Abstract
This study was undertaken to investigate cyclooxygenase-2 (COX-2) expression in follicular cells of the human thyroid. COX-2 expression was studied immunohistochemically in a total of 174 samples. COX-2 immunoreactivity was confined to the cell cytoplasm with the nuclei remaining unlabelled. COX-2 expression was observed in five cases (17.2%) of normal follicular cells and in one case (16.6%) of solid cell nests. Follicular carcinoma expressed COX-2 more frequently than follicular adenoma (93.4% vs 21.1%) (p≤0.001). A higher percentage of cases of papillary microcarcinomas up-regulated COX-2 in comparison with all papillary carcinomas (p≤0.05). However, we could not establish any relationships among COX-2, patients’ ages or lymph node metastases in papillary carcinomas. COX-2 expression was found in 12 (92.3%) poorly differentiated carcinomas and in 13 (92.8%) undifferentiated carcinomas. We found that COX-2 is not always useful as a marker of malignancy. Our results suggest that COX-2 plays a role in progression of all thyroid carcinomas, but in papillary carcinomas, seems more important only in the early stages. COX-2 expression in the undifferentiated carcinoma deserves special consideration due to its prognosis and to the fact that selective COX-2 inhibitors were found to enhance tumour response to radiation in some studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are two isoforms of cyclooxygenase (COX), COX-1 and COX-2, involved in the synthesis of prostaglandins from arachidonic acid [29]. The COX-1 enzyme is expressed constitutively in most mammalian tissues where, although often undetected, it can be rapidly induced. The COX-2 gene is an early-response gene that is up-regulated by proinflammatory stimuli, growth factors, oncogenes and tumour-promoting phorbol esters [10, 13, 22].
COX-2 seems to play a role in carcinogenesis as well as in carcinoma progression; COX-2 is up-regulated in transformed cells [24], in colorectal cancers [20, 22], ulcerative colitis-associated neoplasia [1], hepatocellular carcinoma [14], oesophageal and gastric carcinomas [25, 34], pulmonary and mammary tumours [22], pancreatic cancer [27], renal cell carcinoma [11], prostate carcinoma [33] and pituitary tumours [30]. COX-2 knockout mice also have a lower incidence of several tumour types including skin papillomas and intestinal polyps [2, 18, 26]. Epidemiological studies have also shown that chronic intake of non-steroidal anti-inflammatory drugs, which function as COX inhibitors, reduces the incidence of gastrointestinal cancer [8, 9, 32].
Enhanced expression of COX-2 in lymphocytic thyroiditis and Hashimoto’s thyroiditis suggests an important role of this enzyme in these inflammatory thyroid processes [5, 17]. Thyroid cancer of follicular cells includes several histological types of tumours with various molecular alterations and different clinical behaviours. Papillary carcinoma is the most common malignant tumour of the thyroid and has a relatively indolent clinical course in contrast to the rapidly fatal course of anaplastic (undifferentiated) carcinoma [6]. Poorly differentiated carcinomas fall between well-differentiated (papillary and follicular) carcinomas and undifferentiated carcinomas in terms of both morphologic appearance and behaviour [6]. Specht et al. [23] reported that COX-2 mRNA level was higher in malignant nodules than in adjacent normal tissue or in benign nodules. Most recently, Ito et al. [12] concluded that the up-regulation of COX-2 may contribute predominantly in the early phase of papillary carcinoma progression while playing a more adjuvant role in follicular carcinoma progression. On the other hand, Siironen et al. [21] showed that COX-2 expression increases with age in papillary thyroid cancer.
In the present study, we examine the expression of COX-2 in a series of normal and hyperplastic follicular cells as well as in well-differentiated (papillary and follicular), poorly differentiated and anaplastic (undifferentiated) carcinomas.
Materials and methods
Tissue specimens
One hundred seventy-four thyroid tissue specimens were obtained from the files of the Department of Pathology, Hospital Clínico Universitario, Santiago de Compostela, Spain. This study was approved by the ethical committee of our institution. Tissue slices had been fixed in 10% formalin and embedded in paraffin wax for histological, immunohistochemical and in situ hybridisation studies. Each case was classified according to the Chan criteria [6]. Specimens were normal thyroid tissue, 29; solid cell nests, 6; Graves’ disease, 10; nodular hyperplasia, 15; follicular adenoma, 33; papillary carcinoma, 39 (13 cases of microcarcinomas, 8 cases of classical type, 6 cases of follicular variant, 1 case of solid variant, 4 cases of cribriform-morular variant, 2 cases of Whartin-tumour-like variant and 5 lymph node metastasis); follicular carcinoma, 8; Hürthle cell follicular carcinoma, 7; poorly differentiated carcinoma, 13 (11 cases of insular carcinoma and 2 cases of columnar cell carcinomas); and undifferentiated carcinoma, 14. Of the normal specimens, 16 were from the autopsy specimens, and the other 13 were “normal” tissue taken from non-tumoural areas of the other specimens.
Immunohistochemistry and in situ hybridisation
Immunohistochemical studies were performed on tissue sections 4 μm thick using a COX-2 monoclonal antibody (Cayman Chemical, Ann Arbor, MI, USA, microwaved, 1:50). The reaction was detected using a universal second antibody kit that utilized a peroxidase-conjugated labelled-dextran polymer (Dako EnVision Peroxidase/DAB; Dako, Glostrup, Denmark) in order to avoid misinterpreting endogenous biotin or biotin-like activity in cell cytoplasms [7] or nuclei [3] as positive staining. Paraffin blocks of colorectal cancer known to be positive for COX-2 were used as positive controls. Sections for each negative control were incubated with PBS instead of the primary antibody. Scoring of the immunohistochemical results was performed according to the methods described by Ito et al. [12], with minor modifications. We selected at least five representative fields. Based on the percentages of immunopositive cells, three subdivisions were made as follows: diffusely positive (++), greater than 50% of cells were positive; heterogeneously positive (+), 10–49% of cells were positive; and negative (−), less than 10% of the cells were positive. Equivocal immunointensity was considered to be negative.
As representative cases, in situ hybridisation was performed on 4-μm sections in four cases of normal thyroid tissue, two cases of solid cell nests, one case of Graves’ disease, four cases of papillary carcinoma, one case of follicular carcinoma, one case of Hürthle cell follicular carcinoma, two cases of insular carcinoma, one case of columnar cell carcinoma and three cases of undifferentiated carcinoma to confirm immunohistochemical results. Before hybridisation, tissue sections were deparaffinized, rehydrated and washed in TE buffer (Tris 10 mM, EDTA 1 mM). Thereafter, the tissues were treated with proteinase K (4 μg/ml), washed in TE buffer and 2×SSC buffer and dehydrated with 96% and 100% ethanol. Hybridisation was carried out using a 3′FITC-labelled oligoprobe (5′-TCATCAACACTGCCTCAATTCAGTCTCTCATCTGCAATAA-3′) directed against the human COX-2 mRNA. Sections were hybridised at 37°C overnight. After hybridisation, stringent washes were performed in 2×SSC, 1×SSC and 0.5×SSC at room temperature. The oligoprobes were detected using an alkaline phosphatase-labelled anti-FITC antibody (Dako) and visualized with nitroblue tetrazolium (NBT)/BCIP until desired stain intensity was achieved. Sections were counterstained with methylene green and mounted in an aqueous media. We used sections of colon cancer as positive controls for COX-2 mRNA. For negative control, we have substituted the oligoprobe hybridisation mix with hybridisation buffer. Estimation of immunohistochemical and in situ hybridisation scores for each case was performed by different observers (MAGG, JC-T and IA), independently, and determined after discussion when scores were discrepant.
Statistical analysis
χ2 analysis and Fisher’s exact probability test were adopted to examine the relationship between variables. Two results were considered significant when p<0.05.
Results
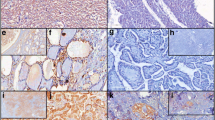
Table 1 summarises the immunohistochemical findings. COX-2 immunoreactivity was confined to the cell cytoplasm with the nuclei remaining unlabelled. Epithelial cells of solid cell nests were negative in five cases and diffusely positive in one case. Although the percentage of immunopositive cells was higher in malignant tumours than in benign ones (p≤0.001), the distribution of COX-2 reactivity was not restricted to tumour cells (Table 1) (Fig. 1). COX-2 expression was observed in five (17.2%) cases of normal thyroid tissue adjacent to three Hürthle cell follicular carcinomas, one follicular carcinoma and one follicular adenoma (Fig. 1a). On the other hand, follicular carcinoma expressed COX-2 more frequently than follicular adenoma (p≤0.001) (Fig. 1e). Twenty-seven (68.8%) of the papillary carcinomas showed COX-2 immunoexpression (Fig. 1b–d). A higher percentage of cases (92.3%) of papillary microcarcinomas up-regulated COX-2 in contrast to all papillary carcinomas (p≤0.05) (Table 2). The mean age in the 23 COX-2-positive papillary carcinomas was 49.9±17.3 in comparison with 42.15±19.6 in the COX-2-negative papillary carcinomas. We could not establish any relationship between COX-2 expression and patients’ age or lymph node metastases.
a–i Immunoexpression of cyclooxygenase-2 (COX-2) in follicular adenoma (×200) (a), papillary microcarcinoma (×200) (b), Whartin-tumour-like variant of papillary carcinoma (×200) (c), classical variant of papillary carcinoma (×400) (d), follicular carcinoma (×200) (e), insular carcinoma (×200) (f) and undifferentiated carcinomas (g–i). A cluster of normal follicular cells adjacent to a follicular adenoma is positive for COX-2 (a). No COX-2 expression is seen in normal thyroid epithelial cell adjacent to a papillary microcarcinoma (b), and follicular carcinoma (e). g Stronger intensity of staining in undifferentiated carcinoma cells in comparison with residual poorly differentiated areas in the same tumour (×200). h and i, Undifferentiated carcinomas showing neoplastic cells positively stained for COX-2 while the entrapped normal follicles are negative (×400)
COX-2 expression was found in 12 (92.3%) poorly differentiated carcinomas and in 13 (92.8%) undifferentiated carcinomas (Fig. 1f–i). In two cases of undifferentiated carcinoma, residual areas of poorly differentiated carcinoma and mucoepidermoid carcinoma were identified showing less immunointensity for COX-2 than the anaplastic areas (Fig. 1g).
In situ hybridisation analysis showed no staining in solid cell nests. A staining pattern supported the immunohistochemical findings in the other types of samples examined (Fig. 2).
Discussion
In this study, we found that COX-2 expression was frequently up-regulated in thyroid carcinomas as previously reported [12, 17, 21, 23], whereas COX-2 expression was limited in normal follicular cells. Solid cell nests of the thyroid, usually considered as normal embryonic remnants of the ultimobranchial body, were recently proposed as thyroid stem cells [19]; in situ hybridisation was negative in epithelial cells of these structures, and weak immunostaining was found in only one case of our series. COX-2 expression in some normal follicular cells was observed in our study and also observed by Ito et al. [12]. We think that this fact, together with negative expression of COX-2 in 30.8% of papillary carcinomas tested, indicates that COX-2 is not always useful as a marker of malignancy.
At least some follicular carcinomas are believed to arise from preexisting adenomas, although this has not yet been confirmed [6]. In our series, the percentage of COX-2 expression in follicular carcinomas was higher than that reported by Ito et al. [12] (93.3% vs 40.9%), but the percentages were similar in follicular adenomas. We cannot discard the possibility that some follicular adenomas up-regulating COX-2 could in fact be “malignant” tumours that have not yet revealed morphologic criteria of malignancy. Papillary carcinomas frequently over-express COX-2 [12, 17, 21]; but this expression is significantly reduced in tumours related to old age, large size, satellite tumours and the presence of a solid, scirrhous or trabecular growth pattern [12], thus, suggesting that the activation of this enzyme is required in the early phase of papillary carcinoma progression [12]. In our study, a significant higher percentage of papillary microcarcinomas up-regulated COX-2 in comparison with all papillary carcinomas. We think that in papillary carcinomas, COX-2 expression seems more important only in the early stages. At the same time, the higher proportion of COX-2 down-regulation in the entire group of papillary carcinomas could be related to the indolent behaviour of these tumours in comparison with other thyroid carcinomas. Siironen et al. [21] postulate that with the older age group having a much worse prognosis and a higher expression of COX-2, COX-2 confers an adverse prognosis via either invasion or metastasis. An association among age, worse prognosis, and higher grade in thyroid pathology is well recognized in the literature [6]. In our series, however, we could not establish any relationship between COX-2 expression and patients’ ages or lymph node metastases.
The cribriform-morular variant of papillary carcinoma is the sporadic counterpart of the thyroid carcinoma that occurs in patients with familial adenomatous polyposis [3, 4, 6]. APC mutations may represent a rare mechanism for COX-2 up-regulation in familial adenomatous polyposis-associated thyroid tumours [18]. However, among our cases of cribriform-morular variant of papillary carcinoma, including one case with germline mutation of the APC gene, we found weak but diffuse COX-2 expression in the only case with somatic, but not germline mutation of the APC gene [4]. A disturbance of the Wnt signal transduction pathway due to β-catenin gene mutations is more significant in the carcinogenesis of the cribriform-morular variant of papillary carcinoma [31].
Solid variant is a rare and poorly characterised variant of papillary thyroid carcinoma [16] whose a less favourable prognosis could correlate with expression of COX-2.
The immunoprofile of the rich lymphoid stroma in Whartin-tumour-like variant of papillary carcinoma is similar to that of chronic lymphocytic thyroiditis [6, 28]. The expression of COX-2 in lymphocytic thyroiditis, Hashimoto’s thyroiditis and some thyroid tumours has been proposed as a basis for the relationship between carcinogenesis and autoimmunity [5, 17, 21].
A stepwise increase in immunoreactivity scores for COX-2 staining was found by Nose et al. [17] from follicular adenomas to papillary carcinomas, well-differentiated and poorly differentiated types and follicular carcinomas (with anaplastic carcinoma included in the poorly differentiated papillary carcinoma group). We found a strong COX-2 expression in more than 90% of both follicular carcinomas and poorly differentiated carcinomas. In our series, up-regulation of COX-2 was found in all cells of 93% of undifferentiated carcinomas, and the stain was stronger in undifferentiated areas in the two cases with residual poorly differentiated foci. These findings deserve special consideration due to the ominous prognosis of undifferentiated thyroid carcinoma [6], as well as to the fact that selective COX-2 inhibitors were found to enhance thoracic tumour response to radiation in preclinical studies [15].
References
Agoff SN, Brentnall TA, Crispin DA, Taylor SL, Raaka S, Haggitt RC, Reed MW, Afonina IA, Rabinovitch PS, Stevens AC, Feng Z, Bronner MP (2000) The role of cyclooxygenase-2 in ulcerative colitis-associated neoplasia. Am J Pathol 157:737–745
Boolbol SK, Dannenberg AJ, Chadburn A, Martucci C, Guo XJ, Ramonetti JT, Abreu-Goris M, Newmark HL, Lipkin ML, DeCosse JJ, Bertagnolli MM (1996) Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res 56:2556–2560
Cameselle-Teijeiro J, Chan JKC (1999) Cribriform-morular variant of papillary carcinoma: a distinctive variant representing the sporadic counterpart of familial adenomatous polyposis-associated thyroid carcinoma? Mod Pathol 12:400–411
Cameselle-Teijeiro J, Ruiz-Ponte C, Loidi L, Suárez-Peñaranda JM, Baltar J, Sobrinho-Simoes M (2001) Somatic but not germline mutation of the APC gene in a case of cribriform-morular variant of papillary thyroid carcinoma. Am J Clin Pathol 115:486–493
Cornetta AJ, Russell JP, Cunnane M, Keane WM, Rothstein JL (2002) Cyclooxygenase-2 expression in human thyroid carcinoma and Hashimoto’s thyroiditis. Laryngoscope 112:238–242
Chan JKC (2000) Tumors of the thyroid and parathyroid glands. In: Fletcher CDM (ed) Diagnostic histopathology of tumors, 2nd edn. Churchill Livingstone, Edinburgh, Scotland pp 959–1056
Cheuk W, Chan JK (2004) Subcellular localization of immunohistochemical signals: knowledge of the ultrastructural or biologic features of the antigens helps predict the signal localization and proper interpretation of immunostains. Int J Surg Pathol 12:185–206
DuBois RN (2000) Review article: cyclooxygenase—a target for colon cancer prevention. Aliment Pharmacol Ther 14(Suppl 1):64–67
Farrow DC, Vaughan TL, Hansten PD, Stanford JL, Risch HA, Gammon MD, Chow WH, Dubrow R, Ahsan H, Mayne ST, Schoenberg JB, West AB, Rotterdam H, Fraumeni JF Jr, Blot WJ (1998) Use of aspirin and other nonsteroidal anti-inflammatory drug and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev 7:97–102
Fosslien E (2000) Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci 30:3–21
Hashimoto Y, Kondo Y, Kimura G, Matsuzawa I, Sato S, Ishizaki M, Imura N, Akimoto M, Hara S (2004) Cyclooxygenase-2 expression and relationship to tumour progression in human renal cell carcinoma. Histopathology 44:353–359
Ito Y, Yoshida H, Nakano K, Takamura Y, Miya A, Kobayashi K, Yokozawa T, Matsuzuka F, Matsuura N, Kuma K, Miyauchi A (2003) Cyclooxygenase-2 expression in thyroid neoplasms. Histopathology 42:492–497
Kelley DJ, Mestre JR, Subbaramaiah K, Sacks PG, Schantz SP, Tanabe T, Inoue H, Ramonetti JT, Dannenberg AJ (1997) Benzo[a]pyrene up-regulates cyclooxygenase-2 gene expression in oral epithelial cell. Carcinogenesis 18:795–799
Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R, Nakashima Y, Nakashima O, Kojiro M, Kurohiji T, Sata M (1999) Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology 29:688–696
Liao Z, Milas L, Komaki R, Stevens C, Cox JD (2003) Combination of a COX-2 inhibitor with radiotherapy or radiochemotherapy in the treatment of thoracic cancer. Am J Clin Oncol 26:S85–S91
Nikiforov YE, Erickson LA, Nikiforova MN, Caudill CM, Lloyd RV (2001) Solid variant of papillary thyroid carcinoma: incidence, clinical-pathologic characteristics, molecular analysis, and biologic behavior. Am J Surg Pathol 25:1478–1484
Nose F, Ichikawa T, Fujiwara M, Okayasu I (2002) Up-regulation of cyclooxygenase-2 expression in lymphocytic thyroiditis and thyroid tumors: significant correlation with inducible nitric oxide synthase. Am J Clin Pathol 117:546–551
Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM (1996) Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase-2 (COX-2). Cell 87:803–809
Preto A, Cameselle-Teijeiro J, Moldes-Boullosa J, Soares P, Cameselle-Teijeiro JF, Silva P, Reis-Filho JS, Reyes-Santias RM, Alfonsin-Barreiro N, Forteza J, Sobrinho-Simoes M (2004) Telomerase expression and proliferative activity suggest a stem cell role for thyroid solid cell nests. Mod Pathol 17:819–826
Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T (1995) Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res 55:3785–3789
Siironen P, Ristimaki A, Nordling S, Louhimo J, Haapiainen R, Haglund C (2004) Expression of COX-2 is increased with age in papillary thyroid cancer. Histopathology 44:490–497
Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT (2000) COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 89:2637–2645
Specht MC, Tucker ON, Hocever M, Gonzalez D, Teng L, Fahey TJ III (2002) Cyclooxygenase-2 expression in thyroid nodules. J Clin Endocrinol Metab 87:358–363
Subbaramaiah K, Telang N, Ramonetti JT, Araki R, DeVito B, Weksler BB, Dannenberg AJ (1996) Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer Res 56:4424–4429
Sung JJ, Leung WK, Go MY, To KF, Cheng AS, Ng EK, Chan FK (2000) Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am J Pathol 157:729–735
Tiano H, Chulada P, Spalding J, Lee C, Loftin C, Mahler J, Morham S, Langenbach R (1997) Effects of cyclooxygenase deficiency on inflammation and papilloma development in mouse skin. Proc Am Assoc Cancer Res 38:1727
Tucker ON, Dannenberg AJ, Yang EK, Fahey TJ III (2004) Bile acids induce cyclooxygenase-2 expression in human pancreatic cancer cell lines. Carcinogenesis 25:419–423
Urano M, Abe M, Kuroda M, Mizoguchi Y, Horibe Y, Kasahara M, Tanaka K, Sudo K, Hirasawa Y (2001) Warthin-like tumor variant of papillary thyroid carcinoma: case report and literature review. Pathol Int 51:707–712
Vane JR, Bakhle YS, Botting RM (1998) Cyclooxygenases 1 and 2 Annu Rev Pharmacol Toxicol 38:97–120
Vidal S, Kovacs K, Bell D, Horvath E, Scheithauer BW, Lloyd RV (2003) Cyclooxygenase-2 expression in human pituitary tumors. Cancer 97:2814–2821
Xu B, Yoshimoto K, Miyauchi A, Kuma S, Mizusawa N, Hirokawa M, Sano T (2003) Cribriform-morular variant of papillary thyroid carcinoma: a pathological and molecular genetic study with evidence of frequent somatic mutations in exon 3 of the beta-catenin gene. J Pathol 199:58–67
Xu XC (2002) COX-2 inhibitors in cancer treatment and prevention, a recent development. Anticancer Drugs 13:127–137
Yoshimura R, Sano H, Masuda C, Kawamura M, Tsubouchi Y, Chargui J, Yoshimura N, Hla T, Wada S (2000) Expression of cyclooxygenase-2 in prostate carcinoma. Cancer 89:589–596
Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schror K (1999) Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res 59:198–204
Acknowledgements
The authors are grateful to Dora C. Insua Santamaría for technical assistance. We also thank Deborah L. Randall for her linguistic supervision. This work was supported by research grant (PGIDE99PXI90201B) sponsored by Xunta de Galicia, Spain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-González, M., Abdulkader, I., Boquete, A.V. et al. Cyclooxygenase-2 in normal, hyperplastic and neoplastic follicular cells of the human thyroid gland. Virchows Arch 447, 12–17 (2005). https://doi.org/10.1007/s00428-005-1235-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-005-1235-1