Abstract
Background
Recently, numerous studies have reported an association between sarcopenia and poor outcomes in various kinds of malignancies. We investigated whether sarcopenia predicts the survival of patients with metastatic urothelial carcinoma who underwent systemic chemotherapy.
Methods
We reviewed 87 metastatic urothelial carcinoma patients who underwent chemotherapy (gemcitabine plus cisplatin or gemcitabine plus carboplatin for cisplatin-unfit patients) between 2007 and 2015. A computed tomography scan prior to chemotherapy was used for evaluating sarcopenia, and we measured three cross-sectional areas of skeletal muscle at the third lumbar vertebra and calculated the skeletal muscle index (SMI), the paraspinal muscle index (PSMI), and the total psoas area (TPA) of each patient. Predictive values of survival were assessed using Cox regression analysis.
Results
The median overall survival (OS) was 16 months (95% CI 13.5–18). Although SMI alone was not a significant predictor of shorter OS (P = 0.117) in univariate analysis, SMI stratified by the value of the body mass index (BMI) was a significant predictor of shorter OS in univariate analysis (P = 0.037) and was also an independent predictor of shorter OS in multivariate analysis (P = 0.026). PSMI and TPA were not significant prognostic factors even when stratified by BMI (P = 0.294 and 0.448), respectively.
Conclusion
Neither PSMI nor TPA could substitute SMI as a predictor for poor outcomes in metastatic urothelial carcinoma patients treated with systemic chemotherapy in our study. SMI stratified by BMI is a useful predictor of prognosis in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, sarcopenia has been widely recognized by physicians and researchers because of its predictive potential for various types of poor clinical outcomes. Originally, the term ‘sarcopenia’ [Greek ‘sarx’ (flesh) + ‘penia (loss)] was put forward by Rosenberg who describes the phenomenon as ‘an age-related loss of muscle mass and function’ [1]. In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) developed a clinical definition and came to a consensus concerning diagnostic criteria for age-related sarcopenia, indicating various causes for developing sarcopenia [2]. As cancer can be one of the causes of sarcopenia, many researchers have investigated the relationship between sarcopenia and prognosis [3,4,5], adverse events from treatments [6, 7], and perioperative morbidity [8, 9] for various kinds of malignancies.
Urothelial carcinoma (UC) is newly diagnosed in approximately 20,000 patients, resulting in 7000 deaths in Japan annually [10]. While systemic chemotherapy, with both a combination of gemcitabine and cisplatin (GC) and a combination of methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) are the standard treatment for advanced UC, GC is preferred due to lower toxicity [11]. However, large numbers of UC patients are elderly and their renal function tends to be impaired by their age and disease; therefore, it is common for cisplatin to be replaced with other substitutes for systemic chemotherapy in advanced UC [12]. Whether sarcopenia influences the outcome of UC patients who may have these frailties is extremely interesting. Thus, we investigated the significance of sarcopenia as a prognostic factor for metastatic UC (mUC) patients who underwent systemic chemotherapy, i.e., GC for normal patients and gemcitabine plus carboplatin (GCb) for cisplatin-unfit patients.
Patients and methods
Patients
We reviewed 87 mUC patients who underwent first-line systemic chemotherapy, namely, GC for patients with normal renal function and good performance status or GCb for patients with renal insufficiency or frailty, at our institutions between June 2007 and December 2015. The study was approved by the local ethics board.
Clinical characteristics at the beginning of GC or GCb therapy were reviewed to account for age, gender, tumor location (upper urinary tract or bladder), prior radical surgery, the Eastern Cooperative Oncology Group performance status (ECOG PS), grade, T stage, lymphovascular invasion (LVI), cisplatin-unfit status (GC or GCb), C-reactive protein (CRP), albumin, Glasgow prognostic score (GPS), and body mass index (BMI).
Imaging analysis
All patients underwent initial diagnostic or follow-up computed tomography (CT). To assess sarcopenia, patients had a CT evaluation within 30 days prior to systemic chemotherapy.
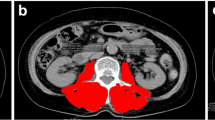
The third lumbar vertebra (L3) was set as a landmark, and the cross-sectional areas of lumbar skeletal muscle that were identified using Hounsfield unit thresholds of − 29 to + 150 were used for evaluating sarcopenia (Fig. 1a) [13]. We assessed three parameters of skeletal muscle mass, namely bilateral psoas muscle (total psoas) (Fig. 1b), paraspinal muscle including total psoas, quadratus lumborum, erector spinae and multifidus muscle (Fig. 1c), and skeletal muscle including paraspinal muscle, transversus abdominus, external and internal obliques and rectus abdominus (Fig. 1d). To evaluate sarcopenia, these muscle areas were normalized with square of the height, total psoas area (TPA) (cm2/m2) (total psoas muscle cross-sectional area at L3), the paraspinal muscle index (PSMI) (cm2/m2) (paraspinal muscle cross-sectional area at L3), and the skeletal muscle index (SMI) (cm2/m2) (skeletal muscle cross-sectional area at L3). Images were analyzed using Image J 1.48 (National Institute of Health, Bethesda, MD, USA, http://rsb.info.nih.gov/ij). Image analysis was performed by one investigator (KT) who was blinded to other variables and patient outcomes.
Three muscle areas were normalized with square of the height and defined as b TPA, c PSMI, and d SMI, respectively. a Axial image of CT scan at L3 (with being skeletal muscle enclosed by color line). b The cross-sectional area at L3 of total psoas muscle (bilateral psoas muscle). c The cross-sectional area at L3 of paraspinal muscle (including total psoas, quadratus lumborum, erector spinae and multifidus muscle). d The cross-sectional area at L3 of skeletal muscle (including paraspinal muscle, transversus abdominus, external and internal obliques and rectus abdominus)
Statistical analysis
Continuous variables were summarized with medians and interquartile range (IQR). Categorical variables were summarized with frequency and percentages. To confirm gender differences in muscle mass, a t test was used for each muscle mass parameter. We chose the cut-off values for SMI defining sarcopenia based on the international consensus (<55 cm2/m2 for men and <39 cm2/m2 for women) [14], and the cut-off values for BMI based on the Japanese consensus for obesity (≥25 kg/m2). Other cut-off values for continuous variables including PSMI and TPA were set at median values. Pearson’s correlation coefficient was used to evaluate correlations between each muscle mass parameter. Survival curves were estimated using the Kaplan–Meier method and differences between groups were evaluated using the log-rank test. Univariate and multivariate Cox hazard models tested the associations between variables and overall survival (OS). All statistical analyses were performed using EZR version 1.33, which is a graphical user interface for R [15], and statistical significance was defined as P < 0.05.
Results
Patient characteristics
During the median follow-up of 15.4 months (range 1.3–69.1), 70 patients (80.5%) died of cancer. All mortal events in this cohort occurred because of cancer progression. The study comprised 65 (74.7%) men and 22 (25.3%) women with a median age of 73 years (IQR 67−77). The primary site of cancer was the upper urinary tract in 41 (47.1%) and the bladder in 46 (52.9%). Forty-two (48.3%) patients underwent radical surgery prior to systemic chemotherapy. Worse ECOG PS (>1) was seen in 25 (28.7%) patients. Grade 3 in the pathological findings was found in 71 (81.6%) patients, and a clinical high stage (>2) was found in 63 (72.4%) patients. Fifty-two (59.8%) patients were positive for LVI, and 44 (50.6%) were unfit to receive cisplatin and underwent chemotherapy with GCb as a substitute treatment for GC. The median CRP value was 0.6 mg/dl (IQR 0.1–1.9) and the median albumin value was 3.6 mg/dl (IQR 3.4–4.0). Forty-four (50.6%) patients had a GPS of 0, 25 (28.7%) of 1, and 16 (18.4%) of 2, and two (2.3%) patients were unknown. The median BMI value was 22.8 kg/m2 (IQR 21.1–24.9) and the median SMI, PSMI, and TPA values were 47.8 cm2/m2 (IQR 42.8–52.7), 26.5 cm2/m2 (IQR 22.9–29.4), and 6.2 cm2/m2 (IQR 4.8–6.9) in men and 39.8 cm2/m2 (IQR 33.2–46.4), 21.2 cm2/m2 (IQR 18.0–25.8), and 4.6 cm2/m2 (IQR 3.8–5.5) in women, respectively (Table 1).
Correlation between muscle indexes
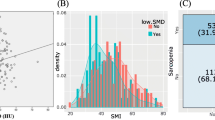
The scatter plots of both PSMI and TPA with SMI to verify the correlation of each index are shown in Fig. 1. The coefficient of determination (r 2) of Pearson’s product-moment correlation for PSMI-SMI and TPA-SMI were 0.808 and 0.598, respectively (Fig. 2a, b). The three-dimensional scatter plot of PSMI, TPA and SMI is shown in Fig. 2c.
Patient survival
Figure 3a shows Kaplan–Meier survival curves of OS. Median OS time was 16 months (95%CI 13.5–18) in total patients. Figure 3b–d shows Kaplan–Meier curves of OS, which were divided into a lower group and a higher group than the cut-off values of each index, respectively. The median OS of the non-sarcopenic group (n = 9) was 23.8 months (95% CI 14.4, not reached), and the sarcopenic group (n = 78) was 15.5 months (95% CI 12.5–18), and there was no significant difference between the two groups (P = 0.11) (Fig. 3b). Similarly, there were no significant differences between the lower and higher groups in terms of both PSMI and TPA (P = 0.45 and 0.38, respectively) (Fig. 3c, d).
We then stratified each sarcopenic and non-sarcopenic group according to BMI (obesity ≥25 kg/m2, non-obesity <25 kg/m2). The median OS of sarcopenic obesity (n = 15), sarcopenic non-obesity (n = 63), non-sarcopenic obesity (n = 5), and non-sarcopenic non-obesity (n = 4) were 15.6 (95% CI 6.2–20.7), 15.4 (95% CI 11.8–18), 23.8 (95% CI 14.4, not reached), and 16.7 (95% CI 15, not reached) months, respectively (P = 0.031) (Fig. 4a). After this stratification, we identified that there was a significant difference between the non-sarcopenic group regardless of obesity status (n = 9) [median OS was 23.8 months (95% CI 14.4-not reached)] and the sarcopenic obesity group (n = 15) [median OS was 15.6 months (95% CI 6.2–20.7)] (P = 0.044) (Fig. 4b). On the other hand, there were no significant differences in the same stratification in terms of PSMI with BMI and TPA with BMI (data not shown here).
Predictor of patient survival
Table 2 shows Cox regression analysis for OS. In univariate analysis, CRP, GPS, and SMI stratified by BMI were candidates for independent prognostic factors (P = 0.001, 0.028, and 0.037, respectively). In multivariate analysis, CRP (P = 0.011, HR 2.401, 95% CI 1.219–4.727) and SMI stratified by BMI (P = 0.026, HR 3.102, 95% CI 1.149–8.374) were independent predictive factors for OS.
Discussion
The term ‘sarcopenia’ was first described in 1997 and means ‘poverty of flesh’ in Greek [1]. Its clinical relevance was defined as the age-related loss of muscle mass and function. Sarcopenia has been well documented in the elderly and is a known mortality risk in this population. The EWGSOP reported on additional clinical characteristics of sarcopenia and created practical diagnostic criteria for use in geriatric assessments. This resulted in categorization of the condition into primary and secondary sarcopenia, with cancer being one of the causes of secondary sarcopenia and cachexia was also identified as a factor in the development of sarcopenia [2]. In 2011, Fearon et al. described a new international consensus on the definition and classification of cancer cachexia, stating that the defining attribute is the loss of skeletal muscle mass which cannot be fully reversed by conventional nutritional support [14]. The EWGSOP’s definition of sarcopenia requires measuring muscle strength (i.e., hand-grip strength) or physical performance (i.e., gait-speed) is also necessary, excluding accurate determination of the loss of total body skeletal muscle using dual-energy X-ray absorptiometry (DXA) or bioelectrical impedance analysis, to diagnose sarcopenia [2]. However, in cancer cachexia, sarcopenia is defined as low skeletal muscle mass measured with the cross-sectional area at L3 [14], following the results of studies showing a correlation between the cross-sectional area of lumber skeletal muscle measured by magnetic resonance imaging (MRI) or CT and total body skeletal muscle measured by whole-body MRI or DXA [16, 17]. As a result of the consensus on the definition of cancer cachexia, several studies have reported on the relationship between sarcopenia and a variety of cancer outcomes [3,4,5], adverse events [6, 7], and complications from cancer treatments [8, 9].
From a urological perspective, cisplatin-based chemotherapy is the standard treatment for mUC. However, we are often forced to choose a substitute because many patients are unfit to receive cisplatin, due mostly to frailty [12]. Thus, we investigated the association between sarcopenia and survival outcomes in order to define the significance of sarcopenia as a prognostic marker of mUC patients treated with gemcitabine doublets, namely GC for normal patients and GCb for cisplatin-unfit patients. However, neither age nor ECOG PS were a significant prognostic factor in Cox regression analysis (P = 0.156 and 0.483, respectively), and although cisplatin-unfit status (GC or GCb) had a tendency, it did not reach significance (P = 0.055) in our study.
To investigate the relationship between sarcopenia and survival outcomes, we measured and calculated two other parameters of muscle area index (excluding SMI) in each patient. In orthopedics, it is known that atrophy of the multifidus muscle and erector spinae, which hold the spine, is associated with the risk of back pain [18]. As such, we considered whether the loss of these muscles could lead to frailty. Thus, we defined a group that included total psoas, the quadratus lumborum, the multifidus muscle and erector spinae as paraspinal muscle that accounts for approximately 50% of the skeletal muscle cross-sectional area at L3. We evaluated this group to identify whether it reflects sarcopenic status. In addition, TPA has been reported as having clinical utility for predicting poor outcomes among several cancers [9, 19]; however, the results of recent studies do not support these findings [20, 21]. We then evaluated the associations of each muscle area index for estimating whether PSMI and TPA are available as substitutes for SMI, which has been proven to reflect sarcopenic status. Although the values of PSMI and TPA significantly correlated with the SMI value, neither of them were associated with the survival outcome of mUC patients treated with gemcitabine doublets, which is similar to the results for SMI in our study. However, SMI stratified by BMI was significantly associated with OS. More importantly, there was a significant difference between the non-sarcopenic (SMI ≥ the gender cut-off value) groups regardless of obese status and sarcopenic obesity (SMI < the gender cut-off value and BMI ≥ 25 kg/m2) group in OS.
Ironically, obesity is known to be associated with better survival rates in patients with cardiovascular disease (CVD) [22], and more recently in patients with some types of cancer as well [23, 24]; hence the label ‘the obesity paradox’, due to limitation when measuring BMI only. However, it was also reported that sarcopenia with obesity may be associated with an increased risk of mortality as opposed to obesity or sarcopenia alone in CVD patients [25]. To date, sarcopenic obesity has not been reported as a significant predictor of cancer survival; however, recent data have suggested that it could be a prognostic marker for cancer patients [26, 27]. These data support that taking skeletal muscle mass with the fat component of the whole body into account can supplement diagnosing sarcopenia by measuring SMI only regarding the consensus of cancer cachexia. In this study, SMI stratified by BMI was a potential predictor of OS in mUC patients treated with gemcitabine doublets. Thus, sarcopenic obesity might also be a prognostic marker for mortality in cancer patients as it is in CVD patients or other types of cancer patients.
Moreover, it is known that inflammatory cytokines play a key role in the development of both primary and secondary sarcopenia [28]. SMI stratified by BMI and inflammatory markers like CRP, which reflect sarcopenic status, can predict survival outcomes. As such, they could be useful for clinical decision-making prior to systemic chemotherapy despite some limitations in our study, namely retrospective, small numbers of patients, and the multi-institutional setting.
In addition, we should pay attention to the reason why only SMI stratified by BMI was a predictor of survival outcome in our study. Sarcopenia is defined as only loss of muscle mass in the definition and classification of cancer cachexia. However, it has been considered by investigators who specialize in sarcopenia that loss of muscle mass by itself is a weak predictor of outcomes, as well as the fact that the link between muscle mass, muscle function, physical performance and other downstream outcomes is not linear. Therefore, the definition of sarcopenia by the EWGSOP requires not only loss of muscle mass but also weak muscle strength or low physical performance when assessing geriatric people. Although most of the patients in this cohort were geriatric and all of them had metastatic cancer, they were still resilient enough to undergo gemcitabine doublets in order to treat their cancer. Therefore, even though we did not assess weight loss during a certain period which is also required when defining cancer cachexia, we do not think all of them were cachectic patients. This characteristic of patients in this study could lead a discrepancy when choosing an appropriate definition of sarcopenia. Although many studies regarding the relationship between sarcopenia and cancer outcomes have adopted the sarcopenia definition of cancer cachexia, we should be concerned about the resilience of the patients in the cohort when investigating it, especially in a small cohort. Thus, our strong messages in this study are that the assessment of loss of muscle mass alone is a weak predictor when researching cancer outcomes and, moreover, the targeted area of muscle mass should not be simplified and should be evaluated with body composition using obesity criteria. Lastly, sarcopenic obesity is a strong predictor of short prognosis in mUC patients treated with chemotherapy.
Finally, post-diagnosis recreational activity has been reported to be associated with better cancer-specific survival outcomes in prostate cancer patients [29]. Thus, there might be a possibility that active intervention, including adequate education about appropriate nutrition and exercise for patients before developing age- or cancer-related sarcopenia, could promote resilience and also improve mortality risk in mUC patients treated with systemic chemotherapy. Further investigations are needed to prove this hypothesis.
Conclusion
SMI stratified by BMI is significantly associated with OS of mUC patients treated with gemcitabine doublets as opposed to PSMI and TPA. Neither PSMI nor TPA could substitute SMI as an active predictor for poor outcomes in our study. Sarcopenic obesity might be a prognostic marker for poor outcomes in mUC patients treated with chemotherapy as it is in CVD and in other types of cancer patients.
Abbreviations
- GC:
-
Gemcitabine plus cisplatin
- GCb:
-
Gemcitabine plus carboplatin
- CT:
-
Computed tomography
- SMI:
-
Skeletal muscle index
- PSMI:
-
Paraspinal muscle index
- TPA:
-
Total psoas area
- OS:
-
Overall survival
- CI:
-
Confidence interval
- BMI:
-
Body mass index
- EWGSOP:
-
European Working Group on Sarcopenia in Older People
- UC:
-
Urothelial carcinoma
- MVAC:
-
Methotrexate, vinblastine, doxorubicin and cisplatin
- mUC:
-
Metastatic urothelial carcinoma
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- LVI:
-
Lymphovascular invasion
- CRP:
-
C-reactive protein
- GPS:
-
Glasgow prognostic score
- L3:
-
Third lumber vertebra
- IQR:
-
Interquartile range
- DXA:
-
Dual-energy X-ray absorptiometry
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- CVD:
-
Cardiovascular disease
References
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127(5 Suppl):990S–991S
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in older people. Age Ageing 39(4):412–423
Psutka SP, Carrasco A, Schmit GD et al (2014) Sarcopenia in patients with bladder cancer undergoing radical cystectomy: impact on cancer-specific and all-cause mortality. Cancer 120(18):2910–2918
Peng P, Hyder O, Firoozmand A et al (2012) Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 16(8):1478–1486
Fukushima H, Nakanishi Y, Kataoka M et al (2016) Prognostic significance of sarcopenia in patients with metastatic renal cell carcinoma. J Urol 195(1):26–32
Huillard O, Mir O, Peyromaure M et al (2013) Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br J Cancer 108(5):1034–1041
Antoun S, Baracos VE, Birdsell L et al (2010) Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol 21(8):1594–1598
Lieffers JR, Bathe OF, Fassbender K et al (2012) Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 107(6):931–936
Smith AB, Deal AM, Yu H et al (2014) Sarcopenia as a predictor of complications and survival following radical cystectomy. J Urol 191(6):1714–1720
Hori M, Matsuda T, Shibata A et al (2015) Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 45(9):884–891
von der Maase H, Sengelov L, Roberts JT et al (2005) Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23(21):4602–4608
Galsky MD, Hahn NM, Rosenberg J et al (2011) Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin-based chemotherapy. J Clin Oncol 29(17):2432–2438
Fukushima H, Yokoyama M, Nakanishi Y et al (2015) Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS One 10(1):e0115895. https://doi.org/10.1371/journal.pone.0115895
Fearon K, Strasser F, Anker SD et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12(5):489–495
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458
Shen W, Punyanitya M, Wang Z et al (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 97(6):2333–2338
Mourtzakis M, Prado CM, Lieffers JR et al (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33(5):997–1006
Fortin M, Battie MC (2012) Quantitative paraspinal muscle measurements: inter-software reliability and agreement using OsiriX and ImageJ. Phys Ther 92(6):853–864
Peng PD, van Vledder MG, Tsai S et al (2011) Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB 13(7):439–446
Taguchi S, Akamatsu N, Nakagawa T et al (2016) Sarcopenia evaluated using the skeletal muscle index is a significant prognostic factor for metastatic urothelial carcinoma. Clin Genitourin Cancer 14(3):237–243
Amini N, Spolverato G, Gupta R et al (2015) Impact total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: a new tool to assess sarcopenia. J Gastrointest Surg 19(9):1593–1602
Angeras O, Albertsson P, Karason K et al (2013) Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J 34(5):345–353
Gonzalez MC, Pastore CA, Orlandi SP et al (2014) Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr 99(5):999–1005
Caan BJ, Meyerhardt JA, Kroenke CH et al (2017) Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev. https://doi.org/10.1158/1055-9965.EPI-17-0200
Wannamethee SG, Atkins JL (2015) Muscle loss and obesity: the health implications of sarcopenia and sarcopenic obesity. Proc Nutr Soc 74(4):405–412
Prado CM, Lieffers JR, McCargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9(7):629–635
Palmela C, Velho S, Agostinho L et al (2017) Body composition as a prognostic factor of neoadjuvant chemotherapy toxicity and outcome in patients with locally advanced gastric cancer. J Gastric Cancer 17(1):74–87
Hall DT, Ma JF, Marco SD et al (2011) Inducible nitric oxide synthase (iNOS) in muscle wasting syndrome, sarcopenia, and cachexia. Aging 3(8):702–715
Friedenreich CM, Wang Q, Neilson HK et al (2016) Physical activity and survival after prostate cancer. Eur Urol 70(4):576–585
Acknowledgements
The authors are grateful to Dr. Ken-Ichiro Yoshida and Dr. Kyoko Arai for constructive suggestions, ServiceScape (https://www.servicescape.com) and Jessica McHale (http://oe-boston.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to declare.
About this article
Cite this article
Abe, H., Takei, K., Uematsu, T. et al. Significance of sarcopenia as a prognostic factor for metastatic urothelial carcinoma patients treated with systemic chemotherapy. Int J Clin Oncol 23, 338–346 (2018). https://doi.org/10.1007/s10147-017-1207-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-017-1207-x