Abstract
Solid cell nests (SCN) associated with Hashimoto’s thyroiditis may show some atypical nuclear features including prominent nuclear grooves, enlarged overlapping nuclei and nuclear clearing. These features are sometimes mistaken for papillary thyroid microcarcinomas especially when the SCN are numerous. We reviewed SCN associated with Hashimoto’s thyroiditis in 12 patients selected from 1,420 archival routinely processed formalin-fixed, paraffin-embedded thyroid specimens of Hashimoto’s thyroiditis in which there was more than ten SCN per slide. In addition to the atypical nuclear features, there was a distinct eosinophilic basement membrane surrounding the SCN. Immunohistochemical analysis showed that the SCN were strongly positive for p63, stained weakly for TTF-1 and were negative for thyroglobulin, HBME-1, and calcitonin. This was compared to papillary thyroid microcarcinomas which were strongly positive for thyroglobulin, TTF-1, HBME-1, and variably positive for p63, while calcitonin and chromogranin were negative. These histological and immunophenotypic features can be used to distinguish SCN from papillary thyroid microcarcinomas associated with Hashimoto’s thyroiditis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solid cell nests (SCN) of the thyroid have interested pathologists since their description by Getzowa in 1907 [1]. SCN are often small, measuring 0.1 mm or less in diameter, and consist of polygonal to ovoid cell with elongated nuclei, finely granular chromatin, and may show nuclear grooves. Other cells with round nuclei and clear cytoplasm may be present. SCN are found in 3% of routinely examined thyroid glands and in one study were identified in 61% of thyroids serially sectioned [2].
Ultrastructural studies of SCN showed microfollicular structures, intracytoplasmic microvacuolar secretory granule features, and ciliated cells, findings that support the suggestion that they may represent remnants of the fifth (ultimobranchial) body [3]. SCN can be found incidentally within the normal thyroid gland in the lateral lobes [4] and can be associated with either neoplastic [5] or non-neoplastic lesions of the thyroid [6, 7]. A combined occurrence of lymphocytic thyroiditis and SCN in the same thyroid gland has been previously noted [8].
Previous studies have described the histological and immunohistochemical features of SCN [2, 3, 6, 9–15]. Four different types of SCN have been reported [2, 7]: (1) SCN type 1 are mainly composed of small round or oval groups of elongated or even spindle shaped cells with centrally located, oval to fusiform nuclei with uneven nuclear envelope showing occasional grooves, and scanty eosinophilic cytoplasm which are called “main cells” (floret-like features), surrounded by collection of lymphocytes; (2) SCN type 2 have large polygonal cells with abundant cytoplasm and distinct cell boundaries (epidermoid-like features); (3) SCN type 3 are characterized by cystic structure lined by flattened or large polygonal cells; and (4) SCN type 4 are composed of structures lined by main cells and follicular epithelium (so-called mixed follicles; Table 1).
SCN type 1 are reported in patients with Hashimoto’s thyroiditis. Multiple foci of SCN type 1 were described by Vollenweider in 20 of 39 [7], by Unger in three of nine [16], by Burstein in two of six [17] cases of Hashimoto’s thyroiditis. Foci of SCN type 1 associated with mixed follicles (SCN type 4) were described by Martin [6] in eight of 81 cases and by Preto [18] in four of six cases of Hashimoto’s thyroiditis.
Some authors reported C-cells in association with main cells. C-cells have accounted for a minor proportion of the solid cell nest population and were characterized by clear cytoplasm and centrally located, small compact nuclei [2, 9, 19].
SCN can be easily recognized when they display large polygonal cells with abundant cytoplasm and distinct cell boundaries (SCN type 2) or are made up of a cystic structure lined by flattened or large polygonal cells (SCN type 3). Distinguishing SCN from papillary thyroid microcarcinoma, however, can be difficult if the SCN are composed of small round or oval groups of elongated cells with centrally located, oval nuclei with irregular nuclear envelope showing occasional nuclear grooves and scanty cytoplasm (SCN type 1).
SCN can also be misinterpreted as squamous metaplasia of follicular thyroid cells, primary or metastatic squamous cell carcinoma, thyroglossal cyst, C-cell hyperplasia, and medullary microcarcinoma [2, 9]. The distinction between these latter lesions and SCN can usually be made on the basis of the hematoxylin and eosin (H&E) appearance, but can be confirmed by immunohistochemistry as SCN were reported as usually negative for thyroglobulin and TTF-1 and strongly reactivity for p63 [20]. The expression of thyroglobulin and TTF-1 has been controversial in SCN [5–7, 9, 14, 20–27].
Currently, there are no well-established criteria for the diagnosis of SCN in these borderline cases mainly associated with Hashimoto’s thyroiditis, which has resulted in diagnostic confusion with papillary thyroid carcinomas in some cases.
To determine if there are histological and immunohistochemical criteria to differentiate cases of SCN associated with Hashimoto’s thyroiditis, from papillary thyroid microcarcinomas, we systematically reviewed the routine H&E-stained slides of cases of Hashimoto’s thyroiditis for the presence of solid structures composed of “main cells”. Cases containing these conspicuous cell groups were investigated immunohistochemically for the expression of thyroglobulin, TTF-1, p63, calcitonin, chromogranin, and HBME-1.
Material and Methods
We examined 1,420 routinely processed archival formalin-fixed, paraffin-embedded thyroid specimens resected for Hashimoto’s thyroiditis during the period of 1955–1985 at Mayo Clinic, Rochester, MN, for the presence of groups of cells with the appearance of SCN type 1.
Institutional Review Board permission was obtained for the study.
Hashimoto’s thyroiditis was diagnosed when 50% or more of the section was occupied by lymphocytic infiltrates. Further essential features were epithelial destruction and oxyphilic change of the follicular cells.
Thirty-six cases from the files had one or more foci of SCN type 1 per slide. We used ten foci of SCN type 1 per slide, as a cut-off selection criterion, because multiple foci of SCN type 1 associated with Hashimoto’s thyroiditis represent the majority of the borderline cases that may be confused with papillary thyroid microcarcinoma in our experience. Moreover, all cases associated with malignant thyroid neoplasms were also excluded from the study. According to our selection criteria, we found 12 cases of SCN of type 1 with ten or more foci of SCN type 1 per slide.
Additional sections were cut from the available blocks and stained with antibodies to p63 (BC4A4, 1/100BRD, Biocare, Concord, CA), TTF-1 (8G7G3/1,1/700 BRD, Dako, Carpinteria, CA, USA), thyroglobulin (2H11/6E1 cocktail, 1/50BRD, Zymed, San Francisco, CA, USA), HBME-1 (HBME-1, 1/40 Dako), calcitonin (polyclonal antibody, 1/6000, Dako) and chromogranin A (LK2H10, predilute, Zymed). Immunohistochemistry was performed according to standard automated immunohistochemical procedure (Ventana Autostainer, Benchmark, XT). Immunoreactions were developed using biotin-free dextran-chain detection system (Envison, dakoCytomation, Carpinteria, CA, USA) and immunoreactions were developed with diaminobenzidine as the chromogen.
Five cases of papillary thyroid carcinomas in patients with Hashimoto’s thyroiditis were used as controls. Follow-up data were available for all patients.
Results
The clinical and pathological data are summarized in Tables 2 and 3.
SCN type 1 was found in 2.5% of routinely examined cases of Hashimoto’s thyroiditis. Of the 36 cases with SCN type 1 associated with Hashimoto’s thyroiditis, only 12 cases met our cut-off selection criterion of at least ten foci per slide (range 10 to 45). The 12 patients were all women (100%) with the mean age of 41.8 year (range, 26–53 years).
Clinically, the patients had symptomatic Hashimoto’s thyroiditis in 33.3% of cases (four of 12), multinodular goiter in 25% (three of 12), thyroid nodule in 33.3% (four of 12) and primary hyperparathyroidism in one case (8.4%) associated with multinodular goiter. Histopathologic evaluation showed Hashimoto’s thyroiditis. There were follicular adenomas in three cases, adenomatous nodules in three cases and a parathyroid adenoma in one case.
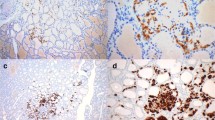
Overall, there was a mean of 12.4 SCN per slide (range, ten to 45) with foci of small round or oval groups of elongated crowded cells with centrally located, oval, overlapping nuclei with irregular nuclear envelope showing grooves, occasional nuclear clearing and scanty cytoplasm (Fig. 1a). In particular, nuclear grooves were found in all 12 cases, whereas nuclear clearing was present in ten of 12 cases (83.3%; Fig. 1b). Cytoplasm invagination into nuclei (intranuclear pink holes) was not observed.
a Solid cell nests (SCN) type 1 in a background of Hashimoto’s thyroiditis. There are small round to oval groups of elongated cells with scanty cytoplasm surrounded by a collection of lymphocytes. (H&E, ×10) b At high magnification, SCN type 1 are composed of main cells that show nuclei with occasional nuclear clearing and grooving. (H&E, ×60) c SCN type 1 cells are typically surrounded by thin eosinophilic basement membrane (black arrows, H&E, ×40). Immunohistochemical analyses showed that the main cells are strongly positive for p63 (d, ×20), stain weakly for TTF-1 (e, ×40) and are negative for thyroglobulin (f, ×40)
SCN type 1 were bordered by eosinophilic, thin basement membrane and surrounded by collections of lymphocytes in all 12 cases (Fig. 1c). Mitotic figures were absent.
Hashimoto’s thyroiditis was present in all cases with a variable degree of inflammation ranging from mild to severe. Seven of 12 cases showed foci of stromal sclerosis.
In all SCN cases, the immunohistochemical reaction with p63 was diffuse and strongly positive in the “main cells” (Fig. 1d) while TTF-1 showed diffuse unequivocal weak positivity (Fig. 1e). Immunostains for thyroglobulin (Fig. 1f), calcitonin, chromogranin, and HBME-1 were all negative in the SCN. In two cases, calcitonin- and chromogranin-containing cells were found between follicular cells and in the inter-follicular spaces, but they showed no special relationship to the solid cell nests.
The above immunohistochemical panel was also performed on five control cases of papillary thyroid microcarcinoma associated with Hashimoto’s thyroiditis.
The neoplastic papillary thyroid carcinoma cells were strongly positive for thyroglobulin, TTF-1 and HBME-1, and negative for calcitonin and chromogranin.
Focal and weak positive staining for p63 was observed in the peripheral cells of papillary thyroid microcarcinoma in one of five cases, with the other four cases being completely negative.
Follow-up data was available for all 12 cases of SCN type 1 (Table 2) and for all five papillary thyroid microcarcinomas. All patients were alive with no evidence of disease after a mean of 68 months (range, 1–244 months) of follow-up.
Discussion
A distinctive form of solid cell nest (SCN type 1), was noted in our 12 cases, consisting of a floret-like arrangement of p63-positive cells with radially projecting fusiform nuclei. These structures were morphologically identical to structures that were described elsewhere in two studies of SCN in Hashimoto’s thyroiditis [7, 17].
We have observed that structures with this histology have been mistaken for papillary thyroid microcarcinoma in some of our consultation cases.
Our findings showed SCN type 1 to be consistently p63- and TTF-1-positive. These floret-like SCN numbered more than ten and ranged from ten to 45 per slide. Most commonly, p63-positive cells in Hashimoto’s thyroiditis were arranged in small, morphologically inconspicuous solid cell clusters in proximity to dense lymphoid infiltrates or germinal centers. Several p63-positive clusters of SCN type 1 in a Hashimoto’s thyroiditis specimen displayed atypical nuclear changes including pale nuclei (ten of 12 cases, 83.3%).
SCN of type 1 may be a source of confusion in Hashimoto’s thyroiditis. SCN type 1 are formed by main cells which show enlarged nuclei with finely dispersed chromatin, inconspicuous nucleoli and frequent nuclear grooves sometimes mimicking a “coffee bean shape”. These features also represented some of the nuclear characteristics described in papillary thyroid carcinoma and hyalinizing trabecular tumor of the thyroid [28].
Features that are unique to SCN type 1 include their distinctly lobular architecture, the surrounding thin eosinophilic basement membrane, and the predominantly oval shape of the nuclei. Cytoplasmic invaginations into nuclei (intranuclear pink holes) were not found in the SCN.
The distinction between SCN and papillary thyroid microcarcinoma can usually be made on the basis of the above morphological appearance on H&E sections, but if needed, one can obtain confirmation with immunohistochemistry (Table 4). SCN showed strong nuclear reactivity for p63 and were negative for thyroglobulin and HBME-1.
Immunohistochemical analysis of SCN reported in the literature is summarized in Table 5.
In contrast with previous studies about the immunophenotype of SCN [20], our 12 cases of SCN type 1 showed a diffuse unequivocal weak positivity for TTF-1 and lacked the scattered reactivity for chromogranin. Weak, but unequivocal, positivity of TTF-1 was also reported in one case of SCN by Ryska [25]. In our immunohistochemical analysis of TTF-1, the same antibody at the same dilution (1/50) and a similar automated immunostainer machine were used as in the Ryska study [25]. Reis-Filho [20] reported SCN to express TTF-1. They used the same TTF-1 antibody but at different dilution (1/100) without an automated immunostainer machine for immunohistochemical analysis, and required a minimum of 20% positivity as a cut-off for all imunohistochemical markers.
A consistent and reproducible strong reactivity for p63 (97.6%) in SCN has been reported [5, 16, 17, 20, 25, 26]. p63 is consistently expressed in basal/stem cells of several types of epithelia and it is usually absent in partially or terminally differentiated cells [29–32].
Our study showed that p63 is a useful marker that is consistently positive in SCN, but also showed a focal weak positivity in the peripheral cells in one of the five papillary thyroid microcarcinomas studied.
In the present study, we showed that the main SCN cells strongly express p63 supporting the suggestion that these cells may have a basal/stem cell features [5, 17, 20].
The expression of thyroglobulin and calcitonin in SCN is controversial. Thyroglobulin and calcitonin positivity has been reported in the main cells in 37.6% and in 29.6% of SCN, respectively [5–7, 9, 13, 14, 19–27, 33–35]. In the present study, the main cells of all 12 cases of SCN were negative for both thyroglobulin and calcitonin. This may be due to different expression of thyroglobulin and calcitonin in the different types of SCN or increasing specificity of the antibodies applied and the limited number of cases examined.
As SCN are thought to be a remnant of the ultimobranchial body [3] and strongly expression p63 one may hypothesize that SCN represent incompletely developed thyroid tissue which could be predisposed to autoimmune thyroid diseases such as Hashimoto’s thyroiditis, because the epithelia from the third and the fourth pouch have the ability to attract lymphocytes [7, 8].
Recently, Cameselle-Teijeiro [5] described an extremely unusual case of SCN hyperplasia coexisting with two papillary microcarcinomas, a follicular adenoma, hyperplastic nodules, and a few lymphoid aggregates. The authors reported morphologic continuity between SCN and one papillary thyroid microcarcinoma and found the same BRAFV600E mutation in both the SCN and the contiguous papillary thyroid microcarcinoma suggesting a histogenetic relationship between the main cells of SCN and the papillary thyroid carcinoma.
Although these findings could support a histogenetic link between SCN and papillary thyroid carcinoma, additional molecular analyses and other studies are needed to support this linkage.
In summary, we examined a series of 12 cases of SCN associated with Hashimoto’s thyroiditis, which consisted of main cells arranged in floret-like structures that showed atypical nuclear features (nuclear clearing and grooving). The SCN showed a distinctive immunophenotype (strong p63 positivity, weak TTF-1 positivity, and HBME-1 negativity) that can be useful to distinguish SCN from papillary thyroid microcarcinoma associated with Hashimoto’s thyroiditis in difficult cases.
Reference
Getzowa S. Ueber die Glandula parathyreoidea, intrathyreoidale Zellhaufen derselben und Reste des postbranchialen köpers. Virchows Arch A Pathol Anat Histopathol 188:181–234, 1907.
Harach HR. Solid cell nests of the thyroid. J Pathol 155:191–200, 1988.
Williams ED, Toyn CE, Harach HR. The ultimobranchial gland and congenital thyroid abnormalities in man. J Pathol 159:135–41, 1989.
Rosai J, Carcangiu ML, DeLellis RA. Tumours of the thyroid gland. In Atlas of tumour pathology, 3rd Series, Fascicle 5.Washington, DC: Armed Forces Institute of Pathology; 1992.
Cameselle-Teijeiro J, Abdulkader I, Pérez-Becerra R, Vázquez-Boquete A, Alberte-Lista L, Ruiz-Ponte C, et al. BRAF mutation in solid cell nest hyperplasia associated with papillary thyroid carcinoma. A precursor lesion? Human Pathol 40(7):1029–35, 2009.
Martin V, Martin L, Viennet G, Challier B, Carbillet J, Fellmann D. Solid cell nests and thyroid pathologies. Retrospective study of 1,390 thyroids. Annu Pathol 20:196–201, 2000.
Vollenweider I, Hedinger C. Solid cell nests (SCN) in Hashimoto’s thyroiditis. Virchows Arch A Pathol Anat Histopathol 412:357–63, 1988.
Prod'hom G, Hedinger C. Relationship between solid cell nests and focal lymphocytic thyroiditis. Ann Pathol 5(4–5):265–70, 1985.
Cameselle-Teijeiro J, Varela-Durán J, Sambade C, Villanueva JP, Varela-Núñez R, Sobrinho-Simoes M. Solid cell nests of the thyroid: light microscopy and immunohistochemical profile. Human Pathol 25:684–93, 1994.
Harach HR. Mixed follicles of the human thyroid gland. Acta Anat (Basel) 129:27–30, 1987
Bykov VL. Tissue of ultimobranchial origin in normal and pathologically altered thyroid gland. Arkh Patol 55:81–84, 1993.
Fraser BA, Duckworth JW. Ultimobranchial body cysts in the human foetal thyroid: pathological implications. J Pathol 127:89–92, 1979.
Mizukami Y, Nonomura A, Michigishi T, Noguchi M, Hashimoto T, Nakamura S, et al. Solid cell nests of the thyroid. A histologic and immunohistochemical study. Am J Clin Pathol 101:186–191, 1994.
Ozaki O, Ito K, Sugino K, Yasuda K, Yamashita T, Toshima K,et al. Solid cell nests of the thyroid gland. Virchows Arch A Pathol Anat Histopathol 418:201–5, 1991.
Beckner ME, Shultz JJ, Richardson T. Solid and cystic ultimobranchial body remnants in the thyroid. Arch Pathol Lab Med 114:1049–52, 1990.
Unger P, Ewart M, Wang BY, Gan L, Kohtz DS, Burstein DE. Expression of p63 in papillary thyroid carcinoma and in Hashimoto’s thyroiditis: a pathobiologic link? Human Path 34:764–9, 2003.
Burstein DE, Nagi C, Wang BY, Unger P. Immunohistochemical detection of p53 homolog p63 in solid cell nests, papillary thyroid carcinoma, and hashimoto's thyroiditis: a stem cell hypothesis of papillary carcinoma oncogenesis. Human Pathol 35(4):465–73, 2004.
Preto A, Cameselle-Teijeiro J, Moldes-Boullosa J, Soares P, Cameselle-Teijeiro JF, Silva P, et al. Telomerase expression and proliferative activity suggest a stem cell role for thyroid solid cell nests. Mod Pathol 17(7):819–26, 2004.
Martin V, Martin L, Viennet G, Hergel M, Carbillet JP, Fellmann D. Ultrastructural features of “solid cell nest” of the human thyroid gland: a study of 8 cases. Ultrastruct Pathol 24:1–8, 2000.
Reis-Filho JS, Preto A, Soares P, Ricardo S, Cameselle-Teijeiro J, Sobrinho-Simoes M. p63 expression in solid cell nests of the thyroid: further evidence for a stem cell origin. Mod Pathol 16:43–48, 2003.
Autelitano F, Santeusanio G, Di Tondo U, Costantino AM, Renda F, Autelitano M. Immunohistochemical study of solid cell nests of the thyroid gland found from an autopsy study. Cancer 59(3):477–83, 1987.
Ozaki O, Ito K, Fujisawa T, Kawano M, Iwabuchi H, Kitamura Y, et al. Solid cell nests of the thyroid in medullary thyroid carcinoma. Histopathology 24(1):77–80, 1994.
Harach HR. Solid cell nests of the thyroid. An anatomical survey and immunohistochemical study for the presence of thyroglobulin. Acta Anat (Basel) 122(4):249–53, 1985.
Michal M, Mukensnabl P, Kazakov DV. Branchial-like cysts of the thyroid associated with solid cell nests. Pathol Int 56(3):150–3, 2006.
Ryska A, Ludvíková M, Rydlová M, Cáp J, Zalud R. Massive squamous metaplasia of the thyroid gland– report of three cases. Pathol Res Pract 202(2):99–106, 2006.
Fellegara G, Dorji T, Bajineta MR, Rosai J. Images in pathology. "Giant" solid cell rest of the thyroid: a hyperplastic change? Int J Surg Pathol 17(3):268–9, 2009.
Janzer RC, Weber E, Hedinger C. The relation between solid cell nests and C cells of the thyroid gland: an immunohistochemical and morphometric investigation. Cell Tissue Res 197:295–312, 1979.
Carney JA, Hirokawa M, Lloyd RV, Papotti M, Sebo TJ. Hyalinizing trabecular tumors of the thyroid gland are almost all benign. Am J Surg Pathol 32:1877–89, 2008.
Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398:714–8, 1999
Levrero M, De Laurenzi V, Costanzo A, Gong J, Wang JY, Melino G. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci 113:1661–70, 2000
Di Como CJ, Urist MJ, Babayan I, Drobnjak M, Hedvat CV, Teruya-Feldstein J, et al. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res 8:494–501, 2002
Reis-Filho JS, Schmitt FC. Taking advantage of basic research: p63 is a reliable myoepithelial and stem cell marker. Adv Anat Pathol 9:280–9, 2002
Harach HR, Vujanic GM, Jasani B. Ultimobranchial bodynests in human fetal thyroid: an autopsy, histological, and immunohistochemical study in relation to solid cell nests and mucoepidermoid carcinoma of the thyroid. J Pathol 169:465–9, 1993.
Faggiano A, Talbot M, Baudin E, Bidart JM, Schlumberger M, Caillou B. Differential expression of galectin 3 in solid cell nests and C cells of human thyroid. J Clin Pathol 56(2):142–3, 2003.
Nadig J, Weber E, Hedinger CH. C-cell in vestiges of the ultimobranchial body in human thyroid glands. Virchows Arch B Cell Pathol 27(2):189–91, 1978.
García-González M, Abdulkader I, Boquete AV, Neo XM, Forteza J, Cameselle-Teijeiro J. Cyclooxygenase-2 in normal, hyperplastic and neoplastic follicular cells of the human thyroid gland. Virchows Arch 447(1):12–7, 2005.
Acknowledgment
The authors express their gratitude to Professor Gianni Bussolati (Department of Biomedical Sciences and Human Oncology University of Turin, Italy) for helping in the review of this paper.
Competing interests
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asioli, S., Erickson, L.A. & Lloyd, R.V. Solid Cell Nests in Hashimoto’s Thyroiditis Sharing Features with Papillary Thyroid Microcarcinoma. Endocr Pathol 20, 197–203 (2009). https://doi.org/10.1007/s12022-009-9095-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-009-9095-x