Abstract
There is a lack of evidence on timing, frequency, and duration of postoperative endocrine, radiologic, and ophthalmologic assessments that should be performed after pituitary surgery (PS). However, it is known that careful optimization of treatment and follow-up strategies as well as a multidisciplinary approach may have a significant impact on long-term outcomes, improving surgical results, minimize complications and facilitate their correct treatment if occurring, and optimize the hormonal, ophthalmological, and radiological reassessment throughout the follow-up. Considering that there are no specific guidelines on the postoperative management of patients with pituitary tumors (PT), we present our protocol for the postoperative management of patients with PT. It has been elaborated by the multidisciplinary team of a Spanish Pituitary Tumor Center of Excellence (PTCE) that includes at least one neurosurgeon, ENT, neuroradiologist, neuro-ophthalmologist, endocrine pathologist and endocrinologist specialized in pituitary diseases. We elaborated this guideline with the aim of sharing our experience with other centers involved in the management of PT thereby facilitating the postoperative management of patients submitted to PS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary tumors (PT) are heterogeneous benign tumors of the central nervous system (CNS). They are present in up to 10% of the general population [1], but do not necessarily result into symptoms, either due to their small size or because they do not secrete hormones in excess. In symptomatic cases, pituitary surgery (PS) by an expert neurosurgeon is usually the initial treatment of choice, with the exception of prolactinomas, in which medical treatment with dopamine agonists is preferred [2].
Nowadays, transsphenoidal surgery is the preferred surgical approach in more than 95% of the cases, even in PT with extensive suprasellar invasion or in some giant PT [3]. Moreover, the transsphenoidal endoscopic approach (EEA) offers a better visualization of hidden structures, which are not usually visible under the direct microsurgical light [4,5,6,7].
The cure rate in the hands of expert pituitary surgeons ranges from 80 to 90% for microadenomas and from 40 to 70% for macroadenomas with a rate of major complications below 1% [8]. However, hyponatremia may occur in up to 10%, and hypopituitarism in 7.5% of patients submitted to experienced pituitary surgeons [9].
In the immediate postoperative period, the development of surgical and endocrine complications must be monitored and the need to continue with glucocorticoids (GC) replacement should be re-evaluated. The minimal setup includes monitoring of hydro-electrolyte balances and natremia, and active screening for potential development of surgical complications such as severe epistaxis, sellar hematoma, fistula of cerebrospinal fluid (CSF), etc. [10]. The omission of treatment with GC, desmopressin, and/or thyroid hormone when indicated leads to significant morbidity and mortality and must be avoided. In patients with acromegaly, Cushing's disease (CD) and prolactinomas, basal serum measurement of growth hormone (GH), cortisol and prolactin, respectively, on days 1–2 postoperatively has been shown to be predictive of early and long-term remission [11,12,13,14,15].
The late postoperative hormonal evaluation should be carried out 4–6 weeks after the intervention and aims to analyze the integrity of the pituitary function, with a special focus on the reevaluation of the corticotropic axis in the case of patients treated with GC. Pathology should be taken into account to plan long-term management since PT with histopathological features of aggressiveness should be followed up more closely [10, 16].
For the reasons outlines above, we truly believe that postoperative management of PT who are suitable candidates to PS optimally require a multidisciplinary team consisting of endocrinologists, neurosurgeons, ENT, neuro-ophthalmologists, neuroradiologists, and endocrine pathologists. The management of these patients in multidisciplinary specialized units with high-volume PT centers, especially in Pituitary Tumors Centers of Excellence (PTCE), provide the best care to patients with PT [17].
The present review based on our clinical practice protocol, recently updated according to available scientific evidence and published consensus of experts in pituitary pathology. We here describe in detail the structured protocol on the multidisciplinary management of patients undergoing PS due to PT, focusing on the postoperative study and management and on surgical treatment and its complications, with the aim of offering guidance to other multidisciplinary teams working in the field. The specific perioperative management of patients with CD and acromegaly is also briefly described.
Material and methods
The physicians involved in the management of patients with PT of Hospital Universitario Ramón y Cajal reviewed systematically current knowledge on the management of PT. The literature review used the online Entrez-PubMed facilities, including only publications in English and Spanish published from 1986 to 2019. More than 5000 papers were reviewed, of which only 85 were used to elaborate the postoperative protocol. The multidisciplinary team elaborated a first draft that was presented and perfected in a formal presentation with other doctors involved in the management of pituitary disease. The protocol was subsequently approved by the Hospital Quality Unit. This protocol is an update of that applied to the management of PT undergoing PS in our center during the last 5 years. Our hospital meets criteria to be considered a PTCE [17], having an experienced neurosurgeon with more than 300 endoscopic pituitary procedures performed, an average of 35–40 PS per year, and more than 330 outpatients under follow-up every year. The neurosurgery group works closely with endocrinologists on an endocrine unit that has a special emphasis on pituitary diseases with the aim of providing the best care for patients, and it is considered a training center for residents in the treatment of pituitary pathologies [17].
Description of our protocol for pituitary surgery
Intraoperative management
Surgical technique, outcomes, and complications
PT should ideally be performed in multidisciplinary specialized units with high-volume PT centers (PTCE) such as ours [17]. The experience of the surgical team is of paramount importance, facilitating long-term remission and gross total resection while minimizing complications [18,19,20]. Nowadays, the most commonly used approach is the transsphenoidal route either microsurgical (MA) or EEA [21]. We use EEA because, compared with the microscope, the endoscope offers the advantage of directly visualizing hidden areas that are not visible under the direct light of the microscope. Despite these theoretical advantages, the superiority of the EEA technique vs the classical MA technique cannot yet be clearly established. A comparison of the two techniques (micro and endoscopic) is limited because few studies are prospective or randomized. Therefore, it is based upon reviews and meta-analyses of studies of the individual techniques [22,23,24,25,26,27,28,29,30,31,32].
PS aims to eliminate excess hormone production (functioning PT (FPT)), avoid or ameliorate tumor mass effects, preserve both pituitary function and adjacent nerve structures (optic and oculomotor nerves), and eliminate or reduce the risk of future recurrences [33]. The cure rate in the hands of expert surgeons is 80–90% for microadenomas and 40–70% for macroadenomas; with an overall rate of major complications (visual, CNS or carotid artery damage, CSF fistula and ophthalmoplegia) below 1% [8]. However, even with expert neurosurgeons, postoperative hyponatremia occurs in up to 10% of patients and hypopituitarism in up to 7.5% of PS [9] (Table 1). The cure rate in our center in the last 10 years is of 75% in acromegaly, 76% in Cushing disease, and the rate of major complications <5%.
Immediate postoperative cares
After PS is concluded, the patient will be transferred to the Intensive Care Unit (high-risk patients) or to the postanesthesia care unit for regular surveillance during the first 12–24 h.
We consider the following as high-risk patients:
-
Elderly patients (over 70 years).
-
High comorbidity.
-
Giant PT with cavernous sinus, III ventricle, or intracranial extension that needed expanded endoscopic approaches.
-
Long-standing CD or acromegaly with serious comorbidities.
-
Intraoperative major complications.
In the early postoperative phase, patients should be monitored for potential surgical (sellar hematoma, meningitis, CSF leakage, epistaxis) and endocrinological complications (diabetes insipidus (DI), hypopituitarism, SIADH). Nurses must check the patient for body temperature, blood pressure, conscience, rhinorrhea, and epistaxis every 8 h, and hydro-electrolytic balance at least daily, until discharge. If neurological symptoms or visual deterioration occurs, an urgent postoperative MRI or CT must be performed [34]. If rhinorrhea is suspected, beta–2 transferrin and beta trace protein test can be measured to confirm CSF leakage [35]. In case of relevant epistaxis, nasal package and reoperation should be done as soon as possible (Table 1).
Perioperative antibiotics should be maintained until removal of nasal packing and, in the absence of complications, the patient may be discharged the next day with home care recommendations (2–3 days after surgery).
Postoperative management of disorders of water metabolism
In the early postoperative period, the disorders of water metabolism are common yet often transient complications. These disorders include DI, SIADH and, although very infrequent, the cerebral salt-wasting syndrome (CSWS).
Central diabetes insipidus and SIADH
Postoperative DI can be transient or permanent and partial or complete, depending on the type and extent of the damage to hypothalamic magnocellular neurons. DI is one of the most common complications after PS [36]. It occurs in ~10–30% of patients undergoing PS, but long-term persistence only happens in 2–7% of cases [18, 37, 38]. The risk of permanent DI is higher in young patients, men, large intrasellar masses, and postoperative CSF leakage [39, 40], patients with a preoperative diagnosis of DI [41, 42], following surgery for Rathke’s cleft cysts or craniopharyngioma [43], and after repeated PS [37]. Because GC are necessary for the excretion of water overload, DI may become first apparent when a patient with secondary adrenal insufficiency (SAI) starts GC therapy. Moreover, other causes of postoperative polyuria, including intraoperative administration of large amounts of fluids, hyperglycemia caused or worsened by GC therapy, and a rapid decrease in GH levels in acromegaly should be ruled out. The presence of serum hyperosmolality and hypernatremia is highly suggestive of DI, but these alterations may be absent in patients in whom water intake is not restricted [44].
DI can appear at any time but usually manifests as the sudden onset of polyuria, accompanied by thirst and polydipsia, within the first 48 h after PS. In most patients, vasopressin secretion recovers, and DI is usually transient, resolving gradually over a period of 3–5 days.
In ~3% of patients, acute postoperative water balance disorders follow a three-phase pattern consisting of an initial polyuric phase (DI) followed by an antidiuretic phase (SIADH) caused by the release of stored vasopressin and a final polyuric phase (DI) that translate depletion of hormone reserves and is usually permanent [39, 45]. Finally, the antidiuretic phase described above is sometimes isolated and represents SIADH. This late-onset of transient hyponatremia can increase due to concomitant hypocortisolism, since patients with untreated adrenal insufficiency may also associate hyponatremia [46].
To screen patients for the potential development of postoperative DI and SIADH, we measure urine output and fluid intake, as well as serum sodium, on a daily basis until discharge (Table 3). Desmopressin is used for DI during the immediate postoperative period, with frequent reassessment of its requirements. Given the frequent transient course, it is important to avoid overtreatment of early postoperative DI in order to reduce the risk of precipitating hyponatremia if followed by a SIADH phase.
Hyponatremia usually develops between postoperative days 5 and 8 [34] and, therefore, PS patients should undergo serum sodium measurements on postoperative days 5–7. The incidence of postoperative hyponatremia appears to be higher in patients with cardiac, renal, and/or thyroid disease, in females, and in patients suffering postoperative CSF drainage [47]. Mild-moderate hyponatremia (134–125 mmol/L) may be treated at the outpatient setting with fluid restriction and frequent circulating sodium controls, whereas more severe hyponatremia (<125 mmol/L) requires hospitalization and the possible use of intravenous infusion of hypertonic saline [48]. On the other hand, a recent study proposes that the prevention of the development of postoperative hyponatremia is possible with 1.0 L daily fluid restriction. They found that in the intervention group none was readmitted for hyponatremia (0/203), compared with 3.41% (20/585) in the control group (p = 0.003) [49]. Our recommendations for treatment are summarized in Table 1.
Cerebral salt-wasting syndrome
Very rarely, postoperative hyponatremia results from CSWS, a disorder characterized by excessive natriuresis and decreased extracellular volume. As opposed to the euvolemic hyponatremia of SIADH, CSWS is characterized by hypovolemic hyponatremia and necessarily requires volume replacement for recovery. Fludrocortisone should be used only if sodium and fluid replacement is unable to counteract excessive natriuresis [50]. The differential diagnosis between SIADH and CSWS is sometimes difficult. Table 2 summarizes the most important characteristics and differences between these two entities [51, 52].
Postoperative reassessment
In the late postoperative period, we reassess the adrenal, thyroid, gonadal, and somatotropic axes. Long term monitoring of visual and pituitary function, coupled with pituitary imaging (preferably MRI) and ENT evaluation, is necessary for all patients who underwent PS.
Postoperative hormonal evaluation
One of the most common complications after PS is hypopituitarism [53], which can be partial or total, and transient or permanent. The hormonal assessment and the management of anterior pituitary hormone deficiencies after PS is summarized in Table 3.
The risk of hypopituitarism after PS varies according to the etiology, ranging from 5–25% in PT to as much as 76% in craniopharyngiomas [54], with its occurrence largely depending on the neurosurgeon’s experience [18, 55]. Other factors, including the size and consistency of the tumor, the extent of the resection, and surgery for recurrent disease, increase the chances of hypopituitarism [18].
Provided that no acute complications have occurred in the immediate postoperative period, we performed the first evaluation of anterior pituitary function 4–6 weeks after surgery (Table 3).
Postoperative imaging evaluation
In most hospitals, including ours, early postsurgical imaging studies are not performed. Unless the occurrence of surgical complications, pituitary imaging should be delayed for 3 to 6 months after surgery, because anatomical changes resulting from surgical manipulation may lead to erroneous interpretation of the findings [56]. To facilitate comparison, postoperative imaging procedures should follow the protocols used before PS.
In the case of complications, imaging studies should be performed immediately. In addition to the usual MRI protocols, the use of GREpT2* sequences (hemorrhages) and MRI-angiography (vascular lesions), as well as even invasive tests such as angiography, may be necessary. In actual emergencies, the usually more accessible brain CT may suffice to diagnose severe complications [56].
-
Microadenomas protocol
-
1.
T1wSE sagittal 2.5–3 mm without intravenous contrast
-
2.
2.5–3 mm coronal T1wSE without intravenous contrast
-
3.
T2wTSE coronal 2.5–3 mm without intravenous contrast
-
4.
Intravenous post contrast coronal dynamic series
-
5.
T1wSE coronal 2.5–3 mm post intravenous contrast
-
6.
T1wSE sagittal 2.5–3 mm post intravenous contrast
-
Macroadenomas protocol
-
1.
T1wSE sagittal 2.5–3 mm without intravenous contrast
-
2.
2.5–3 mm coronal T1wSE without intravenous contrast
-
3.
T2wTSE coronal 2.5–3 mm without intravenous contrast
-
4.
T1wSE coronal 2.5–3 mm post intravenous contrast
-
5.
T1wSE sagittal 2.5-3 mm post intravenous contrast
Postoperative neuroophthalmological evaluation
In our center, postoperative assessment includes visual acuity (VA), pupil and ocular motility, fundus examination, visual field (VF) testing and optical coherence tomography (OCT).
Resection of PT improves visual function in up to 80–90% of patients [57]. VA recovers rapidly after surgical decompression and further improvements may continue over the next few months. According to Kerrison [58], VF improvement follows a triphasic manner—translating release of the conduction block, re-myelination, and neuronal plasticity—and takes months to years to recover.
Postoperative visual improvement may be observed even in patients presenting with pituitary apoplexy. Rates of improvement of VA, VF defect, and ocular palsy of 92.9%, 94.7%, and 100% have been recently reported, with full VA, VF, and ocular palsy recovery being observed in 57.1%, 36.8% and 96.4% of cases, respectively [59].
Although there are no guidelines for long-term follow-up, we suggest an initial postoperative visit at 3 months followed by examinations every 6 months until visual function stabilizes, followed by annual monitoring [60].
Postoperative ENT evaluation
The removal of the nasal tamponade and a nasal endoscopy to assess the state of the surgical site are recommended 48–72 h after surgery. Three weeks later, we remove the silastic plates left from PS, to avoid nasal synechiae and an endoscopic revision is repeated.
Nasal complications and sequelae have little clinical expression and are considered minor complications [61]. Smell disorders are not frequent except in cases of involvement of the cribriform plate [62]. To assess the nasal status and the anatomical and functional repercussion of the PS approach, the tests that were performed preoperatively are repeated at 3 and 6 months after surgery, including endoscopy, visual analogic scales of obstruction, rhinorrhea, facial pain and anosmia, functional tests, SNOT 22 questionnaire, and smell test.
Assessment of pathology reports
Fresh pituitary specimens should be submitted to Pathology immediately upon removal from the patient. Diagnostic material is fixed in formalin and nonessential tissue for diagnosis is processed for biobank storage under pathologist’s supervision. Of note, adequate fixation is crucial for histological and immunohistochemical assessment. The most important prognostic factor that pathologists can provide is an accurate diagnosis.
We use the current World Health Organisation (WHO) classification of pituitary neuroendocrine tumors (PitNET) [63]. This classification relies on the expression of pituitary-specific transcription factors such as Pituitary transcription factor 1 (Pit-1), T-box family member TBX19 and steroidogenic factor 1, and anterior pituitary hormones, as assessed by immunohistochemistry. The main advantage of this classification is the concordance with patients’ clinical course and prognosis [64]. Sparsely granulated silent somatotroph adenomas are associated with a poor response to somatostatin analogs. Densely granulated lactotroph adenomas, silent corticotroph adenomas, Crooke cell adenomas, and plurihormonal poorly differentiated Pit-1 positive adenomas are associated with more aggressive features and a higher risk of relapse. A Ki67 proliferation index above 3% or the presence of more than 2 mitoses per 10 microscopic high-power fields are also associated with a higher risk of relapse [64,65,66]. On the contrary, the prognostic value of p53 expression is unclear at present [64]. Other prognostic markers that are currently under study and may be useful in the near future are estrogen receptor alpha, E-cadherin, O6-methylguanine DNA methyltransferase, and aryl hydrocarbon receptor interacting protein (AIP) [67].
Pathology also served for the differential diagnosis of other pathologies and tumors originated in the pituitary gland and of metastatic disease, especially in the context of silent tumors. In our usual clinical practice, we generally do not guide postsurgical treatment based on the pathology report, but we use this information to plan follow-up: in those PT with data suggesting a more aggressive behavior we perform a closer follow-up consisting of visits every 3–6 months (depending on the growth rate and proximity to vital structures) for at least 2–3 years, and subsequently annual visits if tumor stability is confirmed by both hormonal and imaging tests [68].
Special cases
Postoperative long-term follow up varies depending on the diagnosis and is most challenging in patients with CD and acromegaly, in whom failure of PS is a major concern.
Cushing’s disease
Patients with CD must receive steroid coverage according to the usual the schedule. Usually, they are treated with hydrocortisone replacement doses at discharge until the hipotalamic pituitary adrenal axis (HPAA) is re-evaluated to assess cure or persistence of cortisol excess.
Successful tumor resection leads to GC deficiency due to the presumed inhibition of ACTH secretion from the remaining normal corticotropic cells by long-standing exposure to hypercortisolism. Hence, postoperative endogenous hypocortisolism is indicative of surgical success [69,70,71]. However, suppression of endogenous ACTH secretion by normal corticotrophs may not happen in patients under medical therapy in whom eucortisolism was attained preoperatively or in those with mild or cyclic preoperative hypercortisolism, leading to normal cortisol levels after total tumor resection. Such patients may not require additional GC therapy, and the tests used for the initial diagnosis of Cushing's syndrome must be performed to confirm remission accurately. Hypocortisolism and GC withdrawal symptoms should be monitored in all patients and managed with physiological GC replacement doses until the HPAA is fully recovered, a milestone that might require years in some cases.
Nowadays there is no universal consensus on the criteria that should be used to establish or predict cure of CD after PS [72,73,74,75,76,77]. Several methods have been used: (1) non-provocative tests including the measurement of morning serum cortisol and/or plasma ACTH levels and/or daily urinary free cortisol (UFC) levels during the immediate postsurgical period; and (2) provocative tests including the overnight or 2-day low-dose dexamethasone suppression test (DST), the CRH stimulation test, and the desmopressin stimulation test within the 3 months after surgery. Nowadays the most used cure criteria in the immediate postoperative period are those also employed in our center, as follows [73]:
-
Morning serum cortisol <1.8 µg/dL (higher long-term remission rates, 85–100% [11,12,13]).
-
Normal UFC.
Although extremely low serum cortisol (<1.8 µg/dL) is the best predictor of cure, some patients with low but detectable concentrations (2–4 µg/dL) that suppress with low-DST may remain in long-term remission [71]. A less stringent postoperative criterion is based on a postoperative serum cortisol concentration <5 µg/dL within 14 days of surgery, which associates initial cure rates of up to 80–90% after transsphenoidal microadenectomy in some studies [78,79,80,81], but long-term remission rates between 65 and 80% in others [81, 82]. Occasionally, some patients appear to have a late remission and the serum cortisol levels decline more gradually [83], possibly indicating the progressive necrosis of remaining tumor cells. Thus, it is important to ensure that cortisol has reached the lowest level before considering additional therapy. Measurement of UFC can provide additional useful information when the serum cortisol level is equivocal. The urine collection should begin at least 24 h after the last dose of hydrocortisone; a small maintenance dose (0.25–0.5 mg) of dexamethasone may be substituted instead of hydrocortisone before and during the collection. Levels above normal range indicate tumor persistence [72, 84, 85].
In our center, we measure serum cortisol and UFC in the immediate postoperative period before discharge (day 4–5 after surgery) for the evaluation of remission. Patients with basal serum cortisol <1.8 mcg/dl and normal UFC are considered cured. In patients with postsurgical eucortisolism, we perform two serum cortisol measurements in the morning within the first 2 weeks after surgery, with exogenous hidrocortisone withdrawal of at least 24 h before the first cortisol measurement. In patients in whom it is not possible to measure cortisol in the absence of steroid treatment within the 2 weeks after surgery, the assessment of the HPAA is performed in outpatient setting 4–6 weeks after discharge. If there is a reason to suspect tumor persistence, we perform a late-night salivary cortisol measurement as well as the DST. For persistent endogenous hypercortisolism (high UFC), we consider additional therapy (medical treatment, repeated PS, and/or radiotherapy). Patients with normal cortisol dynamics, in whom normal corticotroph cells may not be suppressed, are carefully monitored.
In patients who fully met criteria for remission, hydrocortisone replacement doses should be administered until complete recovery of the HPAA, a process that may take months or even years). The management of patients with intermediate postoperative values of cortisol should be individualized. All patients should be re-evaluated at least annually for several years and less frequently thereafter, and they should be advised to consider reassessment at any time if they experience a recurrence of their previous symptoms.
Acromegaly
In patients with GH-secreting PT, random IGF-1, and GH concentrations should be measured 12 weeks after surgery.
The postsurgical remission criteria that we use are:
-
IGF-1 within the normal range adjusted for age and sex; and
-
GH <0.14 ng/ml.
GH levels <0.14 ng/ml suggest “surgical remission”, whereas GH levels <1 ng/ml indicate “control” and normalization of mortality risk [14, 15]. In those patients presenting with IGF-1 above the age- and sex-adjusted normal range, or those with baseline GH > 1 ng/ml, serum GH levels should be measured during an 75 g oral glucose tolerance test (OGTT) [14]. A normal response is based on a GH during the OGTT < 1 ng/ml (or <0.4 ng/ml with ultrasensitive assays as ours).
In patients with persistent disease after surgery, an individualized treatment strategy based on medical therapy, repeated PS, and/or radiotherapy should be considered.
Long-term follow up by the different specialists
Data from all patients undergoing PS are included in a database designed for this purpose after giving appropriate written informed consent. This register follows all recommendations of local and European data protection law and has been approved by the local ethics committee.
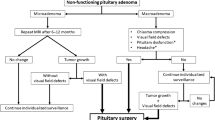
In usual conditions, outpatient follow-up visits in Neurosurgery and Endocrinology are carried out 1, 6, and 12 months after PS, and then at an annual basis. This schedule may be modified according to the specific characteristics of each case. In addition, they are followed-up at the outpatient Rhinology facilities at least during the first postsurgical year (Fig. 1).
Conclusions
The optimal postoperative management of PT submitted to PS requires a multidisciplinary approach involving a team of endocrinologists, neurosurgeons, ENT, neuro-ophthalmologists, neuroradiologists, and pathologists with experience in pituitary diseases. Such teams improve surgical results, minimize complications and facilitate their correct treatment if occurring, and optimize the hormonal, ophthalmological, and radiological reassessment throughout the follow-up. Through this multidisciplinary protocol on postoperative management of PT, currently in use at a PTCE, we aimed to share our experience with other centers that treat these patients, in order to facilitate the management of patients undergoing PS.
References
W.A. Hall, M.G. Luciano, J.L. Doppman, N.J. Patronas, E.H. Oldfield, Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann. Intern. Med. 120(10), 817–820 (1994)
P.U. Freda, A.M. Beckers, L. Katznelson, M.E. Molitch, V.M. Montori, K.D. Post et al. Pituitary incidentaloma: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 96(4), 894–904 (2011). https://doi.org/10.1210/jc.2010-1048
G. Zada, R. Du, E.R. Laws, Defining the ‘edge of the envelope’: patient selection in treating complex sellar-based neoplasms via transsphenoidal versus open craniotomy. J. Neurosurg. 114(2), 286–300 (2011). https://doi.org/10.3171/2010.8.JNS10520. Epub 2010 Sep 3
A. Tabaee, A. Anand, V.K. Barrón, Y. Hiltzik, D.D.H. Brown, M. Seth et al. Endoscopic pituitary surgery: a systematic review and meta-analysis. J. Neurosurg. 2009. https://doi.org/10.3171/2007.12.17635
M. Ammirati, L. Wei, I. Ciric, Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 84(8), 843–849 (2013). https://doi.org/10.1136/jnnp-2012-303194. Epub 2012 Dec 15
R.D. Almutairi, I.S. Muskens, D.J. Cote, M.D. Dijkman, V.K. Kavouridis, E. Crocker et al. Gross total resection of pituitary adenomas after endoscopic vs. microscopic transsphenoidal surgery: a meta-analysis. Acta Neurochir. 160(5), 1005–1021 (2018). https://doi.org/10.1007/s00701-017-3438-z. Epub 2018 Jan 6
Y. Gao, C. Zhong, Y. Wang, S. Xu, Y. Guo, C. Dai et al. Endoscopic versus microscopic transsphenoidal pituitary adenoma surgery: a meta-analysis. World J. Surg. Oncol. 12(4), 94 (2014). https://doi.org/10.1186/1477-7819-12-94
M.E. Molitch, Diagnosis and treatment of pituitary adenomas: a review. JAMA 317(5), 516–524 (2017). https://doi.org/10.1001/jama.2016.19699
D.J. Cote, A. Alzarea, M.A. Acosta, M.M. Hulou, K.T. Huang, H. Almutairi et al. Predictors and rates of delayed symptomatic hyponatremia after transsphenoidal surgery: asystemastic review. World Neurosurg. 88, 1–6 (2016). https://doi.org/10.1016/j.wneu.2016.01.022. Epub 2016 Jan 22
W.W. Woodmansee, J. Carmichael, D. Kelly, L. Katznelson, American association of clinical endocrinologists and american college of endocrinology disease state clinical review: postoperative management following pituitary surgery. Endocr. Pract. 21(7), 832–838 (2015). https://doi.org/10.4158/EP14541.DSCR
J. Estrada, J. García-Uría, C. Lamas, J. Alfaro, T. Lucas, S. Diez et al. The complete normalization of the adrenocortical function as the criterion of cure after transsphenoidal surgery for Cushing’s disease. J. Clin. Endocrinol. Metab. 86(12), 5695–5699 (2001)
J. Newell-Price, Transsphenoidal surgery for Cushing’s disease: defining cure and following outcome. Clin. Endocrinol. 56(1), 19–21 (2002)
L.B. Yap, H.E. Turner, C.B.T. Adams, J.A.H. Wass, Undetectable postoperative cortisol does not always predict long-term remission in Cushing’s disease: a single centre audit. Clin. Endocrinol. 56(1), 25–31 (2002)
L. Katznelson, E.R. Laws Jr, S. Melmed, M.E. Molitch, M.H. Murad, A. Utz et al. Acromegaly: an endocrine society clinical practice guidelinesummary of recommendations. J. Clin. Endocrinol. Metab. 99(11), 3933–3951 (2014). https://doi.org/10.1210/jc.2014-2700. Epub 2014 Oct 30
E.H. Kim, M.C. Oh, E.J. Lee, S.H. Kim, Predicting long-term remission by measuring immediate postoperative growth hormone levels and oral glucose tolerance test in acromegaly. Neurosurgery 70(5), 1106–1113 (2012). https://doi.org/10.1227/NEU.0b013e31823f5c16. discussion 1113
A. Jahangiri, J. Wagner, S.W. Han, M.T. Tran, L.M. Miller, M.W. Tom et al. Rate and time course of improvement in endocrine function after more than 1000 pituitary operations. Neurosurgery 61(Suppl 1), 163–166 (2014). https://doi.org/10.1227/NEU.0000000000000405
F.F. Casanueva, A.L. Barkan, M. Buchfelder, A. Klibanski, E.R. Laws, J.S. Loeffler et al. Criteria for the definition of Pituitary Tumor Centers of Excellence (PTCOE): a Pituitary Society statement. Pituitary 20(5), 489–498 (2017). https://doi.org/10.1007/s11102-017-0838-2
I. Ciric, A. Ragin, C. Baumgartner, D. Pierce, Complications of transsphenoidal surgery: Results of a national survey, review of the literature, and personal experience. Neurosurgery 40(2), 225–236 (1997)
F.G. Barker, A. Klibanski, B. Swearingen, Transsphenoidal surgery for pituitary tumors in the United States, 1996-2000: mortality, morbidity, and the effects of hospital and surgeon volume. J. Clin. Endocrinol. Metab. 88(10), 4709–4719 (2003)
K. Shahlaie, N. McLaughlin, A.B. Kassam, D.F. Kelly, The role of outcomes data for assessing the expertise of a pituitary surgeon. Curr. Opin. Endocrinol. Diabetes Obes. 17(4), 369–376 (2010). https://doi.org/10.1097/MED.0b013e32833abcba
P. Iglesias, V. Rodríguez Berrocal, J.J. Díez, Giant pituitary adenoma: histological types, clinical features and therapeutic approaches. Endocrine 61(3), 407–421 (2018). https://doi.org/10.1007/s12020-018-1645-x. Epub 2018 Jun 16
T.R. Deklotz, S.H. Chia, W. Lu, K.H. Makambi, E. Aulisi, Z. Deeb, Meta-analysis of endoscopic versus sublabial pituitary surgery. Laryngoscope 122(3), 511–518 (2012). https://doi.org/10.1002/lary.22479. Epub 2012 Jan 17
S. Dhandapani, H. Singh, H.M. Negm, S. Cohen, V.K. Anand, T.H. Schwartz, Cavernous sinus invasion in pituitary adenomas: systematic review and pooled data meta-analysis of radiologic criteria and comparison of endoscopic and microscopic surgery. World Neurosurg. 96, 36–46 (2016). https://doi.org/10.1016/j.wneu.2016.08.088. Epub 2016 Aug 30
B. Rotenberg, S. Tam, W.H.A. Ryu, N. Duggal, Microscopic versus endoscopic pituitary surgery: a systematic review. Laryngoscope 120(7), 1292–1297 (2010). https://doi.org/10.1002/lary.20949
K. Phan, J. Xu, R. Reddy, P. Kalakoti, A. Nanda, J. Fairhall, Endoscopic endonasal versus microsurgical transsphenoidal approach for growth hormone–secreting pituitary adenomas—systematic review and meta-analysis. World Neurosurg. 97, 398–406 (2017). https://doi.org/10.1016/j.wneu.2016.10.029. Epub 2016 Oct 15
L.H.A. Broersen, N.R. Biermasz, W.R. van Furth, F. de Vries, M.J.T. Verstegen, O.M. Dekkers et al. “Endoscopic vs. microscopic transsphenoidal surgery for Cushing’s disease: a systematic review and meta-analysis. Pituitary 21(5), 524–534 (2018). https://doi.org/10.1007/s11102-018-0893-3
R.J. Komotar, R.M. Starke, D.M.S. Raper, V.K. Anand, T.H. Schwartz, Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of giant pituitary adenomas. Pituitary 15(2), 150–159 (2012). https://doi.org/10.1007/s11102-011-0359-3
Y. Esquenazi, W.I. Essayed, H. Singh, E. Mauer, M. Ahmed, P.J. Christos et al. Endoscopic endonasal versus microscopic transsphenoidal surgery for recurrent and/or residual pituitary adenomas. World Neurosurg. 101, 186–195 (2017). https://doi.org/10.1016/j.wneu.2017.01.110. Epub 2017 Feb 6
J.K. Goudakos, K.D. Markou, C. Georgalas, Endoscopic versus microscopic trans-sphenoidal pituitary surgery: a systematic review and meta-analysis. Clin. Otolaryngol. 36(3), 212–220 (2011). https://doi.org/10.1111/j.1749-4486.2011.02331.x
J. Strychowsky, S. Nayan, K. Reddy, F. Farrokhyar, D. Sommer, Purely endoscopic transsphenoidal surgery versus traditional microsurgery for resection of pituitary adenomas: systematic review. J. Otolaryngol. Head. Neck Surg. 40(2), 175–185 (2011)
J. Coburger, R. König, K. Seitz, U. Bäzner, C.R. Wirtz, M. Hlavac, Determining the utility of intraoperative magnetic resonance imaging for transsphenoidal surgery: a retrospective study. J. Neurosurg. 120(2), 346–356 (2014). https://doi.org/10.3171/2013.9.JNS122207. Epub 2013 Dec 13
S. Berkmann, S. Schlaffer, C. Nimsky, R. Fahlbusch, M. Buchfelder, Intraoperative high-field MRI for transsphenoidal reoperations of nonfunctioning pituitary adenoma. J. Neurosurg. 121(5), 1166–1175 (2014). https://doi.org/10.3171/2014.6.JNS131994. Epub 2014 Aug 15
D. Esposito, D.S. Olsson, O. Ragnarsson, M. Buchfelder, T. Skoglund, G. Johannsson, Non-functioning pituitary adenomas: indications for pituitary surgery and post-surgical management. Pituitary 22(4), 422–434 (2019). https://doi.org/10.1007/s11102-019-00960-0
W.W. Woodmansee, J. Carmichael, D. Kelly, L. Katznelson, American association of clinical endocrinologists and american college of endocrinology disease state clinical review: postoperative management following pituitary surgery. Endocr. Pract. 21(7), 832–838 (2015). https://doi.org/10.4158/EP14541.DSCR
C. Meco, G. Oberascher, E. Arrer, G. Moser, K. Albegger, β-trace protein test: new guidelines for the reliable diagnosis of cerebrospinal fluid fistula. Otolaryngol. Head. Neck Surg. 129(5), 508–517 (2003)
I. Singer, J.R. Oster, L.M. Fishman, The management of diabetes insipidus in adults. Arch. Intern. Med. 157(12), 1293–1301 (1997)
R. Pivonello, M. De Leo, A. Cozzolino, A. Colao, The treatment of cushing’s disease. Endocr. Rev. 36(4), 385–486 (2015). https://doi.org/10.1210/er.2013-1048. Epub 2015 Jun 11
E.C. Nemergut, Z. Zuo, J.A. Jane, E.R. Laws, Predictors of diabetes insipidus after transsphenoidal surgery: a review of 881 patients. J. Neurosurg. 103(3), 448–454 (2005)
J.A. Loh, J.G. Verbalis, Diabetes insipidus as a complication after pituitary surgery. Nat. Clin. Pract. Endocrinol. Metab. 3(6), 489–494 (2007)
J. Hensen, A. Henig, R. Fahlbusch, M. Meyer, M. Boehnert, M. Buchfelder, Prevalence, predictors and patterns of postoperative polyuria and hyponatraemia in the immediate course after transsphenoidal surgery for pituitary adenomas. Clin. Endocrinol. 50(4), 431–439 (1999)
K. Eguchi, T. Uozumi, K. Arita, K. Kurisu, T. Yano, M. Sumida et al. Pituitary function in patients with rathke’s cleft cyst: significance of surgical management. Endocr. J. 41(5), 535–540 (1994)
P. Mortini, M. Losa, G. Pozzobon, R. Barzaghi, M. Riva, S. Acerno et al. Neurosurgical treatment of craniopharyngioma in adults and children: early and long-term results in a large case series. J. Neurosurg. 114(5), 1350–1359 (2011). https://doi.org/10.3171/2010.11.JNS10670. Epub 2011 Jan 7
I. Halac, D. Zimmerman, Endocrine manifestations of craniopharyngioma. Childs Nerv. Syst. 21(8–9), 640–648 (2005). Epub 2005 Jul 27
W. Fenske, B. Allolio, Current state and future perspectives in the diagnosis of diabetes insipidus: a clinical review. J. Clin. Endocrinol. Metab. 97(10), 3426–3437 (2012). https://doi.org/10.1210/jc.2012-1981. Epub 2012 Aug 1
J.A. Loh, J.G. Verbalis, Disorders of water and salt metabolism associated with pituitary disease. Endocrinol. Metab. Clin. North Am. 37(1), 213–234 (2008). https://doi.org/10.1016/j.ecl.2007.10.008
B.R. Olson, D. Rubino, J. Gumowski, E.H. Oldfield, Isolated hyponatremia after transsphenoidal pituitary surgery. J. Clin. Endocrinol. Metab. 80(1), 85–91 (1995)
S. Barber, B. Liebelt, D. Baskin, Incidence, etiology and outcomes of hyponatremia after transsphenoidal surgery: experience with 344 consecutive patients at a Single Tertiary Center. J. Clin. Med. 3(4), 1199–1219 (2014). https://doi.org/10.3390/jcm3041199
G. Spasovski, R. Vanholder, B. Allolio, D. Annane, S. Ball, D. Bichet et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol. Dial. Transplant. 29(Suppl 2), i1–i39 (2014). https://doi.org/10.1093/ndt/gfu040. Epub 2014 Feb 25
W.T. Burke, D.J. Cote, S.I. Iuliano, H.A. Zaidi, E.R. Laws, A practical method for prevention of readmission for symptomatic hyponatremia following transsphenoidal surgery. Pituitary 21(1), 25–31 (2018)
C.E. Taplin, C.T. Cowell, M. Silink, G.R. Ambler, Fludrocortisone therapy in cerebral salt wasting. Pediatrics 118(6), e1904–e1908 (2006). Epub 2006 Nov 13
R. Guerrero, A. Pumar, A. Soto, M.A. Pomares, S. Palma, M.A. Mangas et al. Early hyponatraemia after pituitary surgery: Cerebral salt-wasting syndrome. Eur. J. Endocrinol. 156(6), 611–616 (2007)
S.L. Atkin, A.M. Coady, M.C. White, B. Mathew, Hyponatraemia secondary to cerebral salt wasting syndrome following routine pituitary surgery. Eur. J. Endocrinol. 135(2), 245–247 (1996)
M. Fleseriu, I.A. Hashim, N. Karavitaki, S. Melmed, M.H. Murad, R. Salvatori et al. Hormonal replacement in hypopituitarism in adults: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 101(11), 3888–3921 (2016). Epub 2016 Oct 13
A.C. Lo, A.F. Howard, A. Nichol, K. Sidhu, F. Abdulsatar, H. Hasan et al. Long-term outcomes and complications in patients with craniopharyngioma: The British Columbia cancer agency experience. Int J. Radiat. Oncol. Biol. Phys. 88(5), 1011–1018 (2014). https://doi.org/10.1016/j.ijrobp.2014.01.019
F. Roelfsema, N.R. Biermasz, A.M. Pereira, Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary 15(1), 71–83 (2012). https://doi.org/10.1007/s11102-011-0347-7
J.F. Bonneville, F. Bonneville, F. Cattin, S. Nagi, MRI of the pituitary gland:indications and results in gynaecology and in obstetrics. Gynecol. Obstet. Fertil. 33(3), 147–153 (2005)
A. Wolf, A. Coros, J. Bierer, S. Goncalves, P. Cooper, S. Van Uum et al. Quantitative evaluation of vision-related and health-related quality of life after endoscopic transsphenoidal surgery for pituitary adenoma. J. Neurosurg. 127(2), 409–416 (2017). https://doi.org/10.3171/2016.7.JNS16200. Epub 2016 Oct 7
J.B. Kerrison, M.J. Lynn, C.A. Baer, S.A. Newman, V. Biousse, N.J. Newman, Stages of improvement in visual fields after pituitary tumor resection. Am. J. Ophthalmol. 130(6), 813–820 (2000)
Y.H. Kim, Y.H. Cho, S.H. Hong, J.H. Kim, M.S. Kim, S.K. Khang et al. Postoperative neurologic outcome in patients with pituitary apoplexy after transsphenoidal surgery. World Neurosurg. 111, e18–e23 (2018). https://doi.org/10.1016/j.wneu.2017.11.124. Epub 2017 Nov 28
C. Cortet-Rudelli, J.F. Bonneville, F. Borson-Chazot, L. Clavier, B. Coche Dequéant, R. Desailloud et al. Post-surgical management of non-functioning pituitary adenoma. Ann. Endocrinol. 76(3), 228–238 (2015). https://doi.org/10.1016/j.ando.2015.04.003. Epub 2015 Jun 23
A.W., D.F., Z.R., N.M., A.A., Endoscopic skull base reconstruction with the use of the nasoseptal flap: the tasmc experience. J. Neurol. Surg. B 77, FP-04-01 (2016). https://doi.org/10.1055/s-0036-1592452
L.X. Yin, C.M. Low, C.L. Puccinelli, E.K. O'Brien, J.K. Stokken, K.M. Van Abel et al. Olfactory outcomes after endoscopic skull base surgery: a systematic review and meta-analysis. Laryngoscope 129(9), 1998–2007 (2019). https://doi.org/10.1002/lary.28003. Epub 2019 Apr 29
S.L. Asa, O. Casar-Borota, P. Chanson, E. Delgrange, P. Earls, S. Ezzat et al. From pituitary adenoma to pituitary neuroendocrine tumor (pitnet): an international pituitary pathology club proposal. Endocr. Relat. Cancer 24(4), C5–C8 (2017). https://doi.org/10.1530/ERC-17-0004
R.V. Lloyd, R.Y. Osamura, G. Klöppel, J Rosai, Pathology and Genetics of Tumours of Endocrine Organs. Fourth edition. IARC WHO Classification of Tumours. Vol 10. (2017)
J. Trouillas, P. Roy, N. Sturm, E. Dantony, C. Cortet-Rudelli, G. Viennet et al. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 126(1), 123–135 (2013). https://doi.org/10.1007/s00401-013-1084-y. Epub 2013 Feb 12
C.P. Miermeister, S. Petersenn, M. Buchfelder, R. Fahlbusch, D.K. Lüdecke, A. Hölsken et al. Histological criteria for atypical pituitary adenomas—data from the German pituitary adenoma registry suggests modifications. Acta Neuropathol. Commun. 3, 50 (2015). https://doi.org/10.1186/s40478-015-0229-8
E. Manojlovic-Gacic, J. Bollerslev, and O. Casar-Borota. Invited Review: Pathology of pituitary neuroendocrine tumours: present status, modern diagnostic approach, controversies and future perspectives from a neuropathological and clinical standpoint. Neuropathol Appl Neurobiol. 2019. https://doi.org/10.1111/nan.12568
G. Raverot, P. Burman, A. McCormack, A. Heaney, S. Petersenn, V. Popovic et al. European society of endocrinology clinical practice guidelines for the management of aggressive pituitary tumours and carcinomas. Eur. J. Endocrinol. 178(1), G1–G24 (2018). https://doi.org/10.1530/EJE-17-0796. Epub 2017 Oct 18
P.J. Trainer, H.S. Lawrie, J. Verhelst, T.A. Howlett, D.G. Lowe, A.B. Grossman et al. Transsphenoidal resection in Cushing’s disease: undetectable serum cortisol as the definition of successful treatment. Clin. Endocrinol. 38(1), 73–78 (1993)
D.R. McCance, D.S. Gordon, T.F. Fannin, D.R. Hadden, L. Kennedy et al. Assessment of endocrine function after transsphenoidal surgery for Cushing’s disease. Clin. Endocrinol. 38(1), 79–86 (1993)
D.R. McCance, M. Besser, A.B. Atkinson, Assessment of cure after transsphenoidal surgery for Cushing’s disease. Clin. Endocrinol. 44(1), 1–6 (1996)
B.M. Biller, A.B. Grossman, P.M. Stewart, S. Melmed, X. Bertagna, J. Bertherat et al. Treatment of adrenocorticotropin-dependent cushing’s syndrome: a consensus statement. J. Clin. Endocrinol. Metab. 93(7), 2454–2462 (2008). https://doi.org/10.1210/jc.2007-2734. Epub 2008 Apr 15
R. Pivonello, M.C. De Martino, M. De Leo, L. Tauchmanovà, A. Faggiano, G. Lombardi et al. Cushing’s syndrome: aftermath of the cure. Arq. Bras. Endocrinol. Metabol. 51(8), 1381–1391 (2007)
N.A. Tritos, B.M.K. Biller, B. Swearingen, Management of Cushing disease. Nat. Rev. Endocrinol. 7(5), 279–289 (2011). https://doi.org/10.1038/nrendo.2011.12. Epub 2011 Feb 8
C. Beauregard, G. Dickstein, A. Lacroix, Classic and recent etiologies of Cushing’s syndrome: diagnosis and therapy. Treat. Endocrinol. 1(2), 79–94 (2002)
M.A. Czepielewski, G.A.F.S. Rollin, A. Casagrande, N.P. Ferreira, Criteria of cure and remission in Cushing’s disease: an update. Arq. Bras. Endocrinol. Metabol. 51(8), 1362–1372 (2007)
A. Ayala, A.J. Manzano, Detection of recurrent Cushing’s disease: proposal for standardized patient monitoring following transsphenoidal surgery. J. Neurooncol. 119(2), 235–242 (2014). https://doi.org/10.1007/s11060-014-1508-0. Epub 2014 Jul 1
R.M. Salassa, E.R. Laws, P.C. Carpenter, R.C. Northcutt, Transsphenoidal removal of pituitary microadenoma in Cushing’s disease. Mayo Clin. Proc. 53(1), 24–28 (1978)
P.C. Carpenter, Cushing’s syndrome: update of diagnosis and management. Mayo Clin. Proc. 61(1), 49–58 (1986)
Z. Ram, L.K. Nieman, G.B. Cutler, G.P. Chrousos, J.L. Doppman, E.H. Oldfield, Early repeat surgery for persistent Cushing’s disease. J. Neurosurg. 80(1), 37–45 (1994)
F. Esposito, J.R. Dusick, P. Cohan, P. Moftakhar, D. McArthur, C. Wang et al. Clinical review: Early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushing’s disease. J. Clin. Endocrinol. Metab. 91(1), 7–13 (2006). Epub 2005 Oct 18
D. Bochicchio, M. Losa, M. Buchfelder, Factors influencing the immediate and late outcome of Cushing’s disease treated by transsphenoidal surgery: a retrospective study by the European Cushing’s Disease Survey Group. J. Clin. Endocrinol. Metab. 80(11), 3114–3120 (1995)
A.M. Pereira, M.O. van Aken, H. van Dulken, P.J. Schutte, N.R. Biermasz, J.W. Smit et al. Long-term predictive value of postsurgical cortisol concentrations for cure and risk of recurrence in Cushing’s disease. J. Clin. Endocrinol. Metab. 88(12), 5858–5864 (2003)
A.L. Serban, E. Sala, G. Carosi, G. Del Sindaco, C. Giavoli, M. Locatelli et al. Recovery of adrenal function after pituitary surgery in patients with Cushing disease: persistent remission or recurrence? Neuroendocrinology 108(3), 211–218 (2019). https://doi.org/10.1159/000496846. Epub 2019 Jan 13
A.B. Atkinson, A. Kennedy, M.I. Wiggam, D.R. McCance, B. Sheridan, Long-term remission rates after pituitary surgery for Cushing’s disease: the need for long-term surveillance. Clin. Endocrinol. 63(5), 549–559 (2005)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Araujo-Castro, M., Pascual-Corrales, E., Martínez San Millan, J.S. et al. Postoperative management of patients with pituitary tumors submitted to pituitary surgery. Experience of a Spanish Pituitary Tumor Center of Excellence. Endocrine 69, 5–17 (2020). https://doi.org/10.1007/s12020-020-02247-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02247-y