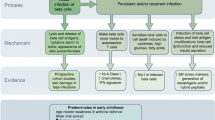
Abstract
There is extensive epidemiological evidence highlighting a possible aetiological role for enteroviral infection in type 1 diabetes. Direct evidence of the presence of enterovirus in the pancreatic islets of type 1 diabetics is, however, limited, due mainly to the paucity of samples from patients diagnosed recently with the disease. This chapter summarises the evidence implicating enteroviral infection in the human pancreas in type 1 diabetes and considers both factors indicating the presence of virus (viral capsid protein, electron microscopic visualisation of viral particles or expression of viral RNA) and the host response to a viral infection (a “viral footprint”). The relationship of these two indicators of viral infection with two apparently different forms of type 1 diabetes (autoimmune versus fulminant) is discussed. It is hypothesised that differing host responses, and perhaps different genetic variation among the viruses involved, may determine whether an enteroviral infection of beta cells causes (1) A rapid lytic cell death—characteristic of fulminant type 1 diabetes and neonatalcoxsackievirus infection, (2) A persistent infection which evokes an autoimmune reaction to beta cells, eventually resulting in their destruction—autoimmune type 1 diabetes, (3) h little host response and little damage.
Overall, it is concluded that the evidence for viral involvement is persuasive but that there is still a long way to go in order to elucidate the precise role of enteroviral infections in the aetiology of type 1 diabetes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Mounting epidemiological evidence has implicated viruses, particularly (but not exclusively) enteroviruses, in the pathogenesis of type 1 diabetes (Andreoletti et al. 1997; Clements et al. 1995; Coutant et al. 2002; Lonnrot et al. 2000; Moya-Suri et al. 2005; Nairn et al. 1999; Richardson et al. 2011; Sarmiento et al. 2007; Schulte et al. 2010; Tauriainen et al. 2011; Yin et al. 2002). However, it is unclear whether viral infection plays a role in the initial development of beta cell autoimmunity or if it accelerates the progression of a pre-existing autoimmune reaction. Indeed, both possibilities may be true depending on the circumstances. In this chapter, we review the evidence implicating viral infection within the pancreas as a causative factor in human type 1 diabetes.
Type 1 diabetes results from destruction of insulin-secreting beta cells in the islets of Langerhans and can occur in two forms. Most commonly, an autoimmune reaction proceeds over a protracted time course leading to the onset of clinical symptoms when ∼75% of beta cells have been destroyed. By contrast, a second form of the disease has been described, principally in Japanese populations, in which the process of beta cell destruction occurs much more quickly, leading to “fulminant” type 1 diabetes. In both cases there is increasing evidence that enteroviral infection may underlie the process of beta cell destruction, but the role of the virus appears to be quite different in these two forms of the disease (Dotta et al. 2007; Richardson et al. 2009; Shibasaki et al. 2010; Tanaka et al. 2009).
In order to examine the relationships between enteroviral infection and the pathogenesis of type 1 diabetes, it is important to conduct studies of the target organ (i.e. the pancreas). Such studies have revealed that an early event within the pancreatic islets in autoimmune diabetes is the expression of interferon-alpha by insulin-secreting beta cells (Foulis et al. 1987b). Interferon-alpha expression is closely associated with Class I MHC hyperexpression by the endocrine cells of the affected islet, a hallmark feature of type 1 diabetes (Bottazzo et al. 1985; Foulis et al. 1987a; Pujol-Borrell et al. 1986). Later there is an infiltrate into the islet of immune cells (insulitis), dominated by cytotoxic CD8+ T lymphocytes, with smaller numbers of B lymphocytes, CD4+ cells and macrophages (Willcox et al. 2009). Beta cells are destroyed within the inflamed islet and, following their loss, the inflammatory cell infiltrate disappears, leaving an insulin-deficient islet containing glucagon-secreting alpha cells, somatostatin-secreting delta cells and pancreatic polypeptide-secreting PP cells, which have not been harmed (Foulis and Stewart 1984). The whole process in the pancreas, from the first to last beta cell being destroyed, is thought to take many months or even years in autoimmune diabetes.
By contrast, in fulminant diabetes, there is a diffuse infiltrate of inflammatory cells within the pancreas resulting in exocrine inflammation (and clinical acute pancreatitis) as well as islet inflammation. The predominant infiltrating cell type in the islets in fulminant type 1 diabetes is the macrophage, closely followed by CD8+ T cells (Shibasaki et al. 2010; Tanaka et al. 2009), and the inflammatory process occurs rapidly with most beta cells (and many alpha cells) being destroyed in a matter of days or weeks (Shibasaki et al. 2010; Tanaka et al. 2009). Within 1 month, the insulitis declines and, after this time, evidence of immune cell infiltration is rarely detected in pancreas biopsy samples.
The presence of interferon-alpha in beta cells in autoimmune type 1 diabetes (Foulis et al. 1987b) and in fulminant type 1 diabetes (Aida et al. 2011) is consistent with the hypothesis that the beta cells might be infected by a virus, as viral infection stimulates beta cells to secrete this cytokine (Chehadeh et al. 2000). Equally, the appearance of massive rapid destruction of the beta cells in fulminant diabetes led some to hypothesise that this was due to a widespread lytic infection by a virus (Foulis et al. 1988). Examination of the literature reveals, however, only limited evidence of direct infection of pancreatic beta cells in patients with type 1 diabetes. At first sight, this appears problematic to the viral hypothesis but, in reality, the paucity of data relates to a still more fundamental problem; namely that pancreatic tissue obtained from patients diagnosed recently with type 1 diabetes is available in only very limited quantity. By contrast, tissue from patients with longer disease duration can be obtained more readily, but this is less profitable to study since, as described above, the pancreas becomes largely depleted of beta cells over the course of time and many of the clues which might hint at the cause of their destruction are lost. Despite these constraints, evidence has been marshalled which supports a viral aetiology for type 1 diabetes.
In the late 1970s, two case studies were published which documented the development of acute-onset diabetes in each of two children who had been infected with a coxsackievirus of the B4 serotype (CVB4) (Gladisch et al. 1976; Yoon et al. 1979). Gladisch et al. (1976) confirmed the presence of virus in the pancreas using FITC-labelled CVB antibody and they also noted extensive insulitis and lysis of islet cells. In the second study, Yoon et al. prepared pancreas homogenates from a diabetic patient and inoculated mouse, monkey and human cell cultures with these extracts. They were then able to isolate and type an enterovirus and found that, again, this was classified as the CVB4 serotype. As in the case studied by Gladisch et al. (1976), analysis of the pancreas revealed evidence of islet inflammation and lysis of beta cells. When used in subsequent mouse studies, the virus isolated by Yoon et al. (1979) was capable of infecting beta cells and causing islet inflammation. This was accompanied by beta cell necrosis and the development of hyperglycaemia in infected mice (Yoon et al. 1979).
Such studies prompted others to search for evidence of the presence of enteroviruses in the pancreases of more patients with type 1 diabetes. In a very comprehensive immunohistochemical analysis of formalin-fixed paraffin-embedded pancreases by Foulis et al. in 1990, 88 patients with recent-onset type 1 diabetes were studied. The investigators sought evidence for the presence of the highly conserved enteroviral capsid protein, VP1, but failed to detect any positive staining among the samples (Foulis et al. 1990). This outcome was especially unexpected given that the technique used was able to detect enteroviral VP1+ cells in the heart and pancreas of neonates who had died of culture proven coxsackie viral myocarditis, which suggested a sensitivity for the immunohistochemical technique similar to that of viral culture from autopsy tissue. This group went on to extract DNA from the samples of diabetic pancreases and performed PCR looking for viral-specific sequences. They also looked for evidence of enteroviral RNA by in situ hybridisation (Foulis et al. 1997). Amplification of extracted DNA from 47 of the patients with primers designed to detect Epstein–Barr virus or cytomegalovirus did not reveal any positive signals. Non-radioactive in situ hybridisation with enteroviral probes revealed infection in coxsackie-infected neonatal pancreas, but no positive signal was observed in the 29 pancreas samples of diabetic patients in which good RNA preservation was established by the detection of insulin mRNA. Subsequently, however, the use of a radioactive enteroviral-specific in situ hybridisation probe did reveal the presence of enteroviral RNA in 4 out of 65 diabetic pancreas samples (Ylipaasto et al. 2004), suggesting that detection of virus in autopsy diabetic pancreases may be possible with more sensitive techniques.
Recently, new antibodies with improved detection efficiency for enteroviral VP1 (notably the 5D8/1 clone marketed by Dako), coupled with the development of heat-induced antigen retrieval (HIER) techniques for immunohistochemistry (which enables antigens that were previously masked to be revealed), have improved the sensitivity of virus detection in fixed archival tissues. Dotta et al. (2007) reported evidence of enteroviral VP1 in the pancreases of two recent-onset type 1 diabetes patients and in a third patient who had undergone whole pancreas graft. The immunohistochemical evidence of VP1 staining was supported by the isolation of a virus (once again belonging to the CVB4 serotype) from the pancreas of one of the patients studied. The VP1 staining was largely confined to beta cells and viral particles were observed in the cytoplasm of these cells when examined under the electron microscope. More importantly, it was revealed that the viral isolate was capable of infecting human islets cultured in vitro and that this resulted in an impairment of insulin secretion (without any change in islet insulin content). This implies that enteroviral infection can lead to functional impairment of beta cells under conditions where the virus does not induce specific beta cell cytotoxicity. In summarising these important findings, it is also worth noting that the virus isolated by Dotta et al. had a high degree of homology with a laboratory reference strain dating from a much earlier period, which has led some to question its provenance (Tracy et al. 2010).
The advent of improved techniques for enteroviral detection in formalin-fixed paraffin-embedded tissue allowed our group to revisit the original collection of recent-onset type 1 diabetic pancreases studied by Foulis et al. (Richardson et al. 2009). Whereas these had previously been thought to be negative for viral protein, we found evidence of enteroviral VP1 immunopositivity in the pancreas of 44 of 72 (61%) of patients. This compared with a total of only 4 positive endocrine cells detected in the pancreas of 3 of 39 (7.7%) non-diabetic paediatric cases. Serial sections showed that the VP1 staining was restricted to insulin-containing islets (ICIs) (insulin-deficient islets being negative), and dual immunofluorescence demonstrated that VP1 was confined to beta cells within these islets (Richardson et al. 2009; Willcox et al. 2011).
Virally infected cells can be expected to mount a specific pattern of molecular responses, the function of which is to minimise the impact of the infection on the cell by, for example, shutting down host protein synthesis. Alteration of the expression of certain markers in a cell may therefore act as a “footprint”, indicating the likely presence of a virus within the cell. One such marker, the inducible pathogen recognition receptor, protein kinase R (PKR) was found to be frequently present in VP1+ islets (Richardson et al. 2009), and more recent work has confirmed that VP1 and PKR co-localise in the beta cells of patients with type 1 diabetes (Richardson, unpublished observations, Fig. 17.1). Interestingly, the level of VP1 expression is much lower in patients with autoimmune type 1 diabetes (Fig. 17.1e) than in those with acute systemic enterovirus infection (Fig. 17.1b). In the autoimmune type 1 cases, VP1 strongly correlates with PKR expression (Fig. 17.1e–f; Richardson unpublished results), which is unlike the situation in the acutely infected pancreas where more VP1+ cells are observed and there appears to be no correlation with PKR (Fig. 17.1b, c).
Immunohistochemical staining for Class I MHC (a and d), VP1 (mAb 5D8/1; b and e) and PKR (c and f) in neonatal coxsackie-infected pancreas (a–c) and serial sections of an autoimmune type 1 diabetes pancreas (d–f). Further staining for CD45 (g) is demonstrated in autoimmune type 1 diabetes. Although both the neonatal coxsackie-infected pancreas and the autoimmune type 1 diabetes pancreas express enteroviral VP1, the islet-specific hyperexpression of Class I MHC and the beta cell selective up-regulation of PKR only occur in autoimmune type 1 diabetes. Endothelial cell and lymphocyte-specific Class I MHC staining is observed in the coxsackie-infected pancreas (a), but the endocrine cells remain largely unstained
Interferon-alpha and hyperexpression of Class I MHC are not present in the islets of non-diabetic controls but are each observed at clinical onset and years after the onset of autoimmune type 1 diabetes, and these markers may form part of a “viral footprint” (Bottazzo et al. 1985; Foulis et al. 1987a; Oikarinen et al. 2008) present in infected islets. However, only a small number of the beta cells within such islets also express enteroviral VP1, suggesting that there may be an underlying persistent enteroviral infection in cells that express little or no VP1 protein, but which can still drive the expression of interferon-alpha and the hyperexpression of Class I MHC. In support of this, enteroviruses can establish persistent infections in human tissues and, when this happens, it is thought that the virus exists in a different but stable molecular form, possibly as double-stranded RNA (Cunningham et al. 1990; Tam and Messner 1999). Under these circumstances VP1 is less likely to be expressed (Klingel et al. 1992), but it seems probable that the occasional islet cells which do express VP1 may represent only the “tip of the iceberg” amongst numerous adjacent beta cells that harbour a more persistent infection.
In fulminant type 1 diabetes, high levels of expression of VP1 were detected in the islets at early time points following disease onset suggesting that the induction of this form of diabetes may result from an acute lytic infection of islet cells leading to damage of the surrounding tissue and promoting a generalised inflammatory response. Thus in this regard, fulminant type 1 diabetes has strong similarities to the acutely infected neonates who died of viral myocarditis (Table 17.1, Fig. 17.1). It is important to emphasise, however, that the number of fulminant diabetes patients examined for VP1 expression remains small with viral protein having been detected in three of the three cases studied (Aida et al. 2011; Tanaka et al. 2009) while enteroviral RNA was found in one of the three cases (Shibasaki et al. 2010); so further studies are necessary to confirm these findings.
It’s All in the Order
As noted previously, it is not known whether an enteroviral infection precedes the onset of autoimmunity or whether it accelerates the disease process once autoimmunity is established. The latter appears to be the case in the NOD mouse model, where a threshold of established insulitis must be present in order that enteroviral infection can hasten the onset of diabetes (Drescher et al. 2004). In humans the immunopathological evidence seems to suggest that the order is subtly different as islets from patients with recent-onset autoimmune type 1 diabetes can be found that are completely devoid of immune cell infiltration, but with evidence of hyperexpression of Class I MHC, expression of interferon-alpha and VP1 protein (Foulis et al. 1987b; Richardson et al. 2009). The detection of VP1 protein in islets in the absence of any signs of insulitis in an autoantibody positive case has also been documented (Oikarinen et al. 2008). Therefore, more research is required in humans to determine whether a viral infection is the “chicken or the egg” in terms of immune-mediated beta cell destruction.
There is still a long way to go in order to elucidate the precise role of enteroviral infections in the aetiology of type 1 diabetes. It appears though that different host responses, and perhaps different genetic variants among the viruses involved, may determine whether an enteroviral infection of beta cells causes (1) rapid lytic cell death, (2) a persistent infection which evokes an autoimmune reaction to beta cells, eventually resulting in their destruction, or (3), a persistent infection with little host response and little damage done.
References
Aida K, Nishida Y, Tanaka S, Maruyama T, Shimada A, Awata T, Suzuki M, Shimura H, Takizawa S, Ichijo M, Akiyama D, Furuya F, Kawaguchi A, Kaneshige M, Itakura J, Fujii H, Endo T, Kobayashi T (2011) RIG-I- and MDA5-initiated innate immunity linked with adaptive immunity accelerates beta-cell death in fulminant type 1 diabetes. Diabetes 60:884–889
Andreoletti L, Hober D, Hober-Vandenberghe C, Belaich S, Vantyghem MC, Lefebvre J, Wattre P (1997) Detection of coxsackie B virus RNA sequences in whole blood samples from adult patients at the onset of type I diabetes mellitus. J Med Virol 52:121–127
Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR (1985) In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med 313:353–360
Chehadeh W, Kerr-Conte J, Pattou F, Alm G, Lefebvre J, Wattre P, Hober D (2000) Persistent infection of human pancreatic islets by coxsackievirus B is associated with alpha interferon synthesis in beta cells. J Virol 74:10153–10164
Clements GB, Galbraith DN, Taylor KW (1995) Coxsackie B virus infection and onset of childhood diabetes. Lancet 346:221–223
Coutant R, Carel JC, Lebon P, Bougneres PF, Palmer P, Cantero-Aguilar L (2002) Detection of enterovirus RNA sequences in serum samples from autoantibody-positive subjects at risk for diabetes. Diabet Med 19:968–969
Cunningham L, Bowles NE, Lane RJ, Dubowitz V, Archard LC (1990) Persistence of enteroviral RNA in chronic fatigue syndrome is associated with the abnormal production of equal amounts of positive and negative strands of enteroviral RNA. J Gen Virol 71:1399–1402
Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P (2007) Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci USA 104:5115–5120
Drescher KM, Kono K, Bopegamage S, Carson SD, Tracy S (2004) Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology 329:381–394
Foulis AK, Stewart JA (1984) The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia 26:456–461
Foulis AK, Farquharson MA, Hardman R (1987a) Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 30:333–343
Foulis AK, Farquharson MA, Meager A (1987b) Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet 2:1423–1427
Foulis AK, Francis ND, Farquharson MA, Boylston A (1988) Massive synchronous B-cell necrosis causing type 1 (insulin-dependent) diabetes—a unique histopathological case report. Diabetologia 31:46–50
Foulis AK, Farquharson MA, Cameron SO, McGill M, Schonke H, Kandolf R (1990) A search for the presence of the enteroviral capsid protein VP1 in pancreases of patients with type 1 (insulin-dependent) diabetes and pancreases and hearts of infants who died of coxsackieviral myocarditis. Diabetologia 33:290–298
Foulis AK, McGill M, Farquharson MA, Hilton DA (1997) A search for evidence of viral infection in pancreases of newly diagnosed patients with IDDM. Diabetologia 40:53–61
Gladisch R, Hofmann W, Waldherr R (1976) Myocarditis and insulitis following coxsackie virus infection. Z Kardiol 65:837–849
Klingel K, Hohenadl C, Canu A, Albrecht M, Seemann M, Mall G, Kandolf R (1992) Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci USA 89:314–318
Lonnrot M, Salminen K, Knip M, Savola K, Kulmala P, Leinikki P, Hyypia T, Akerblom HK, Hyoty H (2000) Enterovirus RNA in serum is a risk factor for beta-cell autoimmunity and clinical type 1 diabetes: a prospective study. Childhood Diabetes in Finland (DiMe) Study Group. J Med Virol 61:214–220
Moya-Suri V, Schlosser M, Zimmermann K, Rjasanowski I, Gurtler L, Mentel R (2005) Enterovirus RNA sequences in sera of schoolchildren in the general population and their association with type 1-diabetes-associated autoantibodies. J Med Microbiol 54:879–883
Nairn C, Galbraith DN, Taylor KW, Clements GB (1999) Enterovirus variants in the serum of children at the onset of type 1 diabetes mellitus. Diabet Med 16:509–513
Oikarinen M, Tauriainen S, Honkanen T, Vuori K, Karhunen P, Vasama-Nolvi C, Oikarinen S, Verbeke C, Blair GE, Rantala I, Ilonen J, Simell O, Knip M, Hyoty H (2008) Analysis of pancreas tissue in a child positive for islet cell antibodies. Diabetologia 51:1796–1802
Pujol-Borrell R, Todd I, Doshi M, Gray D, Feldmann M, Bottazzo GF (1986) Differential expression and regulation of MHC products in the endocrine and exocrine cells of the human pancreas. Clin Exp Immunol 65:128–139
Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG (2009) The prevalence of enteroviral capsid protein VP1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 52:1143–1151
Richardson SJ, Willcox A, Bone AJ, Morgan NG, Foulis AK (2011) Immunopathology of the human pancreas in type-I diabetes. Semin Immunopathol 33:9–21
Sarmiento L, Cabrera-Rode E, Lekuleni L, Cuba I, Molina G, Fonseca M, Heng-Hung L, Borroto AD, Gonzalez P, Mas-Lago P, Diaz-Horta O (2007) Occurrence of enterovirus RNA in serum of children with newly diagnosed type 1 diabetes and islet cell autoantibody-positive subjects in a population with a low incidence of type 1 diabetes. Autoimmunity 40:540–545
Schulte BM, Bakkers J, Lanke KH, Melchers WJ, Westerlaken C, Allebes W, Aanstoot HJ, Bruining GJ, Adema GJ, Van Kuppeveld FJ, Galama JM (2010) Detection of enterovirus RNA in peripheral blood mononuclear cells of type 1 diabetic patients beyond the stage of acute infection. Viral Immunol 23:99–104
Shibasaki S, Imagawa A, Tauriainen S, Iino M, Oikarinen M, Abiru H, Tamaki K, Seino H, Nishi K, Takase I, Okada Y, Uno S, Murase-Mishiba Y, Terasaki J, Makino H, Shimomura I, Hyoty H, Hanafusa T (2010) Expression of Toll-like receptors in the pancreas of recent-onset fulminant type 1 diabetes. Endocr J 57:211–219
Tam PE, Messner RP (1999) Molecular mechanisms of coxsackievirus persistence in chronic inflammatory myopathy: viral RNA persists through formation of a double-stranded complex without associated genomic mutations or evolution. J Virol 73:10113–10121
Tanaka S, Nishida Y, Aida K, Maruyama T, Shimada A, Suzuki M, Shimura H, Takizawa S, Takahashi M, Akiyama D, Arai-Yamashita S, Furuya F, Kawaguchi A, Kaneshige M, Katoh R, Endo T, Kobayashi T (2009) Enterovirus infection, CXC chemokine ligand 10 (CXCL10), and CXCR3 circuit: a mechanism of accelerated beta-cell failure in fulminant type 1 diabetes. Diabetes 58:2285–2291
Tauriainen S, Oikarinen S, Oikarinen M, Hyoty H (2011) Enteroviruses in the pathogenesis of type 1 diabetes. Semin Immunopathol 33:45–55
Tracy S, Drescher KM, Jackson JD, Kim K, Kono K (2010) Enteroviruses, type 1 diabetes and hygiene: a complex relationship. Rev Med Virol 20:106–116
Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG (2009) Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 155:173–181
Willcox A, Richardson SJ, Bone AJ, Foulis A, Morgan NG (2011) Immunohistochemical analysis of the relationship between islet cell proliferation and the production of the enteroviral capsid protein, VP1, in the islets of patients with recent-onset type 1 diabetes. Diabetologia 54:2417–2420
Yin H, Berg AK, Tuvemo T, Frisk G (2002) Enterovirus RNA is found in peripheral blood mononuclear cells in a majority of type 1 diabetic children at onset. Diabetes 51:1964–1971
Ylipaasto P, Klingel K, Lindberg AM, Otonkoski T, Kandolf R, Hovi T, Roivainen M (2004) Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 47:225–239
Yoon JW, Austin M, Onodera T, Notkins AL (1979) Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med 300:1173–1179
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Richardson, S.J., Willcox, A., Bone, A.J., Morgan, N.G., Foulis, A.K. (2013). Viruses in the Human Pancreas. In: Taylor, K., Hyöty, H., Toniolo, A., Zuckerman, A. (eds) Diabetes and Viruses. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4051-2_17
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4051-2_17
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4050-5
Online ISBN: 978-1-4614-4051-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)