Abstract
Purpose
Among patients with various cancers receiving anticancer drugs, sarcopenia is associated with poor survival and treatment outcomes. We conducted an observational study using skeletal muscle index (SMI) evaluation to investigate the association between sarcopenia and treatment outcomes of tyrosine kinase inhibitors (TKIs) in metastatic thyroid cancer patients.
Methods
We included 54 patients (19 men, 35 women; age, 66.5 ± 10.9 years) with differentiated thyroid carcinoma (DTC) or medullary thyroid carcinoma (MTC). The records of patients with metastatic DTC and MTC treated with TKIs were retrospectively reviewed. Patients were divided into sarcopenia and non-sarcopenia groups based on SMI. The SMI cutoff values for sarcopenia were 42 and 38 (cm2/m2) for males and females, respectively. Thirty-three patients had sarcopenia before TKI treatment.
Results
The sarcopenia group had more females and a lower body mass index. The median progression-free survival (PFS) durations were 13.6 (95% confidence interval (CI): 6.1–29.9) and 41.9 (95% CI: 25.2–not estimable) months in the sarcopenia and non-sarcopenia groups (p= 0.017), respectively. Univariate analysis showed that sarcopenia was significantly associated with PFS (p= 0.037). Sex, age, and performance status did not affect PFS. Multivariate analysis showed that sarcopenia was the only independent prognostic factor for PFS (hazard ratio: 2.488, 95% CI: 1.058–5.846, p= 0.037).
Conclusions
Sarcopenia could be a predictive factor of TKI treatment outcomes in patients with metastatic thyroid cancer as well as intervention target to improve prognosis. Further prospective investigations are needed to confirm these preliminary data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2012, ~230,000 new cases of thyroid cancer were diagnosed among women and 70,000 among men, with age-standardized (world population) rates being 6.10 and 1.90 per 100,000 persons, respectively [1]. Papillary thyroid carcinoma and follicular thyroid carcinoma are types of differentiated thyroid cancer (DTC); they, account for >90% of all thyroid carcinomas [2]. DTC has a good prognosis and the disease-specific survival rate of the affected patients is >90% [3]. Medullary thyroid carcinoma (MTC) is a malignant tumor of the parafollicular C cells of the thyroid gland; it accounts for ~5% of all thyroid cancers. In patients with MTC, the 10-year overall survival rate is ~75% [4]. In general, the prognosis for DTC or MTC is relatively good. However, the prognosis is poor for patients with unresectable, advanced DTC or MTC; thus, development of effective treatment approaches for these patients is important. Tyrosine kinase inhibitors (TKIs) exhibit high efficacy against unresectable, advanced DTC and MTC [4, 5]. Lenvatinib causes inhibition of vascular endothelial growth factor (VEGF) receptors 1, 2, and 3, fibroblast growth factor receptors 1–4, and platelet-derived growth factor receptor a, RET, and KIT signaling networks [5]. The global phase 3 Study of (E7080) Lenvatinib in Differentiated Cancer of the Thyroid (SELECT) reported a significant antitumor effect of lenvatinib, with median progression-free survival (PFS) durations of 18.3 and 3.6 months in the lenvatinib and placebo groups, respectively (hazard ratio for progression or death, 0.21; 99% confidence interval (CI), 0.14–0.31; p< 0.001) [5]. Furthermore, the efficacy of lenvatinib has been reported in Japanese clinical practice for DTC patents [6]. Vandetanib causes inhibition of RET, VEGF receptor, and endothelial growth factor receptor. An international, randomized, placebo-controlled, double-blind, phase III study (ZETA) reported a significant antitumor effect of vandetanib with PFS prolongation compared with placebo [4].
Sarcopenia is a syndrome characterized by a progressive and generalized loss of skeletal muscle mass and strength, with adverse outcomes including physical disability, poor quality of life, and death [7]. Sarcopenia is associated with poor survival and treatment outcomes of various anticancer drugs in several cancers [8–10].
At present, only little information is available on the association of sarcopenia with treatment outcomes of TKIs or on the prognosis of thyroid cancer. Thus, we conducted an observational study using the evaluation of skeletal muscle index (SMI) (defined as the sum of muscle area at the level of the third lumbar vertebra [L3] divided by the square of the height) to investigate the association between sarcopenia and treatment outcomes of TKIs in metastatic DTC and MTC patients.
Materials and methods
Patients
This study included 54 patients (19 men, 35 women; median age, 66.5 ± 10.9 years). Thirty-three patients (65%) had sarcopenia before starting TKI treatment. The records of patients with DTC and MTC treated with TKIs between May 2015 and May 2019 were retrospectively reviewed. The inclusion criteria were DTC and MTC confirmed by pathology, age ≥ 18 years, and use of radioactive iodine for DTCs before treatment with a TKI. Patients with anaplastic thyroid cancer and those who did not undergo abdominal computed tomography (CT) before starting TKI treatment were excluded from the study. The study was approved by Ethics Committees of Ito Hospital (approval number 252). Consent has been obtained from each patient after full explanation of the purpose and nature of all procedures used.
The following demographic and clinicopathological information were collected for analysis: sex, age, Eastern Cooperative Oncology Group performance status (PS), height, body weight, body mass index (BMI), histological type of thyroid cancer, distant metastasis sites, clinical response to TKI, and PFS duration.
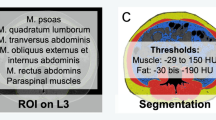
CT is the gold standard method for analyzing skeletal muscle mass, because it can be performed as part of routine clinical practice. Measurements of the cross-sectional area of skeletal muscles on abdominal CT were performed at the L3 level, including seven muscles: psoas, erector spinae, quadratus lumborum, transversus abdominis, external obliques, internal obliques, and rectus abdominis, as this is one of the international standards for measuring sarcopenia [11]. The cross-sectional area of the skeletal muscle at the L3 level was measured using Ziostation2 (Ziosoft, Tokyo, Japan) before starting TKI. The skeletal muscle area at the L3 level was normalized for height using the following equation: skeletal muscle index (SMI) (cm2/m2) = cross-sectional area of skeletal muscles (cm2)/height2 (m2). The SMI cutoff values for sarcopenia in our study were 42 (cm2/m2) for males and 38 (cm2/m2) for females based on a previous report defining sarcopenia in Japanese adults [12]. In this study, the definition of sarcopenia was based only on skeletal muscle mass. Patients were divided into two groups, the sarcopenia group and the non-sarcopenia group, based on the SMI cutoff values.
The initial dose of lenvatinib was 24 mg/day and that of vandetanib was 300 mg/day. The dose was adjusted according to the occurrence of adverse events.
Definitions
The clinical response to TKI was evaluated by CT using the Response Evaluation Criteria in Solid Tumors, version 1.1 [13]. The clinical response was classified into the following four categories: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The objective response rate was defined as the proportion of patients with a CR or PR. Disease control rate was defined as the proportion of patients with a CR, PR, or SD. PFS was calculated as the time from the start of TKI to disease progression or the date of death from any cause.
Statistical analysis
All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [14]. Between-group comparisons were made to identify any significant imbalances in patient characteristics. Categorical variables were compared using Fisher exact test and continuous variables using Student’s t-test. A PFS curve was constructed using the Kaplan–Meier method and between-group differences were assessed using log-rank test. Cox proportional hazards model was used to determine the factors associated with PFS. A p-value of <0.05 was considered significant.
Results
Study population
The patient characteristics were shown in Table 1. There were significant differences in sex (p= 0.045) and BMI (p< 0.001) between the two groups. Almost all the patients included in this study had DTC (84%) and a small number of patients with poorly DTC (PDTC) (9%) and MTC (7%) were also included. We found no significant differences in terms of other categories including the histologic type (p= 0.499), the kind of TKI (p= 0.148), target lesion size (p = 0.239), and distant metastasis (p = 0.312).
Efficacy and tolerability
The treatment outcomes are shown in Table 2. In the sarcopenia and non-sarcopenia groups, the response rates were 45% and 57%, respectively (p= 0.577), and the disease control rates were 73% and 81%, respectively (p= 0.536). The median PFS durations was 13.6 (95% CI: 6.1–29.9) months in the sarcopenia group and 41.9 (95% CI: 25.2–not estimable) months in the non-sarcopenia group (p= 0.017) (Fig. 1). Any treatment interruption was required in 32 (97%) patients in the sarcopenia group and 20 (95%) patients in the non-sarcopenia group (p= 1); also, any dose reduction was required in 27 (82%) patients in the sarcopenia group and 19 (90%) patients in the non-sarcopenia group (p= 0.461).
Prognostic factors
Results of univariate and multivariate analyses of the prognostic factors for PFS are summarized in Table 3. The univariate analysis showed that sarcopenia was significantly associated with PFS (p= 0.037). Sex, age, and PS did not affect PFS. The multivariate analysis showed that sarcopenia was the only independent prognostic factor for PFS (hazard ratio: 2.488, 95% CI: 1.058–5.846, p= 0.037) (Table 3).
Discussion
In this study, we compared the clinical response and PFS duration of TKI treatment between thyroid cancer patients with sarcopenia and without sarcopenia. We found that sarcopenia might be a poor prognostic factor in TKI treatment for advanced thyroid cancer.
The prevalence of pretherapeutic sarcopenia was 38.6% in patients with cancer [15]. However, the same prevalence in patients with thyroid cancer has not been reported. In this study, we found no significant differences in terms of the histologic types and the kinds of TKI between the two groups. The small numbers of patients with PDTC and MTC may have contributed to these results. In addition, there were no significant differences in target lesion size and distant metastasis between the two groups, and it was considered that tumor burden was not different between two groups when TKI treatment started.
Associations between sarcopenia and outcomes of drug therapy have been reported not only in chemotherapy but also in molecular targeted therapy and immunotherapy. In a study of sorafenib treatment for advanced hepatocellular carcinoma, it was reported that sarcopenia was a significant prognostic factor. Additionally, sarcopenia was proposed as a possible target of treatment intervention to improve prognosis [16]. In addition, in studies of sunitinib and everolimus treatment for renal cell carcinoma, sarcopenia was reported as a prognostic factor [17, 18]. In a study of pembrolizumab treatment for advanced non-small cell lung cancer, sarcopenia at baseline, determined using CT, was found to be a significant predictor of worse outcome [10]. This study indicated that sarcopenia was also a poor prognostic factor in patients receiving TKI treatments for thyroid cancer.
Bruce et al. [19] reported that the potential factors associated with PFS of lenvatinib were body weight, PS, baseline tumor size, and treatment intensity. However, in our study, there were no significant differences between PS and target lesion size. Although we found no significant differences in terms of treatment interruption or dose reduction rates between the two groups, PFS was significantly worse in the sarcopenia group. This may be due to the association between sarcopenia and the occurrence of severe adverse events [20]. The other possibility is that patients with sarcopenia may have low sensitivity for chemotherapy, and the TKI efficacy may not last long [20]. In patients with sporadic MTC, a subgroup analysis of PFS for M918T mutation suggested that M918T mutation-positive patients had a higher response rate to vandetanib compared with M918T mutation-negative patients [4]. However, the mutation status of four patients with MTC included in our study was unknown.
Many efforts have been made to reduce the incidence of sarcopenia. Reportedly, preoperative exercises and nutritional support programs can be effective measures to reduce sarcopenia [21]. Although the patients in this study did not receive any medical intervention to treat sarcopenia, appropriate nutritional support may prevent the occurrence of severe adverse events [20]. Patients may have better treatment outcomes if sarcopenia is treated before actually starting the TKI treatment.
Several definitions and measures of sarcopenia have been published [7, 22, 23]. The measurement of the SMI is one of the standards for measuring sarcopenia. Some studies used the cross-section of the psoas alone and the psoas muscle index at the L3 level has been used as a surrogate marker of skeletal muscle mass [24, 25]. Furthermore, sarcopenia has been defined based on the criteria originally proposed by the 2010 European Working Group on Sarcopenia in Older People [7]. However, the cutoff values would most likely be different from those calculated in Asian populations, due to variances in body size, lifestyles, and ethnicities. Therefore, we defined sarcopenia in this study using the SMI cutoff values obtained from Japanese data [12]. In Japanese patients, lenvatinib showed similar clinical outcomes to the overall SELECT population, but some differences in adverse event frequencies and dose modifications were observed [26]. Such differences in the incidence of adverse events of TKIs may also be due to the differences in ethnicities.
This study had some limitations. First, this study was a retrospective study with a small number of patients and it was conducted at a single center. Second, patients were excluded from our analysis if an abdominal CT scan was not performed at baseline, which may lead to selection bias. Finally, we were unable to evaluate the physical function of patients. Recent studies have defined sarcopenia only as skeletal muscle loss assessed using CT scans, but sarcopenia is based on the function of all muscles rather than muscle mass alone. Therefore, assessments of muscle strength and physical performance are vital to establish the presence of sarcopenia.
To the best of our knowledge, this is the first study to investigate the association between sarcopenia and treatment outcomes of TKIs in thyroid cancer patients. TKIs are costly drugs and have been associated with severe adverse events. Therefore, it is important to establish predictive factors for TKI treatment.
For advanced thyroid cancer patients undergoing TKI treatment, sarcopenia may be a useful predictive factor. Further, sarcopenia could potentially be the target of treatment interventions to improve the prognosis in this patient population. Further prospective investigations are needed to confirm these preliminary data.
References
C. La Vecchia, M. Malvezzi, C. Bosetti, W. Garavello, P. Bertuccio, F. Levi, E. Negri, Thyroid cancer mortality and incidence: a global overview. Int. J. Cancer 136, 2187–2195 (2015)
B. Aschebrook-Kilfoy, M.H. Ward, M.M. Sabra, S.S. Devesa, Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 21, 125–134 (2011)
K. Matsuzu, K. Sugino, K. Masudo, M. Nagahama, W. Kitagawa, H. Shibuya, K. Ohkuwa, T. Uruno, A. Suzuki, S. Magoshi, J. Akaishi, Thyroid lobectomy for papillary thyroid cancer: long-term follow-up study of 1,088 cases. World J. Surg. 38, 68–79 (2014)
S.A. Wells Jr, B.G. Robinson, R.F. Gagel, H. Dralle, J.A. Fagin, M. Santoro, E. Baudin, R. Elisei, B. Jarzab, J.R. Vasselli, J. Read, Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J. Clin. Oncol. 30, 134–141 (2012)
M. Schlumberger, M. Tahara, L.J. Wirth, B. Robinson, M.S. Brose, R. Elisei, M.A. Habra, K. Newbold, M.H. Shah, A.O. Hoff, A.G. Gianoukakis, Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 372, 621–630 (2015)
H. Iwasaki, H. Yamazaki, H. Takasaki, N. Suganuma, R. Sakai, H. Nakayama, S. Hatori, S. Toda, K. Masudo, Treatment outcomes of differentiated thyroid cancer with distant metastasis improve by tyrosine kinase inhibitors. Oncol. Lett. 17, 5292–5300 (2019)
A.J. Cruz-Jentoft, J.P. Baeyens, J.M. Bauer, Y. Boirie, T. Cederholm, F. Landi, F.C. Martin, J.P. Michel, Y. Rolland, S.M. Schneider, E. Topinková, Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age. Ageing 39, 412–423 (2010)
S.E. Park, I.G. Hwang, C.H. Choi, H. Kang, B.G. Kim, B.K. Park, S.J. Cha, J.S. Jang, J.H. Choi, Sarcopenia is poor prognostic factor in older patients with locally advanced rectal cancer who received preoperative or postoperative chemoradiotherapy. Medicine (Baltim.) 97, e13363 (2018)
T. Ota, T. Ishikawa, Y. Endo, S. Matsumura, J. Yoshida, T. Yasuda, T. Okayama, K. Inoue, O. Dohi, N. Yoshida, N. Sakamoto, Skeletal muscle mass as a predictor of the response to neo-adjuvant chemotherapy in locally advanced esophageal cancer. Med. Oncol. 36, 15 (2019)
T. Shiroyama, I. Nagatomo, S. Koyama, H. Hirata, S. Nishida, K. Miyake, K. Fukushima, Y. Shirai, Y. Mitsui, S. Takata, K. Masuhiro, Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with PD-1 inhibitors: a preliminary retrospective study. Sci. Rep. 9, 2447 (2019)
M. Mourtzakis, C.M. Prado, J.R. Lieffers, T. Reiman, L.J. McCargar, V.E. Baracos, A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 33, 997–1006 (2008)
H. Nishikawa, M. Shiraki, A. Hiramatsu, K. Moriya, K. Hino, S. Nishiguchi, Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 46, 951–963 (2016)
P. Therasse, S.G. Arbuck, E.A. Eisenhauer, J. Wanders, R.S. Kaplan, L. Rubinstein, J. Verweij, M. Van Glabbeke, A.T. van Oosterom, M.C. Christian, S.G. Gwyther, New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 92, 205–216 (2000)
Y. Kanda, Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48, 452–458 (2013)
F. Pamoukdjian, T. Bouillet, V. Lévy, M. Soussan, L. Zelek, E. Paillaud, Prevalence and predictive value of pretherapeutic sarcopenia in cancer patients: a systematic review. Clin. Nutr. 37, 1101–1113 (2018)
H. Takada, M. Kurosaki, H. Nakanishi, Y. Takahashi, J. Itakura, K. Tsuchiya, Y. Yasui, N. Tamaki, K. Takaura, Y. Komiyama, M. Higuchi, Y. Kubota, W. Wang, M. Okada, N. Enomoto, N. Izumi, Impact of pre-sarcopenia in sorafenib treatment for advanced hepatocellular carcinoma. PLoS ONE 13, e0198812 (2018)
H. Ishihara, T. Kondo, K. Omae, T. Takagi, J. Iizuka, H. Kobayashi, K: Tanabe, Sarcopenia and the modified Glasgow prognostic score are significant predictors of survival among patients with metastatic renal cell carcinoma who are receiving first-line sunitinib treatment. Target. Oncol. 11, 605–617 (2016)
E. Auclin, C. Bourillon, E. De Maio, M.A. By, S. Seddik, L. Fournier, M. Auvray, A. Dautruche, Y.A. Vano, C. Thibault, F. Joly, L. Brunereau, C. Gomez-Roca, C. Chevreau, R. Elaidi, S. Oudard, Prediction of everolimus toxicity and prognostic value of skeletal muscle index in patients with metastatic renal cell carcinoma. Clin. Genitourin. Cancer 15, 350–355 (2017)
B. Robinson, M. Schlumberger, M. Wirth, C. Dutcus, J. Song, M. Taylor, S. Kim, M. Krzyzanowska, J. Capdevila, S. Sherman, M. Tahara, Characterization of tumor size changes over time from the phase 3 study of lenvatinib in thyroid cancer. J. Clin. Endocrinol. Metab. 101, 4103–4109 (2016)
S. Sato, C. Kunisaki, H. Suematsu, Y. Tanaka, H. Miyamoto, T. Kosaka, N. Yukawa, K. Tanaka, K. Sato, H. Akiyama, I. Endo, Impact of sarcopenia in patients with unresectable locally advanced esophageal cancer receiving chemoradiotherapy. In Vivo. 32, 603–610 (2018)
K. Yamamoto, Y. Nagatsuma, Y. Fukuda, M. Hirao, K. Nishikawa, A. Miyamoto, M. Ikeda, S. Nakamori, M. Sekimoto, K. Fujitani, T. Tsujinaka, Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer 20, 913–918 (2017)
L.K. Chen, L.K. Liu, J. Woo, P. Assantachai, T.W. Auyeung, K.S. Bahyah, M.Y. Chou, L.Y. Chen, P.S. Hsu, O. Krairit, J.S. Lee, W.J. Lee, Y. Lee, C.K. Liang, P. Limpawattana, C.S. Lin, L.N. Peng, S. Satake, T. Suzuki, C.W. Won, C.H. Wu, S.N. Wu, T. Zhang, P. Zeng, M. Akishita, H. Arai, Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 15, 95–101 (2014)
M. Kimura, T. Naito, H. Kenmotsu, T. Taira, K. Wakuda, T. Oyakawa, Y. Hisamatsu, T. Tokito, H. Imai, H. Akamatsu, A. Ono, K. Kaira, H. Murakami, M. Endo, K. Mori, T. Takahashi, N. Yamamoto, Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support. Care. Cancer 23, 1699–1708 (2015)
Y. Hamaguchi, T. Kaido, S. Okumura, A. Kobayashi, A. Hammad, Y. Tamai, N. Inagaki, S: Uemoto, Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 32, 1200–1205 (2016)
A. Hiraoka, M. Hirooka, Y. Koizumi, H. Izumoto, H. Ueki, M. Kaneto, S. Kitahata, T. Aibiki, H. Tomida, Y. Miyamoto, H. Yamago, Y. Suga, R. Iwasaki, K. Mori, H. Miyata, E. Tsubouchi, M. Kishida, T. Ninomiya, M. Abe, B. Matsuura, H. Kawasaki, Y. Hiasa, K. Michitaka, Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol. Res. 47, 558–565 (2017)
N. Kiyota, M. Schlumberger, K. Muro, Y. Ando, S. Takahashi, Y. Kawai, L. Wirth, B. Robinson, S. Sherman, T. Suzuki, K. Fujino, A. Gupta, S. Hayato, M. Tahara, Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci. 106, 1714–1721 (2015)
Acknowledgements
We thank Enago (https://www.enago.jp/) for editing this manuscript.
Funding
No specific grant was received from any funding agency in the public, commercial, or not-for-profit sector for the publication of this report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving Human Participants and/or Animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Ito Hospital (approval number 252) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consents
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamazaki, H., Sugino, K., Matsuzu, K. et al. Sarcopenia is a prognostic factor for TKIs in metastatic thyroid carcinomas. Endocrine 68, 132–137 (2020). https://doi.org/10.1007/s12020-019-02162-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02162-x