Abstract
Purpose
Some preliminary studies reported a link between GLP-1 receptor agonists (GLP-1RAs) and thyroid/pancreatic neoplasms, while its human relevance remained undetermined. The present meta-analysis was performed to collect information on cancers associated with GLP-1RAs in patients with type 2 diabetes mellitus (T2DM).
Methods
Medline, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science and ClinicalTrials.gov were extensively searched to identify randomized controlled trials that reported cancer events in T2DM patients treated with GLP-1RAs for at least 52 weeks, up to March 18, 2019. Odds ratio (OR) with 95% Confidence Interval (CI) was calculated for overall cancer (primary outcome), thyroid and pancreatic cancer.
Results
A total of 37 eligible trials were identified. The OR for overall cancer associated with GLP-1RAs was 1.03 (95% CI 0.95–1.12; p = 0.41) compared with comparators. Subgroup analyses showed that treatment with albiglutide was associated with a lower risk of overall cancer (OR 0.76 [95% CI 0.60–0.97]; p = 0.03), and no elevated risk of overall cancer was identified for other GLP-1RAs. No significant differences in the risks of thyroid nor pancreatic cancer were disclosed between GLP-1RAs and comparators.
Conclusions
This meta-analysis did not suggest any increased risk of cancers associated with GLP-1RAs use in T2DM. The reduction in the risk of overall cancer associated with albiglutide needs to be examined further.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growing evidence suggests the association of type 2 diabetes mellitus (T2DM) and an increased risk of cancer [1]. Although the underlying mechanisms are not fully understood, potential mechanisms involve overproduction of reactive oxygen species, insulin and insulin-like growth factor receptor-1 [2]. On the other hand, hyperglycemia and concurrent obesity also impair immunosurveillance and blunt antitumor responses [3]. Currently multiple classes of antidiabetic agents are available for the treatment of T2DM, and it is critical that antidiabetic drugs do not increase the risk of cancer.
Native glucagon-like peptide-1 (GLP-1), secreted from intestinal enteroendocrine L cells in response to meal challenges, augments the biosynthesis of insulin and stimulates insulin secretion in a glucose-dependent manner. Complementary to its insulinotropic effects, GLP-1 also suppresses postprandial glucagon excursions, delays gastric emptying and intestinal mobility, induces satiety, and promotes weight loss, exerting a potent glucose-lowering activity. Furthermore, a β-cell-preserving effect of GLP-1 was observed in animal studies [4]. Easily degraded by dipeptidyl peptidase-4, endogenous GLP-1 has a short elimination half-life. Two approaches were developed to potentiate GLP-1 action, including exogenous administration of GLP-1 receptor agonists (GLP-1RAs) with a prolonged duration of action, and pharmacological inhibition of proteolytic degradation [5].
GLP-1RAs, including exenatide, liraglutide, albiglutide, dulaglutide, semaglutide, lixisenatide, and taspoglutide, are a fairly new class of incretin-based antidiabetic drugs indicated for the treatment of T2DM, especially those with poor weight control, showing clinical advantages in improving glycemic control and weight management with minimal risks of hypoglycemia. However, some unfavorable findings in preliminary experiments as well as in signal generation analyses of adverse event reporting system (AERS) database, and a lack of adequate evidence of their long-term safety in humans raised concerns about their pancreatic and thyroid safety profiles [6, 7]. To address this issue, an updated meta-analysis aimed at evaluating the risk of overall cancer, including pancreatic and thyroid cancer respectively, associated with GLP-1RAs, was performed in this study, collecting data from randomized controlled trials.
Methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [8], and was registered with PROSPERO (number CRD42019133712). The PRISMA checklist is provided in Supplementary Table 1.
Data sources and searches
An extensive literature search was performed in PubMed, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL) and Web of Science, using the main terms “(exenatide OR liraglutide OR lixisenatide OR albiglutide OR dulaglutide OR taspoglutide OR semaglutide OR GLP-1 receptor agonist* OR GLP-1 analog* OR incretin-based therapy* OR incretin mimetic*) AND (T2DM OR insulin-independent diabetes mellitus) AND (neoplasm or neoplasms or cancer or cancers or carcinoma or carcinomas or tumor or tumorsor neoplasia or neoplasias or malignancy or malignancies) to search for relevant studies up to March 18, 2019. Detailed search strategy is listed in Supplementary Table 2. Reference lists of all retrieved trials, reviews, and conference abstracts were searched manually for eligibility to supplement the computer search.
Study selection
All available randomized controlled trials (RCTs) that compared GLP-1RAs with placebo or active comparators in all age groups of T2DM patients, and that reported the incidence of cancers were included. Inclusion criteria: (1) randomized clinical trials comparing a GLP-1RA with a non-GLP-1RA active comparator or/and a placebo, (2) recruiting patients with T2DM only, (3) with durations of at least 52 weeks, (4) providing an estimate on cancers associated with GLP-1RAs use, and (5) published in English. Studies using the fixed-ratio combination containing GLP-1RAs, and those comparing different formulations or subclasses of GLP-1RAs were excluded.
Data extraction and quality assessment
The primary outcome was overall incidence of any cancer defined as ‘Neoplasms benign, malignant and unspecified (including cysts and polyps)’ according to the MedDRA dictionary. Secondary endpoints included incidence of thyroid and pancreatic cancer. Cancer events reported in the publications served as the primary source of information. When multiple reports based on the same population was identified, the most recent or informative one was selected. All other sources, including relevant reviews and pooled analysis reporting results of individual studies, were searched for complementary information on results of published trials, when not available in publications. If cancer events were not reported in the manuscripts, data from the ‘Serious Adverse Events’ section on ClinicalTrials.gov were extracted, and assumed to be zero if not reported on ClinicalTrials.gov. Editorials, letters, articles without treatment-emergent adverse events, and animal experimental studies were excluded. The following data was extracted independently by two reviewers: the first author, publication year, NCT number, trial duration, characteristics of participants (sample size, background therapy, mean age and percentage of women), intervention (type and regimen of GLP-1RAs), comparators, and outcomes of interest. Any discrepancy was resolved by consensus or by the third investigator.
The Cochrane risk of bias tool was used to assess the risk of bias of each study, based on the following aspects: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other source of bias. Two independent reviewers evaluated each item as low, high, or unclear risk of bias.
Jadad score [9, 10] was used to evaluate the methodologic quality of the included studies. The quality scale included randomization (1 point), method to generate randomization sequence was described (computer-generated randomization, or tables of random numbers) (1 point), double blind (1 point), placebo (1 point), numbers and reasons for withdrawal (1 point), and analysis by intent-to-treat (1 point). When more than one reference was found for the same trial, methodological quality assessment was based on the total set of information. A score of ≥4 was considered of good quality.
Data synthesis and analysis
Odds ratios with 95% confidence interval (95% CI) were calculated for all the adverse events aforementioned, using the DerSimonian and Laird method with a random-effects model. We chose the random-effects model because of the relatively small number of component studies and resultant limited validity of tests of heterogeneity. Between-study heterogeneity was calculated by using the chi-square test and quantified by the I2 statistic, with a significance threshold set at p < 0.10. The following prespecified subgroup analyses were performed to explore the source of heterogeneity: (1) types of GLP-1RAs, (2) mean age (≥60 years vs. <60 years), (3) mean percentage of females (≥50% vs. <50%), (4) methodologic quality of study (Jadad score of ≥4 vs. <4), and (5) publication year (recent 5 years vs. recent 5–10 years vs. recent 10–15 years). For primary outcome, a sensitivity analysis was performed by removing one study at each time. Funnel plots and Egger’s linear regression method were used to screen for potential disclosure bias, and the analyses mentioned above were performed with STATA 12.0 (Stata Corporation, College Station, TX, USA).
Results
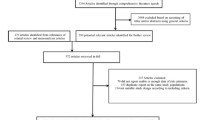
A total of 1218 citations were retrieved from Pubmed/Embase/CENTRAL/Web of Science. 890 studies remained after automatic and manual de-duplication. Of which, 266 potentially eligible studies were identified by reviewing the titles and abstracts. After retrieving the full text and searching on ClinicalTrials.gov, 37 eligible RCTs were finally identified (Supplementary Fig. 1) [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47].
The study characteristics are shown in Supplementary Table 3. In total, 64817 subjects were randomly assigned to one of the GLP-1RAs or comparators. The majority of studies (30 out of 37) provided high-quality evidences. The risk of bias was summarized as follows (Supplementary Fig. 2): 15 RCTs provided adequate descriptions of random sequence generation; 28 RCTs reported adequate allocation concealment for treatment assignment; performance bias was high in 13 trials, which were open-label in the core trials; in 34 RCTs, drop-outs were basically balanced across groups and detailed reasons were provided; methods for intention-to-treat analyses and handling missing data were adequately described in 31 RCTs; finally, RCTs were judged as unclear for selective reporting in 20 RCTs, unless cancer events were included as a safety outcome (none of these studies included cancers as outcomes of interest).
Overall cancer
The 37 studies included a safety population of 63,594 patients with T2DM, with a total exposure of 150,001 patient × years (77,888 for GLP-1RAs and 72,113 for comparators), reporting 1342 and 1223 neoplasms in GLP-1RAs and comparators as treatment-emergent serious adverse events, corresponding to a crude yearly rate of 1.72% and 1.70% in GLP-1RA and comparator groups respectively.
Out of the 37 eligible studies, one trial reported zero events of the primary outcome. In the remaining 36 trials which reported at least one case of cancer, GLP-1RAs were not associated with an increased risk of overall cancer in comparison with comparators (OR 1.03 [95% CI 0.95–1.12]; p = 0.41) (Fig. 1). The results of subgroup analysis are shown in Table 1. When the OR for overall cancer with individual GLP-1RA was analyzed, albiglutide was significantly associated with a lower risk of cancer than comparators (OR 0.76 [95% CI 0.60–0.97]; p = 0.03), whereas no significant differences between other GLP-1RAs and comparators were observed in the risk of overall cancer. The heterogeneity among studies was low (I2 range 0–34.5%). In the sensitivity analysis, the results remained consistent to the removal of each study in turn (Supplementary Fig. 3). Furthermore, no obvious bias was revealed by the Egger’s regression test (p = 0.72) and the visual inspection of the funnel plot (Supplementary Fig. 4).
Thyroid cancer
Of the 37 trials included, 22 reported zero events of thyroid cancer in both the GLP-1RA and comparator groups. In trials reporting at least one case of thyroid cancer (N = 15 trials), the overall risk of thyroid cancer was not different between GLP-1RAs and comparators (OR 1.49 [95% CI 0.83–2.66]; p = 0.18) (Fig. 2a). The Egger’s test showed no significant disclosure bias for thyroid cancer (p = 0.15).
Pancreatic cancer
Sixteen out of 37 trials reported cases of pancreatic cancer. Forty-eight patients treated with GLP-1RAs were diagnosed with pancreatic cancer, compared with 41 patients in comparator groups, yielding an OR of 1.05 [0.68–1.60] (P = 0.83) for pancreatic cancer associated with GLP-1RAs use (Fig. 2b). No obvious disclosure bias was found for the pancreatic cancer, based on the Egger’s test (p = 0.89).
Discussion
Our results, pooling data from RCTs, demonstrated no increased risks of overall, thyroid or pancreatic cancer associated with GLP-1RA therapies, and confirmed the findings of a previous meta-analysis based on a smaller number of trials [48]. Subgroup analysis indicated a protective effect of albiglutide against overall cancer risk. Absence of sufficient studies did not guarantee a comprehensive cancer risk assessment, and therefore the results needs to be interpreted with caution.
Concerns regarding the association between GLP-1RAs use and thyroid cancer, medullary thyroid cancer (MTC) in particular, rose predominantly from murine models. However, species-specific differences in several aspects limited the extrapolation of preliminary findings to humans, including GLP-1 receptor system in C cells and the predisposition to MTC [6, 49, 50]. Moreover, analyses of sequential changes of calcitonin in 5000 participants identified no consistent pattern of relationship between liraglutide and calcitonin concentration [51]. Furthermore, given the rare incidence of MTC, the number needed to treat to appreciate the tumorigenic potential, if any, would be enormous to disclose the difference [52]. Despite the uncertainty, the apparent risk is minimal. On the other hand, MTC accounted only for a small fraction of all thyroid cancers in humans. With respect to other types of thyroid cancer, neither hyperplastic nor papillary human thyroid cancer expressed GLP-1 receptors [53]. Consistently, postmarketing reports did not indicate an elevated risk of thyroid cancer associated with incretin treatment [7].
Another safety concern was a potentially higher risk of pancreatic abnormality. Alarming signals coming from postmarketing reports based on the FDA AERS database, which indicated >6-fold and 2.9-fold excess of risks of pancreatitis and pancreatic cancer, respectively, in patients using exenatide compared with users of other hypoglycemic agents, from 2004–2009 [7]. Indeed, the AERS database exhibited several limitations intrinsic to spontaneous reporting analysis, restricting its clinical implication. Being incomplete and not all independently adjudicated, a reporting bias could not be ruled out. In addition, confounders including alcohol exposure, smoking, diet and comorbidities were not comprehensively taken into consideration in that study [7]. When the time-trend axis was incorporated into the analysis of FDA AERS database from 2004–2009, a significant disproportionality signal of pancreatitis for exenatide appeared only in the first quarter of 2008, right after the FDA alert on exenatide, highlighting a remarkable influence of notoriety bias [54]. For this reason, we also performed a subgroup analysis based on the time of publication, and the results remained consistent across publication year categories.
Evidence collected from randomized trials is generally considered superior to that coming from observational studies, because the process of randomization minimizes confounding bias. The baseline characteristics between groups were highly matched in trials included in this meta-analysis. In fact, concerns also came from autopsy studies, in which increased exocrine preneoplastic lesions and a potential for evolution to neuroendocrine tumors were observed in pancreas of incretin users [55]. However, not only the baseline characteristics of incretin users and nonusers in that study were not matched (e.g., age), but the confounding of comorbidities, limited sample sizes, and no evidence of a previously tumor-free pancreata also blurred the relationship between incretin use and the alteration of pancreatic histology. Moreover, though current data remained conflicting, most cohort studies revealed that incretin treatment was not associated with increased risks of pancreatic events [56, 57].
Though no signal of overall cancer risk associated with GLP-1RA use was detected, the small number of cases prevent further subgroup analyses on other specific types of cancer events. Population-based cohort studies did not indicate a tumor promoter effect of GLP-1RAs with regard to cholangiocarcinoma, colorectal and breast cancer [58,59,60]. Prospective studies with overall tumor or specific ones as primary outcomes would be needed for a reliable assessment of certain cancer risk.
The small sample size suggests caution in the interpretation of the results of the subgroup analysis. On the other hand, while no evidence existed regarding the antitumor effect of albiglutide, preliminary studies showed a growth-inhibiting and apoptosis-promoting effect of liraglutide on human pancreatic cancer cell lines in vitro and in tumor implantation experiments [61]. However, not only the pharmacokinetics, hypoglycemic effect and immunogenicity but also the tolerability and safety of individual GLP-1RA might vary with different molecular structures [62]. Considering the potential existence of mechanism-based safety profiles but a lack of adequate studies, no decisive conclusion can be drawn until further researches concentrated on the anti-tumor activities of albiglutide provide more information.
Our study had several limitations. First, individuals enrolled in RCTs are usually healthier and with less comorbidities than T2DM patients in the general population, being at a lower risk for developing the adverse events of cancer. Moreover, selection bias could not be readily ruled out for some cancer types, in particular MTC (a family or personal history of MTC is a specific exclusion criterion in most RCTs) and pancreatic cancer (patients with a history of pancreatitis or alcohol abuse, being at a higher risk or with low adherence, are usually excluded from trials). Second, none of the studies included oncology events as primary outcomes, resulting in an unclear risk of reporting bias. However, many studies set a panel of oncological experts, who were unaware of the treatment assignments, to adjudicate any cancer event, minimizing potential bias. Third, the durations of the included trials were not long enough for cancer surveillance. Finally, compared with cohort studies, the number of cancer events in this study was relatively small, owing to the nature of RCTs. Also, given the low incidence of cancer events (as is the case of MTC or even pancreatic cancer), the number of participants lost to follow-up is considerably higher than those reporting the outcome of interests, and therefore, an attrition bias could not be easily eliminated. Further studies that provide more conclusive information about the oncological safety of incretin-based therapies in diabetic patients are warranted.
In summary, when pooling data from randomized clinical trials, the risks of overall, thyroid or pancreatic cancer were not significantly different between GLP-1RAs and comparators in T2DM patients.
References
K.K. Tsilidis, J.C. Kasimis, D.S. Lopez, E.E. Ntzani, J.P. Ioannidis, Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 350, g7607 (2015). https://doi.org/10.1136/bmj.g7607
S. O’Neill, L. O’Driscoll, Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes. Rev. 16(1), 1–12 (2015). https://doi.org/10.1111/obr.12229
X. Michelet, L. Dyck, A. Hogan, R.M. Loftus, D. Duquette, K. Wei, S. Beyaz, A. Tavakkoli, C. Foley, R. Donnelly, C. O'Farrelly, M. Raverdeau, A. Vernon, W. Pettee, D. O'Shea, B.S. Nikolajczyk, K.H.G. Mills, M.B. Brenner, D. Finlay, L. Lynch, Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 19(12), 1330–1340 (2018). https://doi.org/10.1038/s41590-018-0251-7
C.Q. Cao, Y.F. Xiang, Z.G. Zhou, The clinical application of linagliptin in Asians. Ther. Clin. Risk Manag 11, 1409–1419 (2015). https://doi.org/10.2147/TCRM.S64402
Y. Zhao, L. Yang, Y. Xiang, L. Liu, G. Huang, Z. Long, X. Li, R.D. Leslie, X. Wang, Z. Zhou, Dipeptidyl peptidase 4 inhibitor sitagliptin maintains beta-cell function in patients with recent-onset latent autoimmune diabetes in adults: one year prospective study. J. Clin. Endocrinol. Metab. 99(5), E876–E880 (2014). https://doi.org/10.1210/jc.2013-3633
L. Bjerre Knudsen, L.W. Madsen, S. Andersen, K. Almholt, A.S. de Boer, D.J. Drucker, C. Gotfredsen, F.L. Egerod, A.C. Hegelund, H. Jacobsen, S.D. Jacobsen, A.C. Moses, A.M. Molck, H.S. Nielsen, J. Nowak, H. Solberg, T.D. Thi, M. Zdravkovic, U. Moerch, Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 151(4), 1473–1486 (2010). https://doi.org/10.1210/en.2009-1272
M. Elashoff, A.V. Matveyenko, B. Gier, R. Elashoff, P.C. Butler, Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 141(1), 150–156 (2011). https://doi.org/10.1053/j.gastro.2011.02.018
D. Moher, A. Liberati, J. Tetzlaff, D.G. Altman, P. Group, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8(5), 336–341 (2010). https://doi.org/10.1016/j.ijsu.2010.02.007
D. Moher, B. Pham, A. Jones, D.J. Cook, A.R. Jadad, M. Moher, P. Tugwell, T.P. Klassen, Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 352(9128), 609–613 (1998). https://doi.org/10.1016/S0140-6736(98)01085-X
A.R. Jadad, R.A. Moore, D. Carroll, C. Jenkinson, D.J. Reynolds, D.J. Gavaghan, H.J. McQuay, Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin. Trials 17(1), 1–12 (1996)
M.C. Bunck, M. Diamant, A. Corner, B. Eliasson, J.L. Malloy, R.M. Shaginian, W. Deng, D.M. Kendall, M.R. Taskinen, U. Smith, H. Yki-Jarvinen, R.J. Heine, One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 32(5), 762–768 (2009). https://doi.org/10.2337/dc08-1797
M. Diamant, L. Van Gaal, B. Guerci, S. Stranks, J. Han, J. Malloy, M.K. Boardman, M.E. Trautmann, Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol. 2(6), 464–473 (2014). https://doi.org/10.1016/S2213-8587(14)70029-4
B. Gallwitz, J. Guzman, F. Dotta, B. Guerci, R. Simo, B.R. Basson, A. Festa, J. Kiljanski, H. Sapin, M. Trautmann, G. Schernthaner, Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet 379(9833), 2270–2278 (2012). https://doi.org/10.1016/S0140-6736(12)60479-6
R.R. Holman, M.A. Bethel, R.J. Mentz, V.P. Thompson, Y. Lokhnygina, J.B. Buse, J.C. Chan, J. Choi, S.M. Gustavson, N. Iqbal, A.P. Maggioni, S.P. Marso, P. Ohman, N.J. Pagidipati, N. Poulter, A. Ramachandran, B. Zinman, A.F. Hernandez, E.S. Group, Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377(13), 1228–1239 (2017). https://doi.org/10.1056/NEJMoa1612917
S.A. Jabbour, J.P. Frias, E. Hardy, A. Ahmed, H. Wang, P. Ohman, C. Guja, Safety and efficacy of exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy: 52-week results of the DURATION-8 randomized controlled trial. Diabetes Care 41(10), 2136–2146 (2018). https://doi.org/10.2337/dc18-0680
M. Jaiswal, C.L. Martin, M.B. Brown, B. Callaghan, J.W. Albers, E.L. Feldman, R. Pop-Busui, Effects of exenatide on measures of diabetic neuropathy in subjects with type 2 diabetes: results from an 18-month proof-of-concept open-label randomized study. J. Diabetes Complicat. 29(8), 1287–1294 (2015). https://doi.org/10.1016/j.jdiacomp.2015.07.013
M.A. Nauck, S. Duran, D. Kim, D. Johns, J. Northrup, A. Festa, R. Brodows, M. Trautmann, A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 50(2), 259–267 (2007). https://doi.org/10.1007/s00125-006-0510-2
M.J. Davies, R. Bergenstal, B. Bode, R.F. Kushner, A. Lewin, T.V. Skjoth, A.H. Andreasen, C.B. Jensen, R.A. DeFronzo, N.N.S. Group, Efficacy of liraglutide for weight loss among patients with type 2 diabetes: The SCALE diabetes randomized clinical trial. JAMA 314(7), 687–699 (2015). https://doi.org/10.1001/jama.2015.9676
A. Garber, R.R. Henry, R. Ratner, P. Hale, C.T. Chang, B. Bode, L.-S. Group, Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes. Metab. 13(4), 348–356 (2011). https://doi.org/10.1111/j.1463-1326.2010.01356.x
S.C. Gough, B.W. Bode, V.C. Woo, H.W. Rodbard, S. Linjawi, M. Zacho, P.D. Reiter, J.B. Buse, One-year efficacy and safety of a fixed combination of insulin degludec and liraglutide in patients with type 2 diabetes: results of a 26-week extension to a 26-week main trial. Diabetes Obes. Metab. 17(10), 965–973 (2015). https://doi.org/10.1111/dom.12498
K. Kaku, M.F. Rasmussen, P. Clauson, Y. Seino, Improved glycaemic control with minimal hypoglycaemia and no weight change with the once-daily human glucagon-like peptide-1 analogue liraglutide as add-on to sulphonylurea in Japanese patients with type 2 diabetes. Diabetes Obes. Metab. 12(4), 341–347 (2010). https://doi.org/10.1111/j.1463-1326.2009.01194.x
K. Kaku, A. Kiyosue, Y. Ono, T. Shiraiwa, S. Kaneko, K. Nishijima, H. Bosch-Traberg, Y. Seino, Liraglutide is effective and well tolerated in combination with an oral antidiabetic drug in Japanese patients with type 2 diabetes: a randomized, 52-week, open-label, parallel-group trial. J. Diabetes Investig. 7(1), 76–84 (2016). https://doi.org/10.1111/jdi.12367
S.P. Marso, G.H. Daniels, K. Brown-Frandsen, P. Kristensen, J.F. Mann, M.A. Nauck, S.E. Nissen, S. Pocock, N.R. Poulter, L.S. Ravn, W.M. Steinberg, M. Stockner, B. Zinman, R.M. Bergenstal, J.B. Buse; L.S. Committee, L.T. Investigators, Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375(4), 311–322 (2016). https://doi.org/10.1056/NEJMoa1603827
M. Nauck, A. Frid, K. Hermansen, A.B. Thomsen, M. During, N. Shah, T. Tankova, I. Mitha, D.R. Matthews, Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes Obes. Metab. 15(3), 204–212 (2013). https://doi.org/10.1111/dom.12012
R. Pratley, M. Nauck, T. Bailey, E. Montanya, R. Cuddihy, S. Filetti, A. Garber, A.B. Thomsen, H. Hartvig, M. Davies, L.-D.-S Group, One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J. Clin. Pract. 65(4), 397–407 (2011). https://doi.org/10.1111/j.1742-1241.2011.02656.x
Y. Seino, M.F. Rasmussen, T. Nishida, K. Kaku, Efficacy and safety of the once-daily human GLP-1 analogue, liraglutide, vs glibenclamide monotherapy in Japanese patients with type 2 diabetes. Curr. Med. Res. Opin. 26(5), 1013–1022 (2010). https://doi.org/10.1185/03007991003672551
B. Ahren, A. Leguizamo Dimas, P. Miossec, S. Saubadu, R. Aronson, Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care 36(9), 2543–2550 (2013). https://doi.org/10.2337/dc12-2006
G.B. Bolli, M. Munteanu, S. Dotsenko, E. Niemoeller, G. Boka, Y. Wu, M. Hanefeld, Efficacy and safety of lixisenatide once daily vs. placebo in people with Type 2 diabetes insufficiently controlled on metformin (GetGoal-F1). Diabet. Med. 31(2), 176–184 (2014). https://doi.org/10.1111/dme.12328
M.A. Pfeffer, B. Claggett, R. Diaz, K. Dickstein, H.C. Gerstein, L.V. Kober, F.C. Lawson, L. Ping, X. Wei, E.F. Lewis, A.P. Maggioni, J.J. McMurray, J.L. Probstfield, M.C. Riddle, S.D. Solomon, J.C. Tardif, E. Investigators, Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373(23), 2247–2257 (2015). https://doi.org/10.1056/NEJMoa1509225
M. Pinget, R. Goldenberg, E. Niemoeller, I. Muehlen-Bartmer, H. Guo, R. Aronson, Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P). Diabetes Obes. Metab. 15(11), 1000–1007 (2013). https://doi.org/10.1111/dom.12121
M.C. Riddle, R. Aronson, P. Home, M. Marre, E. Niemoeller, P. Miossec, L. Ping, J. Ye, J. Rosenstock, Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care 36(9), 2489–2496 (2013). https://doi.org/10.2337/dc12-2454
J. Rosenstock, M. Hanefeld, P. Shamanna, K.W. Min, G. Boka, P. Miossec, T. Zhou, I. Muehlen-Bartmer, R.E. Ratner, Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-S). J. Diabetes Complicat. 28(3), 386–392 (2014). https://doi.org/10.1016/j.jdiacomp.2014.01.012
B. Ahren, L. Masmiquel, H. Kumar, M. Sargin, J.D. Karsbol, S.H. Jacobsen, F. Chow, Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 5(5), 341–354 (2017). https://doi.org/10.1016/S2213-8587(17)30092-X
K. Kaku, Y. Yamada, H. Watada, A. Abiko, T. Nishida, J. Zacho, A. Kiyosue, Safety and efficacy of once-weekly semaglutide vs additional oral antidiabetic drugs in Japanese people with inadequately controlled type 2 diabetes: a randomized trial. Diabetes Obes. Metab. 20(5), 1202–1212 (2018). https://doi.org/10.1111/dom.13218
S.P. Marso, S.C. Bain, A. Consoli, F.G. Eliaschewitz, E. Jodar, L.A. Leiter, I. Lingvay, J. Rosenstock, J. Seufert, M.L. Warren, V. Woo, O. Hansen, A.G. Holst, J. Pettersson, T. Vilsboll; S.-. Investigators, Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375(19), 1834–1844 (2016). https://doi.org/10.1056/NEJMoa1607141
B. Ahren, S.L. Johnson, M. Stewart, D.T. Cirkel, F. Yang, C. Perry, M.N. Feinglos, H.S. Group, HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care 37(8), 2141–2148 (2014). https://doi.org/10.2337/dc14-0024
A.F. Hernandez, J.B. Green, S. Janmohamed, R.B. D′Agostino Sr., C.B. Granger, N.P. Jones, L.A. Leiter, A.E. Rosenberg, K.N. Sigmon, M.C. Somerville, K.M. Thorpe, J.J.V. McMurray, S. Del Prato; c. Harmony Outcomes, investigators, Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392(10157), 1519–1529 (2018). https://doi.org/10.1016/S0140-6736(18)32261-X
P.D. Home, P. Shamanna, M. Stewart, F. Yang, M. Miller, C. Perry, M.C. Carr, Efficacy and tolerability of albiglutide versus placebo or pioglitazone over 1 year in people with type 2 diabetes currently taking metformin and glimepiride: HARMONY 5. Diabetes Obes. Metab. 17(2), 179–187 (2015). https://doi.org/10.1111/dom.12414
L.A. Leiter, M.C. Carr, M. Stewart, A. Jones-Leone, R. Scott, F. Yang, Y. Handelsman, Efficacy and safety of the once-weekly GLP-1 receptor agonist albiglutide versus sitagliptin in patients with type 2 diabetes and renal impairment: a randomized phase III study. Diabetes Care 37(10), 2723–2730 (2014). https://doi.org/10.2337/dc13-2855
L.A. Leiter, J.L. Gross, F. Chow, D. Miller, S. Johnson, B. Ahren, H.S. Group, Once weekly glucagon-like peptide-1 receptor agonist albiglutide vs. prandial insulin added to basal insulin in patients with type 2 diabetes mellitus: Results over 52 weeks. J. Diabetes Complicat. 31(8), 1283–1285 (2017). https://doi.org/10.1016/j.jdiacomp.2017.05.010
M.A. Nauck, M.W. Stewart, C. Perkins, A. Jones-Leone, F. Yang, C. Perry, R.R. Reinhardt, M. Rendell, Efficacy and safety of once-weekly GLP-1 receptor agonist albiglutide (HARMONY 2): 52 week primary endpoint results from a randomised, placebo-controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetologia 59(2), 266–274 (2016). https://doi.org/10.1007/s00125-015-3795-1
P.N. Weissman, M.C. Carr, J. Ye, D.T. Cirkel, M. Stewart, C. Perry, R. Pratley, HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia 57(12), 2475–2484 (2014). https://doi.org/10.1007/s00125-014-3360-3
L. Blonde, J. Jendle, J. Gross, V. Woo, H. Jiang, J.L. Fahrbach, Z. Milicevic, Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet 385(9982), 2057–2066 (2015). https://doi.org/10.1016/S0140-6736(15)60936-9
F. Giorgino, M. Benroubi, J.H. Sun, A.G. Zimmermann, V. Pechtner, Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2). Diabetes Care 38(12), 2241–2249 (2015). https://doi.org/10.2337/dc14-1625
K.R. Tuttle, M.C. Lakshmanan, B. Rayner, R.S. Busch, A.G. Zimmermann, D.B. Woodward, F.T. Botros, Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 6(8), 605–617 (2018). https://doi.org/10.1016/S2213-8587(18)30104-9
G. Umpierrez, S. Tofe Povedano, F. Perez Manghi, L. Shurzinske, V. Pechtner, Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care 37(8), 2168–2176 (2014). https://doi.org/10.2337/dc13-2759
R.S. Weinstock, B. Guerci, G. Umpierrez, M.A. Nauck, Z. Skrivanek, Z. Milicevic, Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes. Metab. 17(9), 849–858 (2015). https://doi.org/10.1111/dom.12479
C. Alves, F. Batel-Marques, A.F. Macedo, A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin. Pract. 98(2), 271–284 (2012). https://doi.org/10.1016/j.diabres.2012.09.008
I. Martin-Lacave, R. Bernab, C. Sampedro, E. Conde, J.M. Fernandez-Santos, M.V. San Martin, A. Beato, H. Galera-Davidson, Correlation between gender and spontaneous C-cell tumors in the thyroid gland of the Wistar rat. Cell Tissue Res. 297(3), 451–457 (1999). https://doi.org/10.1007/s004410051371
B. Gier, P.C. Butler, C.K. Lai, D. Kirakossian, M.M. DeNicola, M.W. Yeh, Glucagon like peptide-1 receptor expression in the human thyroid gland. J. Clin. Endocrinol. Metab. 97(1), 121–131 (2012). https://doi.org/10.1210/jc.2011-2407
L. Hegedus, A.C. Moses, M. Zdravkovic, T. Le Thi, G.H. Daniels, GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J. Clin. Endocrinol. Metab. 96(3), 853–860 (2011). https://doi.org/10.1210/jc.2010-2318
A. Noone, N. Howlader, M. Krapcho. SEER Cancer Statistics Review, 1975–2015 (National Cancer Institute, Bethesda, MD, 2018), https://seer.cancer.gov/csr/1975_2015/. Accessed 22 Oct 2018.
B. Waser, A. Blank, E. Karamitopoulou, A. Perren, J.C. Reubi, Glucagon-like-peptide-1 receptor expression in normal and diseased human thyroid and pancreas. Mod. Pathol. 28(3), 391–402 (2015). https://doi.org/10.1038/modpathol.2014.113
E. Raschi, C. Piccinni, E. Poluzzi, G. Marchesini, F. De Ponti, The association of pancreatitis with antidiabetic drug use: gaining insight through the FDA pharmacovigilance database. Acta Diabetol. 50(4), 569–577 (2013). https://doi.org/10.1007/s00592-011-0340-7
A.E. Butler, M. Campbell-Thompson, T. Gurlo, D.W. Dawson, M. Atkinson, P.C. Butler, Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 62(7), 2595–2604 (2013). https://doi.org/10.2337/db12-1686
J.A. Romley, D.P. Goldman, M. Solomon, D. McFadden, A.L. Peters, Exenatide therapy and the risk of pancreatitis and pancreatic cancer in a privately insured population. Diabetes Technol. Ther. 14(10), 904–911 (2012). https://doi.org/10.1089/dia.2012.0075
L. Azoulay, K.B. Filion, R.W. Platt, M. Dahl, C.R. Dormuth, K.K. Clemens, M. Durand, D.N. Juurlink, L.E. Targownik, T.C. Turin, J.M. Paterson, P. Ernst; I. Canadian Network for Observational Drug Effect Studies, Incretin based drugs and the risk of pancreatic cancer: international multicentre cohort study. BMJ 352, i581 (2016). https://doi.org/10.1136/bmj.i581
D. Abrahami, A. Douros, H. Yin, O.H. Yu, J.L. Faillie, F. Montastruc, R.W. Platt, N. Bouganim, L. Azoulay, Incretin based drugs and risk of cholangiocarcinoma among patients with type 2 diabetes: population based cohort study. BMJ 363, k4880 (2018). https://doi.org/10.1136/bmj.k4880
D. Abrahami, H. Yin, O.H.Y. Yu, M.N. Pollak, L. Azoulay, Incretin-based drugs and the incidence of colorectal cancer in patients with type 2 diabetes. Epidemiology 29(2), 246–253 (2018). https://doi.org/10.1097/EDE.0000000000000793
B.M. Hicks, H. Yin, O.H. Yu, M.N. Pollak, R.W. Platt, L. Azoulay, Glucagon-like peptide-1 analogues and risk of breast cancer in women with type 2 diabetes: population based cohort study using the UK Clinical Practice Research Datalink. BMJ 355, i5340 (2016). https://doi.org/10.1136/bmj.i5340
H. Zhao, R. Wei, L. Wang, Q. Tian, M. Tao, J. Ke, Y. Liu, W. Hou, L. Zhang, J. Yang, T. Hong, Activation of glucagon-like peptide-1 receptor inhibits growth and promotes apoptosis of human pancreatic cancer cells in a cAMP-dependent manner. Am. J. Physiol. Endocrinol. Metab. 306(12), E1431–E1441 (2014). https://doi.org/10.1152/ajpendo.00017.2014
R. Gentilella, V. Pechtner, A. Corcos, A. Consoli, Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: are they all the same? Diabetes Metab. Res Rev. 35(1), e3070 (2019). https://doi.org/10.1002/dmrr.3070
Author contributions
All authors collectively planned the study. C.C. designed the study and wrote the paper. C.C. and S.Y. collected, extracted, assessed, and analyzed the data. The first draft of the paper was written by C.C., and Z.Z. revised the paper. All authors reviewed the paper for intellectual content and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Cao, C., Yang, S. & Zhou, Z. GLP-1 receptor agonists and risk of cancer in type 2 diabetes: an updated meta-analysis of randomized controlled trials. Endocrine 66, 157–165 (2019). https://doi.org/10.1007/s12020-019-02055-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02055-z