Abstract
Purpose
Adrenal incidentalomas (AI) are discovered after work-up unrelated to adrenal gland diseases; up to 30% of AI show subclinical endogenous cortisol excess (SH), frequently associated to hypertension, obesity, metabolic disorders and increased incidence of cardiovascular events (CVEs).
Methods
We analysed 628 AI patients divided into two groups: 471 non-functional adrenal adenoma (NFA) and 157 SH. All patients underwent complete examinations, 24-h ambulatory blood pressure monitoring, biohumoral parameters and vascular damage markers, such as c-IMT and ankle brachial index. After long-term follow-up, we registered newly onset of CVEs such as myocardial infarction (MI), percutaneous stenting and surgical bypass (PTA/CABG), stroke, overall/cardiovascular mortality. Moreover, SH patients underwent to surgical (SSH) or pharmacological treatment (MSH).
Results
SH patients showed higher prevalence of metabolic syndrome, diabetes mellitus, and previous CVEs respect NFA at baseline. After follow-up MSH group showed higher recurrence of major CV events compared with NFA and SSH (RR 2.27 MSH vs NFA for MI; RR 2.30 MSH vs NFA for PTA/CABG; RR 2.41 MSH vs NFA for stroke). In SSH there was a significant reduction of the number of antihypertensive medications needed to reach target blood pressure levels (2.3 ± 1.0 to 1.5 ± 0.4 drugs). None differences were found in SH patients, distinguished in relation to cortisol plasma levels after dexamethasone suppression test (1.8–5 µg/dL, above 5 µg/dL).
Conclusions
SH is linked to relevant cardiovascular and metabolic alterations, leading to worsen clinical outcomes. In eligible patients, adrenalectomy is valid and safe option to treat SH, reducing cardiometabolic abnormalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidentally discovered adrenal mass during exams not strictly related to adrenal disorders is defined “adrenal incidentaloma” (AI) [1, 2]. High availability and routinely use of radiological imaging leads to discover AI in more than 10% of adults, up to 30% in subjects older than 80 years [2, 3]. Standardized and widespread hormonal evaluation allowed to find in more than 30% of AI an autonomous overproduction of cortisol from AI, not related to ACTH levels, without clinical signs or symptoms of Cushing’s disease, condition called “subclinical Cushing’s syndrome” or “subclinical hypercortisolism” (SH) [4]. In the past decades, many international Guidelines have suggested different hormonal assessment in order to identify SH, but recently the use of 1-mg overnight dexamethasone suppression test (DST) is the pivotal test in detection of SH [5].
As well as it is well established that overt hypercortisolism is associated to several comorbidities, also SH has been associated with several metabolic and cardiovascular (CV) comorbidities [6], as hypertension, impairment of glucose metabolism (until type 2 diabetes), inducing high overall CV events and CV mortality [7]. In literature, despite heterogeneity of methodological approaches (retrospective or prospective models of study, different criteria for SH diagnosis, duration of follow-up, data recorded), it is obvious that SH is related to worsen CV and metabolic profile, with higher prevalence of CV events (almost 3-times) and CV mortality (more than 10-times) in SH patients respect non-functional AI (NFAI) [8]. Previously in SH it was found the significant improvement of 24-h blood pressure profile and reduction of metabolic syndrome (MS) prevalence after follow-up of 12 months from surgical treatment (unilateral adrenalectomy) [9]. Moreover, Di Dalmazi et al. showed that subjects with worsened secreting patterns of endogenous cortisol secretion after DST are characterized by higher rate of CV events and lower survival rates for all-cause mortality respect NFAI and patients with stable cortisol secretion [3].
The aim of this study was to evaluate, through a prospective study, the natural history of NFAI and SH subjects in a long-term follow-up setting. Moreover, we intend to evaluate different ongoings of SH patients treated by surgical removal of cortisol-secreting adrenal adenoma (adrenalectomy) compared with patients pharmacologically treated.
Material and Methods
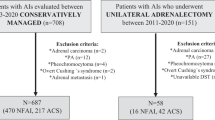
From January 2000 to June 2018, we consecutively enrolled 765 patients referred to Tertiary Centre of Secondary Arterial Hypertension Unit for AI. The diagnosis of AI was based on imaging procedures [abdominal computed tomography (CT) or magnetic nuclear resonance] performed during evaluations not related to adrenal diseases. The radiological features used to define a benign adrenal mass were precontrast Hounsfield units (≤10 HU), 15-min washout after contrast infusion (≥50%), and regular margins [7, 10]. We excluded patients with known or suspected malignancies, renal disease, or cysts. We performed complete clinical and hormonal assessment in order to exclude pheochromocytoma, primary hyperaldosteronism, overt clinical signs of hypercortisolism or hyperandrogenism. We also excluded patients with an history of extended steroid intake or those patients without complete follow-up. Therefore, 137 patients were excluded from the study for the above reasons (18%).
Serum cortisol levels post dexamethasone (≤50 nmol/L or ≤1.8 μg/dL) was used as a diagnostic criterion for the exclusion of autonomous cortisol secretion. SH was defined with serum cortisol levels after 1 mg of DST greater than 1.8 mg/dL (or >50 nmol/L) plus one abnormal hormonal tests of hypothalamic-pituitary-adrenal axis: (a) urinary free cortisol (UFC) level >100 mcg/24 h; (b) morning (8:00 h) plasma ACTH levels <10 pg/ml) [1]. Moreover, we have distinguished SH patients in two groups in relation to cortisol levels post dexamethasone test: between 1.8 and 5 µg/dL and above 5 µg/dL.
ACTH was measured with radioimmunoassay method (RIA), normal limit 10–90 pg/ml, inter/intra-assay CV 8.3/6.2; plasma cortisol was measured with RIA, normal limit 9.6–26 lg/dl, inter/intra-assay CV 5.5/4.5; UFC was measured with RIA, normal limit 1.37–7.53 lg/24 h, inter/intra-assay CV 5.5/4.5. All hormone assays were performed with commercially available kits.
Each patient received detailed description of the study protocol and all subjects signed the informed consent form and gave written approval to be included in this study population, according to the latest version of the World Medical Declaration of Helsinki (2013).
Anthropometric and laboratory data were retrieved in all patients at baseline and during follow-up. The MS was defined by ATPIII-NCEP criteria [11]. The presence of diabetes was evaluated by serum glucose behaviors, Hba1c or oral glucose tolerance test. Ambulatory blood pressure monitoring (ABPM) was performed using the oscillometric technique, which involves a portable lightweight, noninvasive monitor with self-insufflating cuff (Spacelabs Medical, 90207, Issequah, WA, USA). ABPM readings were obtained at 15-min intervals from 6 AM to midnight and at 30-min intervals from midnight to 6 AM. The definitions of “dipper” and “non-dipper” were established where night-time SBP and DBP decrease was >10 and <10%, respectively. Ambulatory hypertension was defined as 24-h BP [125/80 mmHg] [12]. Subjects without a complete 24-h BP measurement (14 diurnals and 7 nocturnal measurements) were excluded from the study.
In all patients we performed by the same operator (AC) eco-color Doppler evaluation of carotid arteries, using Hewlett-Packard Sonor 5500 Ultrasound system (Hewlett Packard, Andover, Massachusetts, USA), equipped with a 3.11 MHz realtime B-mode scanner was used for the evaluation. Imaging of the right common carotid artery (CCA) was performed with the individuals turning their head 45° to the left. The high-resolution images were analysed to calculate cIMT, defined as thickness of the vascular intima-media complex obtained in five consecutive regions of the wall of the CCA, every 4–5 mm beginning close to the bifurcation. The value attributed to each individual was the average value among the cIMT measurement, five from the left and five from the right carotid artery.
In patients with biochemical evidence of SH, clinical indication to surgical treatment [related to presence at least of two relevant comorbidities potentially related to autonomous cortisol secretion (as arterial hypertension, glucose intolerance or type 2 diabetes mellitus, MS, obesity, dyslipidaemia), at least one of these poorly controlled by medical measures], and expressed consent to its procedure, we performed an adrenocortical scintigraphy specific for the adrenal cortex using radiopharmaceuticals (131-I-6b-iodio-methyl-nor-cholesterol) or 18F-fluorodeoxyglucose (FDG) PET/CT total body (in relation to availability); unilateral uptake on the side of the adrenal mass (concordant up-take) confirmed unappropriated cortisol secretion due to single adrenal adenoma [13, 14]. We avoided to perform scintigraphy or FDG PET/CT in patients with large adrenal mass with absolute indication to surgical treatment (i.e., >6 cm).
In 39 SH patients we performed unilateral adrenalectomy (SSH group) with biochemical remission of cortisol excess evaluated during follow-up (thought normalization of serum cortisol after DST and 24-h urinary cortisol excretion); of these, 29 patients completed the entire period of follow-up of 36 months (at baseline, 78% with serum cortisol levels after DST between 1.8–5 µg/dL or 50–138 nmol/L; 22% over 5 µg/dL or 138 nmol/L). On the other hand, 118 SH patients did not undergo to adrenalectomy despite clinical indication because of different reasons (lack of unilateral and concordant uptake on scintigraphy or FDG PET/TC, refusal of surgical treatment, personal preference), and then were pharmacologically treated with optimized therapy in order to reduce metabolic and CV altered parameters (higher doses of drug tolerated by patients or fulfill dosed to get control) (MSH group) (at baseline, 70% with serum cortisol levels after DST between 1.8 and 5 µg/dL or 50 and 138 nmol/L; 30% over 5 µg/dL or 138 nmol/L).
Evaluation during follow-up
The same specialized physicians (CL, LP, GDT, FO) assessed patients every 12–15 months for the first 3 years after diagnosis through clinic evaluation (by hormonal and instrumental tests), and subsequently at 24–36 months through interview until a follow-up of 15 years. We recalled by telephone the participants who missed a scheduled follow-up visit. If a patient had died, we obtained date and cause of death by telephone contact with family or the patient’s family physician.
During follow-up we evaluated the state of hypertension, dyslipidaemia, type 2 diabetes, and newly onset of major cardiovascular events (CVEs), and concomitant treatments.
We used long-term follow-up (15 years) to define Kaplan–Meier curves regarding newly CV events and CV mortality.
Statistical analysis
All data are expressed as mean standard deviation. Differences between means were assessed by the Student’s t test or the Mann–Whitney U test in non-normally distributed data for two-sample comparison, or by one-way analysis of variance applying the Fisher least significant difference post hoc test for multiple comparisons. X2 statistics were used to assess differences between categorical variables.
Univariate, multiple linear regression analysis and Cox proportional-hazards were made with Backward Stepwise Regression method to evaluate models for CV events and CV mortality.
We have evaluated several hormonal parameters (plasma cortisol post dexamethasone test, UFC, ACTH plasma levels), and plotted then on the receiver operating characteristic (ROC) curve for evaluate CV and metabolic outcomes in patients surgically or conservatively treated. The most appropriate cutoff values were established as the ones with higher result of the sum of sensitivity and specificity.
P values less than 0.05 were taken as statistically significant. Statistical analysis was performed using dedicated statistical software SPSS (Statistical Package for Social Sciences, software, version 24; SPSS Inc, Chicago, Illinois, USA) and e GraphPad (version 5.0; GraphPad Software, Inc, La Jolla, California, USA).
Results
We enrolled a total of 628 patients with AI (321 M, 253 F; mean age 60.2 ± 12.2 yrs), distinguished in 471 NFA (282 M, 189 F; mean age 59.6 ± 12.5), and 157 SH (93 M; 64 F; mean age 62.9 ± 11.1); bilateral AIs were found in 83 (13.2%) patients. The mean dimension of adrenal lesions was 22.8 ± 12.5 mm, without significant differences on mean diameter between NFA and SH patients (22.4 ± 12.7 mm vs 24.5 ± 13.6 mm, respectively).
Table 1 shows parameters of overall patients enrolled at baseline. SH patients show higher office systolic and diastolic blood pressure (SBP and DBP) compared with NFA patients (139 ± 20.9 mmHg vs 145 ± 2.4 mmHg, p < 0.01; 82.9 ± 10.9 mmHg vs 85.7 ± 12.5 mmHg, p < 0.02; respectively). SH patients showed higher levels of uric acid respect NFA patients (5.2 ± 1.5 mg/dl vs 4.8 ± 1.6 mg/dl; p < 0.05). Moreover, at baseline we found significantly higher prevalence of MS (classified by ATP III criteria) (42.8%) and diabetes mellitus (19%) in SH patients respect NFA group (12.7 and 7%, p < 0.05; respectively). None differences of number of medications used were observed (lipid-lowering, antihypertensive drugs and oral antidiabetic drugs) in all subjects, albeit we found an increasing trend to use more antihypertensive drugs in SH patients respect NFA group (2.4 ± 1.2 vs 2.0 ± 1.1).
At baseline, in overall subjects enrolled the anamnestic evaluation of previously diagnosed major CVEs leads to find a significant higher prevalence for all main categories in SH patients compared with NFA patients: myocardial infarction (MI) (6.4 vs 1.5%, p < 0.05), percutaneous transluminal angioplasty/coronary artery bypass graft (4.5 vs 0.4%, p < 0.05), and stroke (5.1 vs 1.5%, p < 0.05) (Table 1).
In total, 29 subjects (10 M, 19 F; mean age 61.7 ± 11.6 yrs) underwent unilateral adrenalectomy with complete restoration of cortisol excess, completing the entire period of follow-up of 36 months; whereas 118 patients (M 50, 68 F; mean age 62.2 ± 11.2 yrs) were pharmacologically treated with optimized therapy in order to reduce metabolic and CV altered parameters. None of patients surgically-treated had peri- and post- procedural major complications.
Table 2 shows demographic, hemodynamic and biochemical parameters in overall patients distinguished in three groups: NFA, MSH, and SSH. Interestingly, at the end of ambulatory follow-up SSH showed significant decrease of waist circumference (100.5 ± 10.8 cm vs 94.1 ± 6.6 cm; p < 0.05) and neck circumference (38.1 ± 3.6 cm vs 34.2 ± 5.1 cm; p < 0.05), whereas MSH group showed increased neck circumference during follow-up (38.2 ± 3.5 cm vs 39.1 ± 3.8 cm; p < 0.05). Both SSH and MSH groups showed significant reduction of office-SBP (131.6 ± 17.4 mmHg vs 123.2 ± 8.45 mmHg, 145.6 ± 21.6 mmHg vs 134.9 ± 16.2 mmHg, respectively; p < 0.05) and office-DBP (80.2 ± 10.7 mmHg vs 73.8 ± 5.3 mmHg, 85.6 ± 12.4 mmHg vs 80.4 ± 9.1 mmHg, respectively; p < 0.05). Regarding biochemical parameters, after ambulatory follow-up in SSH patients we found significant reduction of total cholesterol (195.2 ± 34 mg/dl vs 189.5 ± 30.2 mg/dl; p < 0.05) and LDL-cholesterol (121.5 ± 32.2 mg/dl vs 112 ± 27.3 mg/dl; p < 0.05); whereas we observed significative increase of uric acid levels in MSH (5.1 ± 1.5 mg/dl vs 5.5 ± 1.3 mg/dl; p < 0.05) and NFA patients (4.8 ± 1.6 mg/dl vs 5.1 ± 1.6 mg/dl; p < 0.05).
Interestingly, in SSH group reduction of blood pressure behaviors was observed during at 24-h ABPM evaluation (Table 3), associated to significant reduction of mean number of anti-hypertensive medications used (2.3 ± 1.0 vs 1.1 ± 0.4; p < 0.05); whereas in MSH and NFA group significant reduction of blood pressure behaviors was not found as well as mean number of anti-hypertensive medications. Furthermore, SSH patients had also an improvement of circadian pressure profile, with significative reduction of “non-dipping pattern” after follow-up (from 48.3 to 27.5%; p < 0.05).
Moreover, after ambulatory follow-up in SSH patients we have described the significative reduction of MS prevalence (42.6 vs 18%; p < 0.05) as well as of diabetes mellitus (20.7 vs 7.4%; p < 0.05). Regarding ATPIII criteria, SSH patients showed a drastic reduction of incidence in hypertension (89.2 to 40.2%; p < 0.05), hypertriglyceridemia (27.8 to 10.8%, p < 0.05) and waist circumference altered (55 vs 35%, p < 0.05). On the other hand, in MSH group we observed significant increase of waist circumference altered (>102 cm for men and >88 cm for women) (46.7 vs 48.9%, p < 0.05). In MSH patients we found a significant thickening of c-IMT (from 0.91 ± 0.5 to 1.0 ± 0.2; p < 0.01) and an increased incidence of plaques (from 20 to 32%, p < 0.01) (Table 4). In brief in Table 5 are reported the main hormonal and clinical characteristics of each patient who underwent surgery should be added.
Figure 1a represents Kaplan–Meier curves showing incidence of newly CVE in overall patients during long-term follow-up (15 years). MSH patients have higher CV events than patients with NFA and SSH, with Unadjusted risk ratio (RR) for CV events in MSH respect NFA and SSH of 2.56 and 2.10, respectively (p < 0.001). Figure 1b represents Kaplan–Meier curves for CV mortality. Compared with patients with NFA and SSH, unadjusted CV mortality at the end of the follow-up gets worse in patients with MSH, with cardio-vascular cumulative survival significantly reduced (RR 2.64 respect NFA and 1.72 respect SSH; p < 0.001). Regarding incidence of newly CVE or CV mortality observed during follow-up, none differences were found in SH patients, either conservatively or surgically treated, distinguished in relation to cortisol plasma levels after DST (in SSH group 78% had values between 1.8 and 5 µg/dL, and 22% above 5 µg/dL; in MSH group 70% had values between 1.8 and 5 µg/dL, and 30% above 5 µg/dL).
Kaplan–Meier Curve showing global cardio-vascular events (a) (nonfatal acute myocardial infarction, percutaneous transluminal coronary angioplasty and surgical bypass for ischemic heart disease, or ischemic stroke) and logarithmic cumulative cardio-vascular survival (b) in patients with NFA, MSH and SSH during follow-up (15-years)
Table 6 shows the results of univariate and Cox proportional-hazards models for CVE; CVE prevalence was independently associated positively with mean value of 24-h systolic blood pressure (p < 0.0001), with an independent contribution of plasma levels of LDL-cholesterol and plasma levels of serum glucose. Whereas, regarding univariate and Cox proportional-hazards models for CV mortality (Table 7), CV mortality was independently associated with plasma levels of serum glucose (p < 0.001). In Fig. 2 the ROC curve analysis shows the association between urinary serum cortisol (UFC) at baseline and (A) the reduction of office systolic blood pressure after surgery [best cutoffs for UFC was set at 109.65 nmol/24 h (AUC 0.720 ± 0.106, p < 0.05; sensibility 76.2%, specificity 37.5%)] and (B) and reduction of waist circumference after surgery [best cutoffs for UFC was set at 132 nmol/24 h (AUC 0.815 ± 0.153, p < 0.05; sensibility 63%, specificity 50%)].
The receiver operating characteristic (ROC) curve analysis shows (a) the diagnostic performance of urinary free cortisol (UFC) at baseline to discriminate Office Systolic Blood pressure (O-SBP) reduction after surgery in SSH patients; the best cutoffs for UFC was set at 109.65 nmol/24 h (AUC 0.720 ± 0.106, p < 0.05; sensibility 76,2%, specificity 37,5%); (b) the association between UFC at baseline and the reduction of waist circumference after surgery in SSH patients. The best cutoffs for UFC was set at 132 nmol/24 h (AUC 0.815 ± 0.153, p < 0.05; sensibility 63%, specificity 50%)
Discussion
Several evidences show that the autonomous secretion of cortisol from AI, defined as SH, despite absence of overt phenotypical features of Cushing’s Syndrome, is associated to metabolic and CV complications inducing reduction of either quality of life and survival, due to higher incidence of CV events and CV mortality [7, 8, 15,16,17,18,19]. In this respect, Di Dalmazi et al. [7] have evaluated in large registry of AI patients, newly onset of CVE and overall and CV-specific mortality, distinguishing the enrolled subjects in relation to response to DST during follow-up. In particular, these Authors revealed that SH patients showed higher incidence of CVE compared with NFAI patients. Moreover, subgroup of SH patients with worsened secreting patterns after DST during follow-up, showed highest incidence of CVE respect to patients with stable cortisol secretion. In this study, beyond age, the main factor related to worsen CV global risk factor was the mean concentrations of cortisol after DST, suggesting in SH patients the pivotal role of cortisol excess in the development of metabolic and CV abnormalities.
Recently, in a systematic review Park et al. [8] have compared all studies performed on AI and SH [7, 15,16,17,18,19]. Despite differences about methodological analysis (retrospective models, different diagnostic criteria for SH, extreme heterogeneity of metabolic and CV outcomes evaluated, short period of evaluation), the Authors have confirmed that SH is associated to higher incidence of CVEs and CV mortality, as well as to higher prevalence of diabetes mellitus and hypertension.
Our study agrees with previous evidences and reinforces thesis that chronic cortisol autonomous secretion by AI is related to progressive worsening of cardio-metabolic phenotype [20, 21].
In particular, we adopted clinical practice published guidelines, that are concordant with use of 1 mg DST for the screening of endogenous hypercortisolism, with cortisol threshold <1.8 µg/dl to define adequate suppression [1], associated to other biochemical abnormalities: low ACTH levels (<10 pg/ml), higher 24-h UFC excretion (over the upper limit of reference values) or missing circadian rhythm of cortisol [1, 21]. Moreover, other strengths of our study are the long-term prospective observation (follow-up more than 15-year), and the specific evaluation of major CV outcomes.
Interestingly, at the time of enrolment we found higher prevalence of MS and diabetes mellitus in SH patients compared with NFAI patients (42.8% vs 12.7% and 19% vs 7%, respectively), as well as higher prevalence of previous major CVE, as MI (6.4 vs 1.5%), stroke (5.1 vs 1.5%) and revascularization procedures (CABG or BPAC) (4.5 vs 0.4%), confirming in SH patients the higher CVE events and the role of metabolic abnormalities, as MS and altered glucose metabolism. Recently, Araujo-Castro M. et all have found in 149 AI patients, followed-up for a mean time of 34.6 months, higher prevalence of diabetes in subjects with plasma cortisol values >1.8 μg/dl after DXM test respect to NFAI (38.0 vs 22.0%, p < 0.04) [22].
Chronic exposure to hypercortisolism can impair glucose homeostasis by modifying the complex network of signaling in adipose tissue biology [23], exerting pleiotropic effects on different fat depots [24]. In case of endogenous or exogenous glucocorticoid excess, dysregulated adipose physiology brings to abdominal obesity and high circulating levels of free fatty acids and triglycerides [25, 26]. Lipid overload is the consequence of altered insulin signaling in adipocytes, increased lypolisis, aberrant adipokine secretion and low-grade inflammation, inducing peripheral insulin resistance, up to overt diabetes mellitus [27, 28]. As consequence, some Authors had proposed screening, by overnight 1 mg DST, on type 2 diabetes population, especially if associated to poor glycemic control and advanced microvascular damage. Recently, Cansu et at. have found significant prevalence of SH (2%) in diabetic patients with high levels of HbA1c (>8%) and macrovascular complications, as nephropathy, retinopathy and polyneuropathy [29].
We previously described impairment of adipokines levels and adipokines expression in intra-abdominal adipose tissue in SH patients; in fact, we found higher tissue expression of Leptin mRNA from fat surrounding adrenal gland in cortisol-producing adrenal adenomas than in NFAs [30]; on the other hand, in cortisol-secreting group, Adiponectin mRNA expression was reduced in fat surrounding cortisol-secreting adenoma of adrenal gland. As regard, in patients with overt Cushing Syndrome, Weise and Masuzaki found positive correlation between Leptin concentrations and BMI [31, 32]. Actually, there are several biochemical evidences that in SH patients adipocytokines as well several pro-inflammatory cytokines (i.e., IL-6) secretion from intra-abdominal fatty tissue (i.e., periadrenal, perirenal or perivisceral fatty tissue), may contribute to influencing long-term CV risk in SH patients [33]. As regard, after a long time follow-up we interestingly found a significant reduction of visceral adiposity, expressed clinically as waist circumference, in those patients surgically treated, due to resolution of autonomous cortisol excess production.
Moreover, Maurice et al. found in patients with Cushing’s Syndrome higher left ventricular mass index associated to higher levels of epicardial adipose tissue, suggesting pathophysiological relationship between epicardial fat tissue, left ventricular function, and cortisol secretion [34].
Leptin is directly responsible to sympathetic activation leading to development or maintenance of arterial hypertension [35]. Overstimulation of sympathetic nerve function [36] and sodium-retaining action of cortisol can contribute to the alteration of the circadian BP rhythm, explaining higher prevalence of non-dipping pattern [9, 12], as well as the restoration of BP rhythm after treatment of cortisol excess.
GCs can affect glucose metabolism by multifactorial mechanisms, demonstrated by in vitro and in vivo studies [37], as increased hepatic glucose production (directly or indirectly antagonizing metabolic actions of insulin), decreased insulin-dependent glucose uptake into peripheral tissues, especially skeletal muscle tissue (reduced expression and phosphorylation of insulin receptor substrate-1, phos-phatidylinositol 3-kinase, and protein kinase B/Atk, reduced migration of glucose trans-porter GLUT-4 to cell surface, reduced glycogen synthesis, stimulated muscle proteolysis), inhibition of insulin secretion from pancreatic b-cells (suppressed expression of GLUT-2 and glucokinase, increased glucose oxidation and decreased protein kinase’s A and C activation) [38].
Second aim of our study was to evaluate if the surgical treatment of cortisol excess (by laparoscopic adrenalectomy) could prevent and reduce CV risk, compared with SH patients undergoing an optimized pharmacological treatment in order to reduce metabolic modifications. As regard it, the debate is still open: while National Institutes of Health and French Society of Endocrinology do not extensively recommend surgery [39], Italian Association of Clinical Endocrinologists and American Association of Clinical Endocrinologists suggest surgical approach [14, 40], because cardiometabolic comorbidities, such as worsening hypertension, abnormal glucose tolerance, dyslipidemia or osteoporosis, are not completely controlled with an intensified pharmacological therapy. Some authors have suggested that surgery should be considered in SH patients in the presence of at least two comorbidities among obesity, arterial hypertension, type 2 diabetes and dyslipidemia [41]. Comparing surgical and conservatively management of SH patients, Chiodini et al. found that medical therapy has relevant side-effects and minor beneficial effects, whereas surgical treatment is associated to significant improvement (more than half of cases) of hypertension, diabetes, obesity and risk of fractures [42,43,44].
As regard it, in an interesting review Iacobone et al. [21] confirmed the beneficial effects of surgery for several metabolic abnormalities in SH patients, despite methodological limitations of evaluated studies (heterogeneity definition of SH, different endpoints). About technical aspects, in large study population conducted in specialized and experienced Centers, de La Villeòn demonstrated that laparoscopic adrenalectomy, besides being valid and adequate in order to improve several metabolic and CV abnormalities, is also safe and risk-free, with low morbidity and no significant mortality [45].
In previous study [9] we observed in selected patients with NFAI and SH undergoing to surgical treatment, after a follow-up of 12 months, the significant reduction in terms of prevalence of obesity, hypertension, MS, and reduction of 24-h average-SBP, as well as reduction of non-dipper pattern and mean number of antihypertensive medications used. Same results were observed by Debono [15] and Patrova [15], with relevant relationship between mortality in patients with autonomous cortisol secretion with cortisol levels post-DXT, age and tumor size; moreover, it was observed increased mortality, beyond CVEs, related to infection disease, as well as and higher incidence of osteoporosis.
In this study, we have expanded our population, added new information on personal history of CVEs (detecting CVEs prior to enrollment), and extended timing of follow-up to 15 years. During follow-up, in SSH patients we have observed a significant reduction of several metabolic and CV parameters as WC, serum behaviors of triglycerides and LDL-cholesterol, up to reduced prevalence of dyslipidaemia, hypertriglyceridemia, MS and type 2 diabetes; whereas in MSH group we have found significant increase of WC and serum uric acid.
Besides the overt damage previously described, there’s more emerging interest on subclinical atherosclerosis markers in SH. In a previous study, we described that subjects with Cushing’s Syndrome had higher values of IMT and ABI, compared with healthy and essential hypertensive subjects [46]. Hence, it has also been discovered that in SH patients, arterial stiffness, IMT, flow mediated dilation, and atherosclerotic plaques, are independently associated with severity and duration of excessive cortisol exposure [47,48,49,50,51,52]. Once subclinical cortisol excess has permanently removed (by surgical treatment), it is clear the potential positive effect of pre-existing vascular damage. About it, in this study during follow-up we found significant thickening of c-IMT (from 0.93 ± 0.3 to 1.0 ± 0.2; p < 0.01) and an increased incidence of plaques (from 21 to 30%, p < 0.01) in subject undergoing medical management (MSH patients), while in SSH patients we did not observe any worsening in vascular indexes in SSH patients. Moreover, using ROC curve analysis, we have found that UFC values higher than 110 and 132 nmol/24 h are able to predict before surgery the significative amelioration of relevant anthropometric and vascular parameters, as reduction of visceral obesity and office systolic blood pressure control in SSH patients.
Several registries and studies have widely demonstrated that patients with SH are at increased risk of CVEs [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. In an Italian multicenter study directed by Morelli in 2014 [19], it has been shown that SH is an independent risk factor for the occurrence of new CVE over time, with an annual rate of CV events of 3.1%, that is substantially similar in population affected by type 2 diabetes, hypertension and dyslipidemia.
Di Dalmazi et al. [7] discovered that the survival rate for all-cause mortality was lower in AI patients with autonomous secretion of cortisol, especially in those with worsening cortisol secretion during follow-up (evaluated by cortisol levels after 1mg-DST) compared with nonsecreting masses (57 vs. 91%). In this report, factors associated with increased mortality were age and mean cortisol levels after 1mg-DST, but not previous coronary heart disease.
In our study, we observed either higher CVEs prevalence (MI, stroke, revascularization procedures) at diagnosis of SH, as well as higher prevalence of MS and DM, either significant reduction of CVEs and mortality in subjects treated with surgical removal of cortisol excess. Main independent factors associated to CV mortality and CVEs were mean value of 24-h average-SBP, plasma levels of LDL-cholesterol and serum glucose, underling the role of these metabolic and vascular alterations strictly related to autonomous cortisol secretion in the development of CV and metabolic complications.
Regarding it, our study confirms and extends literature evidences: cortisol excess in SH is associated to worse lipid and glicometabolic profile, and a globally increased recurrence of MS. As regard, in our casuistry those patients with lack of suppression cortisol levels after DST (>1.8 µg/dL or >50 nmol/L), identified by Guideline as “possible autonomous cortisol secretion”, in presence of coexistence of CV co-morbidities (arterial hypertension poorly treated with medications, glucose intolerance or type 2 diabetes mellitus, MS, obesity, dyslipidemia) show higher prevalence of main CVEs and mortality, similar to patients with cortisol levels after DSM test >5.0 µg/dL or >138 nmol/L), highlighting the additive action of CV comorbidities associated to autonomous cortisol secretion into development of main CV complications. SH patients who received a pharmacological management of their comorbidities, compared with patients who went to laparoscopic adrenalectomy showed a dramatic recurrence of major CVE, with unadjusted RR for CVE in MSH respect NFA and SSH of 2.56 and 2.10, respectively (p < 0.001).
After multivariate analysis, cortisol secretion was not a major factor that influenced CV events and CV mortality as shown in Tables 5 and 6. This result could be addressed to two different aspects. First, we have taken into account the overall population (SH was represented by 25% of patients enrolled); second, in the statistic model we have analyzed the continuous variables and not the excessive cortisol secretion status as a dichotomic variable. These two factors may have reduced the statistical power of cortisol secretion influencing CV events and mortality in our analysis.
Because of high prevalence of subclinical organ damage and metabolic abnormalities in SH patients, future efforts should not be in addressing to surgery only those patients with overt CV damage, or whit previously major CVE; but once clearly biochemically and clinically defined SH, physicians should be inclined to identify subclinical damage, an essential part of hypertension-mediate organ damage in SH patients [51, 52]. Thus, it will be desirable to recognize those subjects who will get maximum benefit from laparoscopic adrenalectomy and will prevent a certain worsening of their metabolic and CV profile.
In our case series, none of our patients who went laparoscopic adrenalectomy had peri and post-procedural complications. Our experience agrees with literature that surgical approach is effective on reducing CV abnormalities and recurrence of new CVE. Probably, for the pathological mechanisms mentioned above, surgical approach has been demonstrated as really way to reduce hypertensive overload, expressed in terms of average 24 h-blood pressure and non-dipping pattern. Moreover, during follow-up, SSH patients used a significantly reduced number of antihypertensive medications (2.3 ± 1.0 vs 1.5 ± 0.4; p < 0.05).
Future studies should be designed in prospective randomized controlled model and should require a long-term follow up to determine real effects on cumulative rate survival and life of these patients.
In conclusion, in our experience patients with SH, especially if associated to CV comorbidities, should be encouraged to strict follow-up and treatment, optimization of pharmacological treatment, until laparoscopic adrenalectomy if indicated, because of its promising effect on CV, heart and cerebro-vascular disease.
References
M. Fassnacht, W. Arlt, I. Bancos, H. Dralle, J. Newell-Price, A. Sahdev, A. Tabarin, M. Terzolo, S. Tsagarakis, O.M. Dekkers, Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 175(2), G1–G34 (2016)
F. Mantero, M. Terzolo, G. Arnaldi, G. Osella, A.M. Masini, A. Alı‘, M. Giovagnetti, G. Opocher, A. Angeli, A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J. Clin. Endocrinol. Metab. 85, 637–644 (2000)
S. Bovio, A. Cataldi, G. Reimondo, P. Sperone, S. Novello, A. Berruti, P. Borasio, C. Fava, L. Dogliotti, G.V. Scagliotti, A. Angeli, M. Terzolo, Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J. Endocrinol. Invest 29(4), 298–302 (2006)
G. Di Dalmazi, R. Pasquali, F. Beuschlein, M. Reincke, Subclinical hypercortisolism: a state, a syndrome, or a disease? Eur. J. Endocrinol. 173(4), M61–M71 (2015)
J. Shen, M. Sun, B. Zhou, J. Yan, Nonconformity in the clinical practice guidelines for subclinical Cushing’s syndrome: which guidelines are trustworthy? Eur. J. Endocrinol. 171, 421–431 (2014)
I.I. Androulakis, G. Kaltsas, G. Piaditis, A.B. Grossman, The clinical significance of adrenal incidentalomas. Eur. J. Clin. Investig. 41, 552–560 (2011)
G. Di Dalmazi, V. Vicennati, S. Garelli, E. Casadio, E. Rinaldi, E. Giampalma, C. Mosconi, R. Golfieri, A. Paccapelo, U. Pagotto, R. Pasquali, Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2(5), 396–405 (2014)
J. Park, A. De Luca, H. Dutton, J.C. Malcolm, M.A. Doyle, Cardiovascular outcomes in autonomous cortisol secretion and nonfunctioning adrenal adenoma: a systematic review. J. Endocr. Soc. 3(5), 996–1008 (2019)
L. Petramala, G. Cavallaro, M. Galassi, C. Marinelli, G. Tonnarini, A. Concistrè, U. Costi, M. Bufi, P. Lucia, G. De Vincentis, G. Iannucci, G. De Toma, C. Letizia, Clinical benefits of unilateral adrenalectomy in patients with subclinical hypercortisolism due to adrenal incidentaloma: results from a single center. High. Blood Press Cardiovasc Prev. 24(1), 69–75 (2017)
W.W. Mayo-Smith, G.W. Boland, R.B. Noto, M.J. Lee, State-of-the-art adrenal imaging. Radiographics 21, 995–1012 (2001)
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 285(19), 2486–2497 (2001)
M. Ceruti, L. Petramala, D. Cotesta, S. Cerci, V. Serra, C. Caliumi, M. Iorio, G. De Toma, A. Ciardi, D. Vitolo, C. Letizia, Ambulatory blood pressure monitoring in secondary arterial hypertension due to adrenal diseases. J. Clin. Hypertens. 8, 642–648 (2006)
C. Fagour, S. Bardet, V. Rohmer, Y. Arimone, P. Lecomte, N. Valli, A. Tabarin, Usefulness of adrenal scintigraphy in the follow-up of adrenocortical incidentalomas: a prospective multicenter study. Eur. J. Endocrinol. 160, 257–264 (2009)
M.A. Zeiger, G.B. Thompson, Q.Y. Duh et al. On behalf of the American Association of Clinical Endocrinologists and the American Association of Endocrine Surgeons. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr. Pract. 15, 1–20 (2009)
M. Debono, M. Bradburn, M. Bull, B. Harrison, R.J. Ross, J. Newell-Price, Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J. Clin. Endocrinol. Metab. 99(12), 4462–4470 (2014)
V. Morelli, S. Palmieri, A. Lania, A. Tresoldi, S. Corbetta, E. Cairoli, C. Eller-Vainicher, M. Arosio, M. Copetti, E. Grossi, I. Chiodini, Cardiovascular events in patients with mild autonomous cortisol secretion: analysis with artificial neural networks. Eur. J. Endocrinol. 177(1), 73–83 (2017)
J. Patrova, M. Kjellman, H. Wahrenberg, H. Falhammar, Increased mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: a 13-year retrospective study from one center. Endocrine 58(2), 267–275 (2017)
S. Yener, S. Ertilav, M. Secil, T. Demir, B. Akinci, L. Kebapcilar, A. Comlekci, F. Bayraktar, S. Yesil, Prospective evaluation of tumor size and hormonal status in adrenal incidentalomas. J. Endocrinol. Invest. 33(1), 32–36 (2010)
V. Morelli, G. Reimondo, R. Giordano, S. Della Casa, C. Policola, S. Palmieri, A.S. Salcuni, A. Dolci, M. Mendola, M. Arosio, B. Ambrosi, A. Scillitani, E. Ghigo, P. Beck-Peccoz, M. Terzolo, I. Chiodini, Long-term follow- up in adrenal incidentalomas: an Italian multicenter study. J. Clin. Endocrinol. Metab. 99(3), 827–834 (2014)
N. Vogelzangs, A.T.F. Beekman, Y. Milaneschi, S. Bandinelli, L. Ferrucci, Penninx BWJH. Urinary cortisol and six-year risk of all-cause and cardiovascular mortality. J. Clin. Endocrinol. Metab. 95, 4959–4964 (2010)
M. Iacobone, M. Citton, M. Scarpa, G. Viel, M. Boscaro, D. Nitti, Systematic review of surgical treatment of subclinical Cushing’s syndrome. Br. J. Surg. 102(4), 318–330 (2015)
S. Araujo-Castro, C. Robles Lázaro, P. Parra Ramírez, M. Cuesta Hernández, M.A. Sampedro Núñez, M. Marazuela, Cardiometabolic profile of non-functioning and autonomous cortisol-secreting adrenal incidentalomas. Is the cardiometabolic risk similar or are there differences? Endocrine 66(3), 650–659 (2019)
M. Kumari, M. Shipley, M. Stafford, M. Kivimaki, Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II Study. J. Clin. Endocrinol. Metab. 96, 1478–1485 (2011)
C. Scaroni, M. Zilio, M. Foti, M. Boscaro, Glucose metabolism abnormalities in cushing syndrome: from molecular basis to clinical management. Endocr. Rev. 38(3), 189–219 (2017)
E.B. Geer, J. Islam, C. Buettner, Mechanisms of glucocorticoid induced insulin resistance: focus on adipose tissue function and lipid metabolism. Endocrinol. Metab. Clin. North Am. 43(1), 75–102 (2014)
A. Rafacho, H. Ortsäter, A. Nadal, I. Quesada, Glucocorticoid treatment and endocrine pancreas function: implications for glucose homeostasis, insulin resistance and diabetes. J. Endocrinol. 223(3), R49–R62 (2014)
H. Hodabandehloo, S. Gorgani-Firuzjaee, G. Panahi, R. Meshkani, Molecular and cellular mechanisms linking inflammation to insulin resistance and b-cell dysfunction. Transl. Res. 167(1), 228–256 (2016)
D. Patsouris, J.G. Neels, W. Fan, P.P. Li, M.T. Nguyen, J.M. Olefsky, Glucocorticoids and thiazolidinediones interfere with adipocyte-mediated macrophage chemotaxis and recruitment. J. Biol. Chem. 284(45), 31223–31235 (2009)
G.B. Cansu, S. Atılgan, M.K. Balcı, R. Sarı, S. Özdem, H.A. Altunbaş, Which type 2 diabetes mellitus patients should be screened for subclinical Cushing’s syndrome? Hormones (Athens) 16(1), 22–32 (2017)
C. Letizia, L. Petramala, C.R. Di Gioia, C. Chiappetta, L. Zinnamosca, C. Marinelli, G. Iannucci, A. Ciardi, G. De Toma, G. Iacobellis, Leptin and adiponectin mRNA expression from the adipose tissue surrounding the adrenal neoplasia. J. Clin. Endocrinol. Metab. 100(1), E101–E104 (2015)
M. Weise, V. Abad, R.V. Considine, L. Nieman, K.I. Rother, Leptin secretion in Cushing’s syndrome: preservation of diurnal rhythm and absent response to corticotropin-releasing hormone. J. Clin. Endocrinol. Metab. 84(6), 2075–2079 (1999)
H. Masuzaki, Y. Ogawa, K. Hosoda, T. Miyawaki, I. Hanaoka, J. Hiraoka, A. Yasuno, H. Nishimura, Y. Yoshimasa, S. Nishi, K. Nakao, Glucocorticoid regulation of leptin synthesis and secretion in humans: elevated plasma leptin levels in Cushing’s syndrome. J. Clin. Endocrinol. Metab. 82(8), 2542–2547 (1997)
A. Babinska, M. Kaszubowski, P. Kmieć, K. Sworczak, Adipokine and cytokine levels in patients with adrenocortical cancer, subclinical Cushing’s syndrome and healthy controls. Steroids 140, 39–44 (2018)
F. Maurice, B. Gaborit, C. Vincentelli, I. Abdesselam, M. Bernard, T. Graillon, F. Kober, T. Brue, F. Castinetti, A. Dutour, Cushing syndrome is associated with subclinical LV dysfunction and increased epicardial adipose tissue. J. Am. Coll. Cardiol. 72(18), 2276–2277 (2018)
M.P. Canale, S. Manca di Villahermosa, G. Martino, V. Rovella, A. Noce, A. De Lorenzo, N. Di Daniele, Obesity-related metabolic syndrome: mechanisms of sympathetic overactivity. Int J. Endocrinol. 2013, 865965 (2013)
U.A. Hawkins, E.P. Gomez-Sanchez, C.M. Gomez-Sanchez, C.E. Gomez- Sanchez, The ubiquitous mineralocorticoid receptor: clinical implications. Curr. Hypertens. Rep. 14, 573–580 (2012)
R. Giordano, F. Guaraldi, R. Berardelli, I. Karamouzis, V. D’Angelo, E. Marinazzo, A. Picu, E. Ghigo, E. Arvat, Glucose metabolism in patients with subclinical Cushing’s syndrome. Endocrine 41, 415–423 (2012)
D. Qi, B. Rodrigues, Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolism. Am. J. Physiol. Endocrinol. Metab. 292, E654–E667 (2007)
M.M. Grumbach, B.M. Biller, G.D. Braunstein, K.K. Campbell, J.A. Carney, P.A. Godley, E.L. Harris, J.K. Lee, Y.C. Oertel, M.C. Posner, J.A. Schlechte, H.S. Wieand, Management of the clinically inapparent adrenal mass (“incidentaloma”). Ann. Intern Med 138(5), 424–429 (2003)
M. Terzolo, A. Stigliano, I. Chiodini, P. Loli, L. Furlani, G. Arnaldi, G. Reimondo, A. Pia, V. Toscano, M. Zini, G. Borretta, E. Papini, P. Garofalo, B. Allolio, B. Dupas, F. Mantero, A. Tabarin, Italian association of clinical endocrinologists. AME position statement on adrenal incidentaloma. Eur. J. Endocrinol. 164(6), 851–870 (2011)
I. Chiodini, A. Albani, A.G. Ambrogio, M. Campo, M.C. De Martino, G. Marcelli, V. Morelli, B. Zampetti, A. Colao, R. Pivonello; ABC Group, Six controversial issues on subclinical Cushing’s syndrome. Endocrine 56(2), 262–266 (2017)
R. Rossi, L. Tauchmanova, A. Luciano, M. Di Martino, C. Battista, L. Del Viscovo, V. Nuzzo, G. Lombardi, Subclinical Cushing’s syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J. Clin. Endocrinol. Metab. 85(4), 1440–1448 (2000)
A. Toniato, I. Merante-Boschin, G. Opocher, M.R. Pelizzo, F. Schiavi, E. Ballotta, Surgical versus conservative management for subclinical Cushing syndrome in adrenal incidentalomas: a prospective randomized study. Ann. Surg. 249(3), 388–391 (2009)
L. Barzon, F. Fallo, N. Sonino, M. Boscaro, Development of overt Cushing’s syndrome in patients with adrenal incidentaloma. Eur. J. Endocrinol. 146(1), 61–66 (2002)
B. de La Villéon, S. Bonnet, H. Gouya, L. Groussin, F. Tenenbaum, S. Gaujoux, B. Dousset, Long-term outcome after adrenalectomy for incidentally diagnosed subclinical cortisol-secreting adenomas. Surgery 160(2), 397–404 (2016)
L. Petramala, D. Lorenzo, G. Iannucci, A. Concistré, L. Zinnamosca, C. Marinelli, G. De Vincentis, A. Ciardi, G. De Toma, C. Letizia, Subclinical Atherosclerosis in Patients with Cushing Syndrome: Evaluation with Carotid Intima-Media Thickness and Ankle-Brachial Index. Endocrinol. Metab. 30(4), 488–493 (2015)
G. Reimondo, B. Allasino, M. Coletta et al. Evaluation of midnight salivary cortisol as a predictor factor for common carotid arteries intima media thickness in patients with clinically inapparent adrenal adenomas. Int J. Endocrinol. 2015, 674734 (2015)
M. Evran, G. Akkus, I. Berk Bozdoğan et al. Carotid intima-media thickness as the cardiometabolic risk indicator in patients with nonfunctional adrenal mass and metabolic syndrome screening. Med Sci. Monit. 22, 991–997 (2016)
N.N. Imga, O. Ucar Elalmis, M. Muslum Tuna et al. The relationship between increased epicardial fat thickness and left ventricular hypertrophy and carotid intima-media thickness in patients with nonfunctional adrenal incidentaloma. Int J. Endocrinol. Metab. 14, e37635 (2016)
R. Lupoli, P. Ambrosino, A. Tortora et al. Markers of atherosclerosis in patients with Cushing’s syndrome: a meta-analysis of literature studies. Ann. Med 49, 206–216 (2017)
P.M. Stewart, Is subclinical Cushing’s syndrome an entity or a statistical fallout from diagnostic testing? Consensus surrounding the diagnosis is required before optimal treatment can be defined. J. Clin. Endocrinol. Metab. 95(6), 2618–2620 (2010)
B. Williams, G. Mancia, W. Spiering, E. Agabiti Rosei, M. Azizi, M. Burnier, D. Clement, A. Coca, G. De Simone, A. Dominiczak, T. Kahan, F. Mahfoud, J. Redon, L. Ruilope, A. Zanchetti, M. Kerins, S. Kjeldsen, R. Kreutz, S. Laurent, G.Y.H. Lip, R. McManus, K. Narkiewicz, F. Ruschitzka, R. Schmieder, E. Shlyakhto, K. Tsioufis, V. Aboyans, I. Desormais, 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press 27(6), 314–340 (2018)
Author information
Authors and Affiliations
Contributions
L.P., C.L., G.D.T., and G.I.: protocol/project development, data collection or management, data analysis, and manuscript writing/editing. L.P. and C.L.: protocol/project development and manuscript writing/editing. F.O., R.R., and A.C.: manuscript writing/editing. G.D.V., M.S., and L.P.: data analysis. F.O., R.R., and A.C.: data collection or management and data analysis. M.S., G.I., and G.D.V.: data collection or management.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Petramala, L., Olmati, F., Concistrè, A. et al. Cardiovascular and metabolic risk factors in patients with subclinical Cushing. Endocrine 70, 150–163 (2020). https://doi.org/10.1007/s12020-020-02297-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02297-2