa = 0.6–0.8, “substantial”; and kappa = 0.8–1, “almost perfect” agreement).
The status of pN was not subjected to statistical analyses as only 20 specimens had limited or selective lymph node sampling.
Results
Demographic, Clinical, and Pathological Features
A total of 43 patients were included in the study for final analysis. Among these patients, 13 (30.2 %) were men and 30 (69.8 %) were women with a mean age of 50.2 (±13.5) years, ranging between 26 and 75 years. Follow-up information regarding the overall survival (OS) and DFS was available in all patients. Only one patient died of disease. The OS range was 10 to 100 months with a median of 40 months; DFS ranged from 0 to 100 months with a median of 60 months. Among the 43 patients, 10 (23.3 %) developed distant metastasis, either at the time of the operation or during the follow-up period. Thirty-three (76.7 %) patients remained negative for distant metastasis. The status of pN was available in 20 specimens, and only 4 of 20 panNETs had lymph node metastasis. The demographic and pathological findings of the metastatic and non-metastatic tumor groups were summarized in Table 1.
The mean tumor size was 4.08 ± 3.53 cm in the entire cohort. In contrast to panNETs without distant metastasis (mean tumor size, 3.11 ± 2.37 cm), panNETs with distant metastasis (mean tumor size, 7.29 ± 4.83 cm) had a larger tumor size (p = 0.001). Eleven (25.6 %) panNETs exhibited vascular invasion characterized by intravascular tumor cells admixed with thrombus. Seven (63.6 %) angioinvasive well-differentiated panNETs developed distant metastasis (p = 0.001). Tumor necrosis was noted only in two panNETs with distant metastasis. Perineural invasion was present in seven (16.3 %) panNETs. The resection margins were intact in 37 (86 %) specimens.
The frequency of vascular invasion, perineural invasion, and surgical margin involvement was significantly higher in the distant metastatic group when compared with the non-metastatic group (p < 0.05) (Table 1).
Conventional Mitotic Count on H&E-Stained Slides
While the numeric data differed among observers, all tumors (100 %) were assigned to a G1 category of mitotic rate (<2 mitoses per 10 HPF) (Table 2). The mean mitotic count was 0.16 ± 0.30 per 10 HPF (range, 0–1.2) on H&E-stained slides. The mean mitotic count was higher in the metastatic group (metastatic tumors, 0.46 ± 0.44; non-metastatic tumors, 0.08 ± 0.17) (p = 0.003). No comparisons could be performed between metastatic and non-metastatic groups because all tumors were clustered within the G1 mitotic category. Interobserver agreement was fair (ICC kappa score = 0.593) for this evaluation. The mean time spent counting mitotic figures was 359.33 ± 74.11 seconds.
PHH3-Assisted Mitotic Count
The mean mitotic count per 10 HPF determined using PHH3 immunohistochemistry was higher (0.52 ± 1.37; range, 0–5.6) than conventional methods (0.16 ± 0.30; range, 0–1.2). However, this was not statistically different (p = 0.62). Unlike conventional mitotic count, three (7 %) tumors were assigned to a G2 mitotic category. The mean PHH3-assisted mitotic count of the metastatic group (1.6 ± 0.26) was not higher than that of the non-metastatic group (0.18 ± 0.24) (p > 0.05). However, the performance of PHH3-assisted mitotic grade category was significant as the three cases with a G2 mitotic category were associated with distant metastasis (p = 0.01) (Table 1).
The interobserver reproducibility was obviously improved and near-perfect agreement was achieved (ICC kappa score = 0.947) when using PHH3 immunohistochemistry. Perfect agreement (Fleiss’ kappa score = 1) was also achieved when grade assignments were based on PHH3-assisted categorization.
The mean time spent to determine mitotic count on PHH3-stained slides (183.88 ± 60.85 seconds) was significantly shorter than that of the conventional method (359.33 ± 74.11 seconds) (p < 0.001).
Ki67 Score
A mean of 1691 tumor cells (range, 1038–2714 cells) were counted. The mean Ki67 score was 3.47 ± 3.05. When Ki67 scores were assigned to a Ki67 grade category, 22 (51 %) and 21 (49 %) cases fell into G1 and G2, respectively. The mean Ki67 score of the metastatic group (5.90 ± 3.31) was significantly higher than that of the non-metastatic group (2.73 ± 2.58) (p = 0.004). Consistently, a Ki67 score of G2 category was also associated with a higher frequency of distant metastasis (8/20, 40 %) than those with a Ki67 score of G1 category (2/23, 8.7 %) (p = 0.028).
Tumor Grade and Performance of Mitotic Count and Ki67 Grade Categories
In this series, 23 and 20 panNETs were classified as G1 and G2, respectively. The Ki67 scores and tumor grade along with conventional and PHH3-assisted mitotic grade categories are shown in Table 2. Comparisons between the categories of conventional mitotic grade, PHH3-assisted mitotic grade, and Ki67 grade were not possible because all cases were allocated to the G1 category when using conventional mitotic count. While a moderate correlation between conventional mitotic count and Ki67 score (r = 0.436) was noted, the PHH3-assisted mitotic count showed a weak correlation with the conventional mitotic count and Ki67 score (r = 0.204, r = 0.220; respectively). A weak correlation was detected between the tumor grade based on PHH3-assisted mitotic count and Ki67 score (r = 0.294).
A G2 mitotic grade category based on PHH3 immunohistochemistry identified tumors associated with distant metastasis with a sensitivity of 30 % and specificity of 100 %. The positive predictive value was 100 % and the negative predictive value was 82.5 %. Grading panNETs based on their Ki67 score predicted metastasis with the sensitivity of 80 % and the specificity of 63.6 %. The positive predictive value was 40 % and the negative predictive value was 91.3 %.
DFS was longer in the G1 tumor group (determined by Ki67 score) than that of G2 (p= 0.028).
Correlates of Distant Metastasis
Among the factors shown to have significant correlation with distant metastasis by univariate analysis (tumor size, vascular invasion, perineural invasion, the status of surgical margin, Ki67 grade category, and PHH3 grade category), presence of vascular invasion and the Ki67 grade category were the factors found to be significantly and independently related to the presence of distant metastasis in multivariate analysis (p = 0.03 and p = 0.004, respectively).
Discussion
This study investigated the impact of PHH3-assisted mitotic count by comparing its performance with conventional mitotic count and Ki67 score as well as the status of distant metastasis.
The paucity of mitotic figures in well-differentiated neuroendocrine tumors, intratumoral proliferative heterogeneity, challenges related to the identification of hot spots, and difficulty to distinguish mitotic figures from apoptotic cells, time demanding nature of the process, interobserver variability, and suboptimal sections due to technical artifacts have been major drawbacks of determining mitotic activity [5–10]. PHH3-assisted mitotic count has been found to be useful in multiple tumor types including breast cancer, malignant melanoma, meningioma, adrenal cortical carcinoma, and pulmonary neuroendocrine tumors [15–19]. To our knowledge, there is only a single cohort that investigated the role of PHH3-assisted grading in surgically resected panNETs [10]; however, additional observations regarding the application of this ancillary tool are still required.
In this study, the grading performance of PHH3-assisted mitotic count was found to be superior to conventional mitotic count that failed to appropriately categorize three panNETs. This series found that the PHH3-assisted mitotic count was highly specific for predicting metastasis as all three patients with a G2 mitotic grade based on PHH3 immunohistochemistry developed distant metastasis, including a single patient who died of disease. However, these results should be interpreted with caution given the relatively small size of our series. Interestingly, Voss et al. [10] also highlighted that the use of PHH3 had a significant value in grading panNETs and predicting aggressive behavior.
It is important to emphasize that PHH3-assisted mitotic count provided better interobserver agreement and better identification of hot spots in the current study. Voss et al. [10] defined a hot spot as “an area at 100× magnification that contains at least 7 mitoses” and reported that the presence of even a single hot spot had excellent correlation with aggressive behavior. They suggested determination of mitotic figures and hot spots at ×100 magnification. However, the current study failed to undertake this approach as matching mitotic figures to tumor cells and distinguishing artifactual staining required an assessment at ×400 magnification.
Owing to the better identification of mitotic cells using PHH3 immunohistochemistry, the mean mitotic count was found to be higher than the mean of conventional mitotic count in our cohort. Voss et al. [10] provided similar results and proposed a higher mitotic cut-off point (4 per 10 HPF) than that of the 2010 WHO grading scheme. Even though we used the cut-off point of 2 per 10 HPF as suggested by the WHO grading scheme, the three panNETs assigned to a G2 mitotic grade category revealed a mitotic count greater than 4 per 10 HPF. While the past years have seen some debate on the cut-off criteria applied to the WHO grading scheme [3], we believe additional observations on larger cohorts with longer follow-up periods are needed to refine the validity of such an approach.
Despite its performance, the PHH3-assisted mitotic count downgraded 17 cases that were classified as G2 based on Ki67 scores in this series. The Ki67 score was either the same or higher than the mitotic grade category in this study. Earlier reports also showed that grades assigned based on conventional mitotic count were generally lower than the grades using Ki67 scores [5, 6, 8]. In contrast to PHH3 immunohistochemistry, Ki67 highlights cells in all active phases of cell cycle as well as the cells in mitosis (M phase) [24]. The cases in which PHH3 mitotic count predicted aggressive behavior with perfect specificity were also cases with higher Ki67 scores; however, the mitotic count had limited prognostic value in cases with low Ki67 scores. As expected, the number of cells in all active phases (G1, S, G2, and M) of cell cycle is much higher than that of the cells in the M phase. As a result, low grade tumors with very low number of Ki67 positive cells contain so scarce mitotic figures that it may be difficult to find without PHH3 immunohistochemistry. Therefore, it is not surprising that Ki67 yields a better overall performance than mitotic count in general and it has been shown to be an independent prognostic marker in large cohorts of panNETs [25–27].
Our results also expand this data by showing that a longer DFS is better predicted by accurate tumor grading, which is mainly determined by Ki67 grade category. While the evaluation of Ki67 scores is not uniform among practicing pathologists, the computerized digital software algorithms and manual Ki67 assessment by counting tumor cells on printed images (as in this series) have solved a significant amount of queries related to this issue [9, 11, 12]
Since the tumor grade impacts the management of patients with panNETs, it is important to accurately grade these tumors. Our results indicate that PHH3-assisted mitotic count facilitates an accurate mitotic count with a perfect agreement among observers. The small size of this cohort is an important limitation of the current study, a G2 mitotic grade category based on PHH3 immunohistochemistry was one of the correlates of panNETs with distant metastasis. While the prognostic impact of PHH3-assisted mitotic count needs to be clarified in larger cohorts, Ki67 scores designated higher grade category in all cases; thus, the latter was the best determinant of the tumor grade. More importantly, the presence of vascular invasion along with the Ki67 grade category was found to be independent predictors of distant metastasis.
References
Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO Classification of Tumours of the Digestive System. IARC Lyon, France
Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, Krasinskas AM, Jang KT, Frankel WL, Balci S, Sigel C, Klimstra DS (2015) The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol 39 (5):683–690. doi:10.1097/pas.0000000000000408
Reid MD, Balci S, Saka B, Adsay NV (2014) Neuroendocrine tumors of the pancreas: current concepts and controversies. Endocr Pathol 25 (1):65–79. doi:10.1007/s12022-013-9295-2
Yang Z, Tang LH, Klimstra DS (2013) Gastroenteropancreatic neuroendocrine neoplasms: historical context and current issues. Semin Diagn Pathol 30 (3):186–196. doi:10.1053/j.semdp.2013.06.005
Goodell PP, Krasinskas AM, Davison JM, Hartman DJ (2012) Comparison of methods for proliferative index analysis for grading pancreatic well-differentiated neuroendocrine tumors. Am J Clin Pathol 137 (4):576–582. doi:10.1309/AJCP92UCXPJMMSDU
Khan MS, Luong TV, Watkins J, Toumpanakis C, Caplin ME, Meyer T (2013) A comparison of Ki-67 and mitotic count as prognostic markers for metastatic pancreatic and midgut neuroendocrine neoplasms. Br J Cancer 108 (9):1838–1845. doi:10.1038/bjc.2013.156
Klimstra DS (2013) Pathology reporting of neuroendocrine tumors: essential elements for accurate diagnosis, classification, and staging. Semin Oncol 40 (1):23–36. doi:10.1053/j.seminoncol.2012.11.001
McCall CM, Shi C, Cornish TC, Klimstra DS, Tang LH, Basturk O, Mun LJ, Ellison TA, Wolfgang CL, Choti MA, Schulick RD, Edil BH, Hruban RH (2013) Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol 37 (11):1671–1677. doi:10.1097/PAS.0000000000000089
Tang LH, Gonen M, Hedvat C, Modlin IM, Klimstra DS (2012) Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: a comparison of digital image analysis with manual methods. Am J Surg Pathol 36 (12):1761–1770. doi:10.1097/PAS.0b013e318263207c
Voss SM, Riley MP, Lokhandwala PM, Wang M, Yang Z (2015) Mitotic count by phosphohistone H3 immunohistochemical staining predicts survival and improves interobserver reproducibility in well-differentiated neuroendocrine tumors of the pancreas. Am J Surg Pathol 39 (1):13–24. doi:10.1097/pas.0000000000000341
Papathomas TG, Pucci E, Giordano TJ, Lu H, Duregon E, Volante M, Papotti M, Lloyd RV, Tischler AS, van Nederveen FH, Nose V, Erickson L, Mete O, Asa SL, Turchini J, Gill AJ, Matias-Guiu X, Skordilis K, Stephenson TJ, Tissier F, Feelders RA, Smid M, Nigg A, Korpershoek E, van der Spek PJ, Dinjens WN, Stubbs AP, de Krijger RR (2015) An International Ki67 Reproducibility Study in Adrenal Cortical Carcinoma. Am J Surg Pathol. doi:10.1097/pas.0000000000000574
Reid MD, Bagci P, Ohike N, Saka B, Erbarut Seven I, Dursun N, Balci S, Gucer H, Jang KT, Tajiri T, Basturk O, Kong SY, Goodman M, Akkas G, Adsay V (2015) Calculation of the Ki67 index in pancreatic neuroendocrine tumors: a comparative analysis of four counting methodologies. Mod Pathol 28 (5):686–694. doi:10.1038/modpathol.2014.156
Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106 (6):348–360
Juan G, Traganos F, James WM, Ray JM, Roberge M, Sauve DM, Anderson H, Darzynkiewicz Z (1998) Histone H3 phosphorylation and expression of cyclins A and B1 measured in individual cells during their progression through G2 and mitosis. Cytometry 32 (2):71–77
Skaland I, Janssen EA, Gudlaugsson E, Klos J, Kjellevold KH, Soiland H, Baak JP (2009) Validating the prognostic value of proliferation measured by Phosphohistone H3 (PPH3) in invasive lymph node-negative breast cancer patients less than 71 years of age. Breast Cancer Res Treat 114 (1):39–45. doi:10.1007/s10549-008-9980-x
Tsuta K, Liu DC, Kalhor N, Wistuba, II, Moran CA (2011) Using the mitosis-specific marker anti-phosphohistone H3 to assess mitosis in pulmonary neuroendocrine carcinomas. Am J Clin Pathol 136 (2):252–259. doi:10.1309/ajcpdxfopxgef0rp
Nielsen PS, Riber-Hansen R, Jensen TO, Schmidt H, Steiniche T (2013) Proliferation indices of phosphohistone H3 and Ki67: strong prognostic markers in a consecutive cohort with stage I/II melanoma. Mod Pathol 26 (3):404–413. doi:10.1038/modpathol.2012.188
Ribalta T, McCutcheon IE, Aldape KD, Bruner JM, Fuller GN (2004) The mitosis-specific antibody anti-phosphohistone-H3 (PHH3) facilitates rapid reliable grading of meningiomas according to WHO 2000 criteria. Am J Surg Pathol 28 (11):1532–1536
Duregon E, Molinaro L, Volante M, Ventura L, Righi L, Bolla S, Terzolo M, Sapino A, Papotti MG (2014) Comparative diagnostic and prognostic performances of the hematoxylin-eosin and phospho-histone H3 mitotic count and Ki-67 index in adrenocortical carcinoma. Mod Pathol 27 (9):1246–1254. doi:10.1038/modpathol.2013.230
Erovic BM, Harris L, Jamali M, Goldstein DP, Irish JC, Asa SL, Mete O (2012) Biomarkers of parathyroid carcinoma. Endocr Pathol 23 (4):221–231. doi:10.1007/s12022-012-9222-y
Mete O, Asa SL (2011) Pathological definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivation. Mod Pathol 24 (12):1545–1552. doi:10.1038/modpathol.2011.119
Erovic BM, Kim D, Cassol C, Goldstein DP, Irish JC, Asa SL, Mete O (2012) Prognostic and predictive markers in medullary thyroid carcinoma. Endocr Pathol 23 (4):232–242. doi:10.1007/s12022-012-9225-8
Araujo PB, Cheng S, Mete O, Serra S, Morin E, Asa SL, Ezzat S (2013) Evaluation of the WHO 2010 grading and AJCC/UICC staging systems in prognostic behavior of intestinal neuroendocrine tumors. PLoS One 8 (4):e61538. doi:10.1371/journal.pone.0061538
Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182 (3):311–322. doi:10.1002/(sici)1097-4652(200003)182:3<311::aid-jcp1>3.0.co;2–9
Ellison TA, Wolfgang CL, Shi C, Cameron JL, Murakami P, Mun LJ, Singhi AD, Cornish TC, Olino K, Meriden Z, Choti M, Diaz LA, Pawlik TM, Schulick RD, Hruban RH, Edil BH (2014) A single institution's 26-year experience with nonfunctional pancreatic neuroendocrine tumors: a validation of current staging systems and a new prognostic nomogram. Ann Surg 259 (2):204–212. doi:10.1097/SLA.0b013e31828f3174
Scarpa A, Mantovani W, Capelli P, Beghelli S, Boninsegna L, Bettini R, Panzuto F, Pederzoli P, delle Fave G, Falconi M (2010) Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol 23 (6):824–833. doi:10.1038/modpathol.2010.58
Martin-Perez E, Capdevila J, Castellano D, Jimenez-Fonseca P, Salazar R, Beguiristain-Gomez A, Alonso-Orduna V, Martinez Del Prado P, Villabona-Artero C, Diaz-Perez JA, Monleon A, Marazuela M, Pachon V, Sastre-Valera J, Sevilla I, Castano A, Garcia-Carbonero R (2013) Prognostic factors and long-term outcome of pancreatic neuroendocrine neoplasms: Ki-67 index shows a greater impact on survival than disease stage. The large experience of the Spanish National Tumor Registry (RGETNE). Neuroendocrinology 98 (2):156–168. doi:10.1159/000355152
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
None
Additional information
Dr. Mine Gulluoglu and Dr. Ozgur Mete are co-senior authors in this manuscript.
Rights and permissions
About this article
Cite this article
Ozturk Sari, S., Taskin, O.C., Gundogdu, G. et al. The Impact of Phosphohistone-H3-Assisted Mitotic Count and Ki67 Score in the Determination of Tumor Grade and Prediction of Distant Metastasis in Well-Differentiated Pancreatic Neuroendocrine Tumors. Endocr Pathol 27, 162–170 (2016). https://doi.org/10.1007/s12022-016-9424-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-016-9424-9