Abstract
This study addressed the immunohistochemical expression of MUC1 in papillary thyroid carcinoma (PTC) of different histotypes, sizes, and morphological features of aggressiveness, and its correlation with the overexpression of cyclin D1, a target molecule of the Wnt pathway. MUC1 expression was examined in a total of 209 PTCs. Cytoplasmic MUC1 expression was elevated in the tall, columnar cell and oncocytic variants (100%), Warthin-like (78%), and conventional PTCs (61%), and in papillary microcarcinoma (PMC) with the conventional growth pattern (52%). On the contrary, it was low in the follicular variant (27%) of PTC and PMCs with follicular architecture (13%). Cytoplasmic MUC1 accumulation did not associate with any clinicopathological features except peritumoral lymphoid infiltration in PTCs and in PMCs with the conventional growth pattern. MUC1 staining correlated with cyclin D1 overexpression in conventional PTCs and PMCs and PMCs with follicular architecture. The results demonstrate that MUC1 expression varies broadly in different histological variants of PTC, being the lowest in tumors with follicular structure. In general, it does not prove to be a prognosticator of PTC aggressiveness. A high correlation between MUC1 and cyclin D1 implies MUC1 involvement in the Wnt cascade functioning in a large subset of human PTCs and PMCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MUC1 is a heavily glycosylated transmembrane protein, a member of the mucin family, expressed on the apical surfaces of epithelial cells in mammary glands and gastrointestinal, respiratory, urinary, and reproductive tracts [1], where it is believed to play a primary mechanical and chemical protective function of epithelial and mesothelial tissue linings [1, 2]. MUC1 overexpression has been found in a variety of human cancers, including thyroid carcinoma [3–7] and thyroid cancer cell lines [8]. In well-differentiated thyroid carcinomas, MUC1 may be a determinant of glycosylation features of tumor cells, but its overexpression did not prove to be a diagnostic marker [3]. Some differences in glycosylation pattern [4] and in alternatively spliced MUC1 mRNA species [5] have been reported in normal and neoplastic thyrocytes. It has also been shown that MUC1 mRNA overexpression is more typical of papillary thyroid cancer (PTC) but not of follicular adenoma, and most PTCs with elevated MUC1 mRNA levels displayed cytoplasmic MUC1 staining [6].
Recently, MUC1 gained an additional interest due to the newly demonstrated ability of its highly conserved cytoplasmic domain to interact with a variety of proteins involved in signal transduction [1, 9–11]. On the apical membrane of normal epithelial cells, MUC1 can act as a reservoir for β-catenin, preventing its release into the cytoplasm [10] and altering β-catenin interaction with cadherin in the formation of adherent junctions, resulting in diminished cell adhesion [10–12]. β-catenin is a key downstream effector of the Wnt signaling pathway that contributes to cell growth and survival [13–15]. The aberrant cytoplasmic accumulation of β-catenin in thyroid cancers was reported to significantly correlate with an overexpression of cyclin D1 [16–18], a transcriptional target of β-catenin and one of the critical cell cycle regulators which drives G1/S phase transition [13].
PTC may present with a wide diversity of clinicopathological features [19–25], but no systematic analysis of MUC1 expression in different PTC histotypes has been done to date. In the present study, we investigated MUC1 immunoexpression in PTCs with different histological architecture, size of primary tumor, and morphological manifestations of aggressiveness, and correlated it with cyclin D1 overexpression.
Materials and Methods
Thyroid Cancer Tissues and Morphology
Two hundred and nine paraffin blocks from 205 patients (33 males and 172 females) with thyroid cancer surgically treated at the Medical Radiological Research Center (MRRC, Obninsk, Russian Federation) during 1994–2005 were available for the study. Among the 209 tumors, 102 measured 1.0 cm and less in the largest dimension [papillary microcarcinoma (PMC), mean size 0.6 cm, mean age of patients 47.1 years], and 107 exceeded 1.0 cm in size (PTC, mean 2.2 cm, mean age of patients 45.8 years).
Histological classification was made according to the criteria described by LiVolsi [19] and Rosai et al. [20] by two pathologists from MRRC (A.A. and E.L.) and two pathologists from Nagasaki University (S.M. and M.N.). Whenever there was a discrepancy between the two groups of pathologists, the case was discussed under a multiple-head microscope and a consensus diagnosis was reached. Peri- and intratumoral lymphoid infiltration and fibrosis were scored as absent, weak, moderate, or strong, but only the samples with moderate and strong indices were considered positive, whereas those with absent or weak features were assigned negative. Clinical staging was done according to DeGroot et al. [26] Data on age, gender of patients, and size of tumor were retrieved from pathology records. Appropriate informed consent was obtained from each individual according to ethical guidelines for the use of human materials for scientific purposes effective in MRRC.
Immunohistochemistry
Paraffin-embedded tissues were dewaxed in xylene and rehydrated in phosphate-buffered saline. Endogenous peroxidase activity was blocked by immersion in 0.3% H2O2/methanol. Normal goat serum (10% v/v) was used to prevent nonspecific binding of the secondary antibody. After microwave antigen retrieval in sodium citrate buffer (pH 6.0), tissues were incubated overnight at 4°C with either anti-MUC1 monoclonal antibody (sc-7313, Santa Cruz Biotech, Santa Cruz, CA, USA, dilution 1:150) or anti-cyclin D1 monoclonal antibody (Zymed Labs, South San Francisco, CA, USA, dilution 1:50). The slides were subsequently incubated with a universal immunoperoxidase polymer anti-mouse/anti-rabbit immunohistochemical staining reagent (Histofine Simple Stain MAX PO MULTI, Nichirei Biosciences, Tokyo, Japan). Immunohistochemical reaction products were visualized with diaminobenzidine. For each series of immunohistochemistry, breast carcinoma and lymph node sections were used as the positive controls for MUC1 and cyclin D1, respectively. Nonimmune mouse IgG instead of the primary antibody was used as a negative control.
To assess MUC1/cyclin D1 correlations, double immunohistochemical staining was done. Briefly, after incubation with primary and secondary antibodies and product reaction visualization with diaminobenzidine, the second immunohistochemical reaction was performed using an alkaline phosphatase-conjugated anti-mouse IgG antibody and 5-bromo-4-chloro-indolyl phosphate/nitroblue tetrazolium chloride mixture (DakoCytomation, Carpenteria, CA, USA) as a substrate. Between the two phases of double immunostaining, sections were soaked overnight in hydrochloride-buffered 0.1 M glycine (pH 2.2) to reduce nontarget binding of the second antibody. For the negative control in the double immunohistochemistry experiments, the primary antibody of the second immunostaining procedure was omitted and incubation was done with the secondary antibody only.
Evaluation of Immunohistochemical Results
Immunohistochemical staining for MUC1 and cyclin D1 was evaluated by three independent observers (AA, SM, and MN). Discrepancies were resolved after discussion at a multiple-head microscope. For MUC1 scoring, only positive cytoplasmic staining was considered. Fine staining of apical membrane of normal thyroid epithelial cells was ignored. The intensity of cytoplasmic staining was recorded as absent, weak, moderate, or strong, and the level of MUC1 expression was graded as absent, focal (less than 50% positive cells), or diffuse (more than 50% positive cells). Cases were regarded as MUC1-positive when tumors displayed strong diffuse or focal or moderate diffuse staining, and MUC1-negative when staining was absent, weak, or moderate focal. For cyclin D1 evaluation, tumors were classified as positive if more than 30% of cells displayed nuclear staining and negative if otherwise, irrespective of staining intensity.
Statistical Analysis
The mean age of patients and the mean size of tumors were expressed as a mean value ± SE. To compare means, one-way ANOVA was used. Other parameters were expressed as either an absolute number of cases associated with a feature concerned or as a ratio of positive observations to total number of cases. Fisher’s exact test was used for categorical variables. Statistical significance level was set to p < 0.05.
Results
MUC1 Expression in Different Morphological Variants of PTC
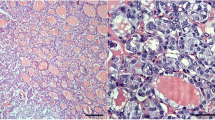
In the cancer tissues, cytoplasmic MUC1 staining was uneven, with the tumor periphery generally stained more often and more strongly than the central part, but the extent and intensity of immunostaining varied in different parts of tumors. Focal protein expression was also found in normal epithelial cells in the areas featuring lymphocytic thyroiditis (Fig. 1).
Different histological types of PTC and MUC1 expression. A conventional papillary carcinoma a, c and a peripheral focus of the solid growth pattern with peritumoral lymphoid infiltration b, d. The follicular variant e and a papillary carcinoma with mixed architecture and stromal lymphocytic infiltration f. The tall cell variant of papillary carcinoma g. A strong diffuse MUC1 immunostaining was observed in the papillary carcinoma with the conventional c, solid d, and mixed growth pattern f, and tall cell variant g. Negative immunostaining for MUC1 in the follicular variant of papillary carcinoma e. Strong focal MUC1 immunostaining detected in the epithelial cells of Hashimoto thyroiditis h. Hematoxylin and eosin staining a, b, immunohistochemistry for MUC1 c–h. Original magnification ×150 a, b, d, f, h and ×300 c, e, g
Positive MUC1 staining was observed in 4/4 (100%) oncocytic variant, 7/7 (100%) tall and columnar cell variants, 7/9 (78%) Warthin-like PTC, 43/71 (61%) conventional type PTC, and 41/79 (52%) PMCs with the conventional growth pattern (Table 1). Less often, MUC1 expression was detected in the follicular (3/11, 27%) and encapsulated (0/5) PTC variants and in PMCs with the follicular growth pattern (3/23, 13%). The difference in MUC1 expression prevalence was statistically significant in the combined follicular/encapsulated variants of PTC vs. combined conventional/all other variants of PTC, between the tall/columnar cell variants vs. conventional PTC, and between the two subgroups of PMCs (with conventional and follicular growth patterns).
The mean size of tumors was larger in the group of follicular (p = 0.01), oncocytic (p = 0.01), and tall and columnar cell variants (p < 0.01), as compared to the conventional PTCs (Table 1). Size of PMCs with the conventional growth pattern was larger than that of PMCs with the follicular architecture (p < 0.01). The prevalence of positive MUC1 staining was significantly higher in the conventional PTCs greater than 10 mm in diameter and PMCs between 5 and 10 mm in diameter compared to conventional PMCs measuring less than 5 mm (Table 2).
MUC1 Expression and Clinicopathological Features of PTC
Analysis of the clinicopathological features of MUC1-positive and -negative cases of conventional PTC did not reveal any associations apart from a significantly higher prevalence of peritumoral lymphoid infiltration and intratumoral fibrosis in MUC1-positive tumors (Table 3). The group of MUC1-positive PMCs with conventional growth pattern also demonstrated a significantly higher number of cases with lymphoid infiltration (peri- and intratumoral) compared to MUC1-negative PMCs. No significant difference in the extent of peri- and intratumoral infiltration, however, was observed between the conventional and follicular variants of PTC (data not shown) at variance with the distinct expression of MUC1 in these two groups. In PMCs, peri- and intratumoral infiltration was detected significantly more often in tumors with the conventional growth pattern than in those with follicular architecture; this difference corresponded with the differing MUC1 patterns in these tumors (data not shown).
Correlation between MUC1 Expression and Cyclin d1 Overexpression
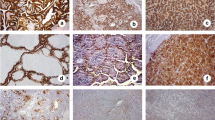
Nuclear cyclin D1 staining was present only in tumors and not in normal thyroid epithelial cells (Fig. 2). The pattern of cyclin D1 immunostaining varied between tumors and the reaction intensity was generally higher at the peripheral parts of widely invasive tumors than in the central tumor regions. The double immunohistochemical staining corroborated the results of separate examination of MUC1 and cyclin D1 expression (Fig. 2).
A solid focus of invasive papillary carcinoma displaying cyclin D1 overexpression a. Double immunostaining for MUC1 (blue cytoplasmic 5-bromo-4-chloro-indolyl-phosphatase/nitroblue tetrazolium staining) and cyclin D1 (brown nuclear diaminobenzidine staining) b in a papillary carcinoma. Original magnification ×300
In Table 3, we summarize data on the association of MUC1 expression with cyclin D1 overexpression in PTCs and PMCs with the conventional growth pattern. The prevalence of cyclin D1 overexpression was significantly higher in MUC1-positive conventional PTCs and PMCs than in the corresponding MUC1-negative tumors (p < 0.01). Similar results were obtained in PMCs with the follicular architecture: MUC1-positive tumors demonstrated cyclin D1 overexpression more frequently than the MUC1-negative group of tumors with the same morphology (p < 0.01, data not shown). In other histological variants of PTC, the association between MUC1 and cyclin D1 was not conducive to statistical analysis due to the small number of such tumors; however, a strong tendency similar to that observed in conventional PTCs was seen (data not shown).
Discussion
Pattern of MUC1 immunoexpression in our series of thyroid tissues differed in the normal and tumor epithelium. In the unaffected follicular cells, we observed a fine MUC1 immunostaining at the apical membrane, whereas in the cancer tissue, it was rather irregular (mixed membrane and cytoplasmic), with staining intensity varying between individual tumors, in line with previous reports on thyroid [6] and breast cancer [27]. It is worth noting that the cytoplasmic MUC1 expression, the only pattern type considered positive in this study, was detected not only in PTC, but also in the epithelial component of lymphocytic thyroiditis with no cancer features. This observation rules out an exclusively specific MUC1 association with malignancy.
MUC1 immunoexpression varied in PTCs of different histotypes. We observed a high prevalence of positive staining in the tall cell, columnar cell, Warthin-like, and oncocytic variants in contrast to the follicular and encapsulated variants of PTC. The encapsulated variant of PTC was intentionally considered here as a separate group, as it differed from other histotypes in terms of the absence of lymph node metastases. According to the original classification of thyroid tumors proposed by Rosai et al., the encapsulated variant of PTC is assigned an individual entity mostly due to an excellent clinical course and prognosis [20]. Despite the fact that the encapsulated and follicular variants of PTC differed from each other by their manifestations of aggressiveness (i.e., unlike the encapsulated variant, the follicular variant of PTC is often accompanied by nodal disease), in both of these groups MUC1 immunoexpression was lower than in the conventional and Warthin-like tumors. The oncocytic and aggressive tall and columnar cell variants of PTC with a very high rate (80–100%) of local invasion and lymph node metastases demonstrated the strongest MUC1 accumulation.
Apparently, MUC1 immunoexpression in all histological variants of PTC but follicular correlated with the morphological features of tumor aggressiveness such as intraglandular spreads and regional metastases. The less aggressive encapsulated variant of PTC displayed lower frequency of MUC1 expression, whereas all cases of the aggressive tall and columnar cell variants were MUC1-positive. Interestingly, the follicular variant of PTC, morphologically not less aggressive than the conventional PTC, demonstrated lower prevalence of MUC1 expression. An assumption that this difference might be due to differing frequency of peritumoral lymphocytic infiltration between these two variants of PTC was not confirmed when statistical analysis was applied. Perhaps the discrepancy in MUC1 accumulation in the follicular and conventional variants of PTC may be rather attributed in part to the mutation-specific gene expression profiles in PTCs driven by RET/PTC, BRAF, RAS, and PAX8-PPARγ oncogenes [28, 29]. Mutated BRAF is known to be more prevalent in PTCs with the conventional morphology, whereas in the follicular variant of PTC, PAX8-PPARγ rearrangements and missense RAS mutations have been reported with high frequency [29, 30]. In our series, BRAF T1799A transversion was observed in 43 and 10% of the conventional and follicular variants of PTC, respectively (data not shown). It is therefore possible that the follicular variant of PTC may result in lower MUC1 levels depending on the variant-associated oncogene, as well as other, so far unidentified factors.
Despite the absence of correlation between MUC1 expression and lymphocytic infiltration observed in the comparison of the follicular and conventional variants of PTC, it is tempting to speculate that inflammation might modulate MUC1 expression in both thyroid cancer and nontransformed epithelial cells. First, there was a focal cytoplasmic MUC1 staining in lymphocytic thyroiditis tissues; second, unlike in larger tumors, there was a significant correlation between MUC1 immunostaining and lymphocytic infiltration in PMCs with the conventional growth pattern as compared to the tumors with the follicular architecture; and third, in several cases of generally MUC1-negative PTC, we found an intensive cytoplasmic MUC1 staining along the needle scar remained after the preoperative fine needle aspiration biopsy diagnostic procedure (not shown). Clearly, inflammation could not be identified as the only feature that determines the aberrant MUC1 accumulation in normal or tumor thyrocytes; however, it possibly influences the affected epithelium. Further studies would be necessary to distinguish whether MUC1 exerts its protective function as a response to inflammation or whether it elicits host immune reaction on the tumor growth in the thyroid.
With regard to the conventional PMCs, our study of a large group of tumors showed a relationship between the prevalence of MUC1 expression and tumor size. The frequency of MUC1 immunoexpression in PMCs sized less than 5 mm was significantly lower than in the microcarcinoma ranging 5–10 mm in size and conventional type of PTC sized greater than 10 mm. Furthermore, the smaller tumors displayed a statistically lower rate of cyclin D1 overexpression as compared with the tumors of larger size being in line with our previous report [18]. An association between MUC1 expression and lymph node metastases was not found in these series of PMCs. Based on these observations, it currently seems unlikely that MUC1 staining may be a predictor of an aggressive behavior of PMC.
A strong correlation between cyclin D1 overexpression and the aberrant expression of β-catenin in PTCs and a subset of PMCs has been shown in previous studies [16–18]. As a potential mechanism, the protein kinase C-phosphorylated cytoplasmic tail of MUC1 has been shown to bind and signal through β-catenin and the mitogen-activated protein kinase pathways [9]. A fragment of the cytoplasmic tail of MUC1 undergoes cytosol-to-nucleus trafficking in association with β-catenin and suggests a MUC1 effect on β-catenin transcriptional coactivator function [11]. A strong correlation between MUC1/β-catenin binding and metastatic progression of the tumor has been shown in breast carcinoma [10].
In view of these data, we examined a correlation between MUC1 and the Wnt pathway in thyroid tumors using cyclin D1 as an endpoint. In the conventional PTCs and in PMCs with the conventional and follicular growth pattern, we observed a significant relationship between the cytoplasmic MUC1 and cyclin D1 immunostaining. In rarer PTCs such as tall and columnar cell, Warthin-like, and oncocytic variants, the same tendency was clearly seen but remained statistically difficult because of the relatively low number of such tumors. These results demonstrate a possible correlation between MUC1 accumulation and regulatory impairment of the Wnt pathway in a substantial proportion of PTC, suggesting that MUC1 has a role in β-catenin/cyclin D1 signaling in this form of human malignancy.
The clinical and prognostic significance of MUC1 expression in thyroid cancer is still an open question. The cytoplasmic MUC1 staining was thought to be associated with PTCs with an unfavorable clinical course and lymph node involvement [6]. An aggressive tall cell variant of PTC that usually has a poorer prognosis has been shown to be MUC1-positive on immunohistochemistry more often than the conventional variant [7]. A recent study demonstrated that MUC1 gene upregulation and MUC1 overexpression could contribute to clinical behavior and outcome of PTC not only in the aggressive tall cell variant but also in some tumors with the conventional growth pattern [7]. In our series, we detected positive MUC1 staining in 61% of PTCs with conventional morphology. Given that 10–15% PTCs exhibit an aggressive behavior manifested in local invasion, distant metastases, treatment resistance, and mortality [31], it would be difficult to fit our results to the above mentioned claims. In addition, we observed a strong MUC1 staining in 78% of cases of the Warthin-like PTC (tumors with an oncocytic cellular composition and extensive lymphoid infiltration). Data from the literature on clinical follow-up of patients with this type of cancer suggest either that its prognosis is not less good than that of typical PTC [24] or that such tumors display about the same morphological manifestations of aggressiveness and may have a rather favorable clinical outcome [25]. For this and other reasons discussed above, we conclude that MUC1 accumulation could not constitute a potent prognostic role for PTC.
In summary, our study demonstrated that MUC1 expression is lower in the encapsulated and follicular variants of PTC compared to other histotypes of thyroid carcinomas. In PMCs with the conventional growth pattern, MUC1 expression was proportional to tumor size, being lower in PMCs measuring less than 5 mm and higher in larger tumors. Cytoplasmic MUC1 accumulation did not correlate with any of the clinicopathological features of the tumors except for lymphoid infiltration, although inflammation per se did not strictly determine MUC1 overexpression. MUC1 pattern correlated with cyclin D1 immunostaining, implying that MUC1 is involved in the Wnt cascade functioning in thyroid cancer.
References
Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia 6:339–53, 2001.
Perez-Vilar J, Hill RL. The structure and assembly of secreted mucins. J Biol Chem 274:31751–4, 1999.
Alves P, Soares P, Fonseca E, et al. Papillary thyroid carcinoma overexpresses fully and underglycosylated mucins together with native and sialylated simple mucin antigens and histo-blood group antigens. Endocr Pathol 10:315–24, 1999.
Magro G, Schiappacassi M, Perissinotto D, et al. Differential expression of mucins 1–6 in papillary thyroid carcinoma: evidence for transformation-dependent post-translational modifications of MUC1 in situ. J Pathol 200:357–69, 2003.
Weiss M, Baruch A, Keydar I, et al. Preoperative diagnosis of thyroid papillary carcinoma by reverse transcriptase polymerase chain reaction of the MUC1 gene. Int J Cancer 66:55–9, 1996.
Bieche I, Ruffet E, Zweibaum A, et al. MUC1 mucin gene, transcripts, and protein in adenomas and papillary carcinomas of the thyroid. Thyroid 7:725–31, 1997.
Wreesmann VB, Sieczka EM, Socci ND, et al. Genome-wide profiling of papillary thyroid cancer identifies MUC1 as an independent prognostic marker. Cancer Res 64:3780–9, 2004.
Patel KN, Maghami E, Wreesmann VB, et al. MUC1 plays a role in tumor maintenance in aggressive thyroid carcinomas. Surgery 138:994–1001; discussion 1001–2, 2005.
Carraway KL, Ramsauer VP, Haq B, et al. Cell signaling through membrane mucins. Bioessays 25:66–71, 2003.
Schroeder JA, Adriance MC, Thompson MC, et al. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene 22:1324–32, 2003.
Wen Y, Caffrey TC, Wheelock MJ, et al. Nuclear association of the cytoplasmic tail of MUC1 and beta-catenin. J Biol Chem 278:38029–39, 2003.
Wesseling J, van der Valk SW, Vos HL, et al. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol 129:255–65, 1995.
Hatsell S, Rowlands T, Hiremath M, et al. Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia 8:145–58, 2003.
Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev 11:3286–305, 1997.
Gumbiner BM. Signal transduction of beta-catenin. Curr Opin Cell Biol 7:634–40, 1995.
Ishigaki K, Namba H, Nakashima M, et al. Aberrant localization of beta-catenin correlates with overexpression of its target gene in human papillary thyroid cancer. J Clin Endocrinol Metab 87:3433–40, 2002.
Nakashima M, Meirmanov S, Naruke Y, et al. Cyclin D1 overexpression in thyroid tumours from a radio-contaminated area and its correlation with Pin1 and aberrant beta-catenin expression. J Pathol 202:446–55, 2004.
Lantsov D, Meirmanov S, Nakashima M, et al. Cyclin D1 overexpression in thyroid papillary microcarcinoma: its association with tumour size and aberrant beta-catenin expression. Histopathology 47:248–56, 2005.
LiVolsi V. Surgical pathology of the thyroid. Philadelphia: Saunders; 1990.
Rosai J, Carciangiu ML, DeLellis RA. Tumors of the thyroid gland. Washington, D.C.: Armed Forces Institute of Pathology; 1992.
LiVolsi VA, Albores-Saavedra J, Asa SL. Papillary carcinoma. In: DeLellis RA, Lloyd R, Hertz PhU, Eng C, editors. WHO classification of tumors. Pathology and genetics of tumors of endocrine organs. Lyon: IARC Press. p. 57–66, 2004.
Khoo ML, Ezzat S, Freeman JL, et al. Cyclin D1 protein expression predicts metastatic behavior in thyroid papillary microcarcinomas but is not associated with gene amplification. J Clin Endocrinol Metab 87:1810–3, 2002.
Hunt JL, LiVolsi VA, Baloch ZW, et al. Microscopic papillary thyroid carcinoma compared with clinical carcinomas by loss of heterozygosity mutational profile. Am J Surg Pathol 27:159–66, 2003.
Apel RL, Asa SL, LiVolsi VA. Papillary Hurthle cell carcinoma with lymphocytic stroma. “Warthin-like tumor” of the thyroid. Am J Surg Pathol 19:810–14, 1995.
Ozaki O, Ito K, Mimura T, et al. Papillary carcinoma of the thyroid. Tall-cell variant with extensive lymphocyte infiltration. Am J Surg Pathol 20:695–8, 1996.
DeGroot LJ, Kaplan EL, McCormick M, et al. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab 71:414–24, 1990.
Rahn JJ, Dabbagh L, Pasdar M, et al. The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer 91:1973–82, 2001.
Giordano TJ, Kuick R, Thomas DG, et al. Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene 24:6646–56, 2005.
Castro P, Rebocho AP, Soares RJ, et al. PAX8-PPARgamma rearrangement is frequently detected in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab 91:213–20, 2006.
Zhu Z, Gandhi M, Nikiforova MN, et al. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol 120:71–7, 2003.
Shaha AR, Shah JP, Loree TR. Patterns of failure in differentiated carcinoma of the thyroid based on risk groups. Head Neck 20:26–30, 1998.
Acknowledgement
This work was supported in part by grant numbers 18591030, 19390253, and 19790651 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abrosimov, A., Saenko, V., Meirmanov, S. et al. The Cytoplasmic Expression of MUC1 in Papillary Thyroid Carcinoma of Different Histological Variants and its Correlation with Cyclin D1 Overexpression. Endocr Pathol 18, 68–75 (2007). https://doi.org/10.1007/s12022-007-0012-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-007-0012-x