Abstract
Background and Objective
Once-weekly glucagon-like peptide-1 receptor agonists (GLP-1RAs) are a novel class of injectable antidiabetic drugs. Previous studies indicated that GLP-1RAs (exenatide and liraglutide) might increase the incidence of pancreatitis and pancreatic cancer. Here, we evaluated the clinical safety of once-weekly GLP-1RAs with respect to tumour risk.
Methods
Relevant studies were selected from ClinicalTrials.gov. Randomized controlled trials that reported the incidences of neoplasms were included in our research. Outcomes were calculated as the risk ratio using the Mantel–Haenszel method and fixed-effects model.
Results
Our analysis included 26 randomized controlled trials with 16,090 patients. Once-weekly GLP-1RAs did not increase the risk for tumours compared with other antidiabetic drugs [risk ratio (RR), 1.02; 95 % confidence interval (CI), 0.74–1.41; p = 0.91]; this finding was independent of the type of GLP-1RA administered (albiglutide, exenatide extended-release and dulaglutide) and duration of the trials (limited to ≥52 weeks). Subgroup analyses revealed that once-weekly GLP-1RAs did not increase tumour risk compared with placebos, exenatide and liraglutide, insulin or oral drugs. Additionally, once-weekly GLP-1RAs did not increase tumour risk in any tissue.
Conclusions
Compared with other antidiabetic drugs, once-weekly GLP-1RAs did not increase the risk for any tumour, and this finding was independent of the type of GLP-1RA administered and treatment duration. However, our study had many limitations, and further longer term trials with larger samples should be conducted in future to confirm our results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A total 26 randomized controlled trials with 16,090 patients and 161 tumour cases were included in our research. |
Our study showed that once-weekly GLP-1RAs did not increase tumour risk compared with other therapies. |
1 Introduction
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are a novel class of injectable antidiabetic drugs with multiple glucoregulatory effects, including enhancement of glucose-dependent insulin secretion, suppression of inappropriately elevated postprandial glucagon secretion and slowing of gastric emptying [1–3]. They have been the focus of much attention during the last years because of their unique mechanisms of action [4–6]. They are beneficial for blood glucose control; their other potential benefits include preservation of beta-cell function and improvements in other diabetes-related co-morbid conditions, such as hypertension, hyperlipidaemia and obesity [7–9]. The potentiation of insulin and glucagon release is glucose-dependent and is therefore associated with a low risk of hypoglycaemia [10–12].
Currently, several once-weekly GLP-1RAs have been approved by the US Food and Drug Administration (FDA) as adjunctive therapy for the management of type 2 diabetes [13–15]. Besides consistent glycaemic control, once-weekly GLP-1RAs offer some advantages over exenatide and liraglutide, including less frequent injections and improved treatment satisfaction. A few of studies indicate that the GLP-1RAs exenatide and liraglude might increase the occurrence of some specific cancers (e.g. pancreatic or thyroid cancer). Compared with liraglutide and exenatide, once-weekly GLP-1RAs have a longer half-life and continual action. Therefore it might be easier to obtain a definite result if we perform a study focused on once-weekly GLP-1RAs. Thus far, no study has evaluated the clinical safety of once-weekly GLP-1RAs with respect to tumour risk. Many randomized controlled trials (RCTs) have been published in PubMed, EMBASE and the Cochrane Library; however, most of these studies did not report findings related to tumour occurrence. Subsequently, we searched RCTs registered in ClinicalTrials.gov. Here, we report the findings of our informal meta-analysis on once-weekly GLP-1RAs and the risk of occurrence of tumours.
2 Methods
2.1 Search Strategy and Selection Criteria
We selected relevant studies registered in ClinicalTrials.gov up to 25 October 2015 using the following keywords: glucagon like peptide 1 receptor agonist OR exenatide OR albiglutide OR taspoglutide OR dulaglutide OR lixisenatide OR semaglutide OR CJC-1131 OR LY315902 OR CJC-1134-PC.
Two independent authors screened trials that could potentially be included in our study one by one. An RCT was considered eligible if the following criteria were met: (1) adult patients with type 2 diabetes were studied; (2) once-weekly GLP-1RAs and other treatments were compared; (3) the incidences of neoplasms (benign, malignant and unspecified) were reported as serious adverse events; (4) the duration of intervention was at least 12 weeks; and (5) there were more than 60 samples in each arm. Exclusion criteria were as follows: (1) observational and retrospective studies; (2) non-clinical studies; and (3) lack of information about the outcome that we analysed in this study.
2.2 Data Extraction
Two independent authors extracted the following data from each selected study: NCT number, study duration, trial sponsors, intention-to-treat (ITT) population, interventions and the number of individuals in the population without tumours.
2.3 Bias Assessment
Most of the RCTs included in our study were well designed and no obvious bias was founded in relevant published papers. However, all the data about tumour occurrence were extracted from ClinicalTrials.gov directly, rather than published papers, and it might be not appropriate for us to assess the risk of bias according to the information in ClinicalTrials.gov. Therefore, we performed this informal meta-analysis without assessment of risk of bias.
2.4 Statistical Analysis
We combined groups to create a single pairwise comparison when a trial contained multiple intervention groups. Outcomes were calculated as risk ratios (RRs) with 95 % confidence intervals (CIs) using the Mantel–Haenszel method and fixed-effects model. I 2 testing was performed to assess the magnitude of the heterogeneity between studies, with values greater than 50 % considered indicative of moderate-to-high heterogeneity [16]. To evaluate the influence of each study on the overall effect size, sensitivity analyses were conducted using the leave-one-out method, i.e. by removing one study at a time and then repeating the analysis. An inverse variance random-effects model was also used to further prove the robustness of the analysis results. Subgroup analyses were also performed according to study duration, tumour location and type of control groups.
We assessed funnel plot asymmetry using Egger tests and defined significant publication bias as p < 0.1. The trim-and-fill computation method was used to estimate the effect of publication bias on the interpretation of the results when publication bias was significant [17]. All these analyses were performed using RevMan 5.1 (Nordic Cochrane Centre) and Stata version 11 software (StataCorp LP, College Station, TX, USA).
3 Results
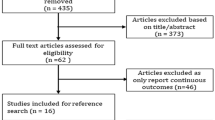
We identified 429 studies from the ClinicalTrials.gov registry, and 26 RCTs with 16,090 patients were included in our analysis [18–43]. The study selection process is shown in Fig. 1. Nine of them evaluated albiglutide [18–26], six evaluated exenatide extended-release (ER) [27–32] and 11 evaluated dulaglutide [33–43]. According to the types of control groups, eight trials compared once-weekly GLP-1RAs with a placebo [18, 20, 23, 24, 26, 28, 40, 41], seven with exenatide and liraglutide [20, 21, 27, 30, 33, 39, 42], seven with insulins [22, 25, 31, 34, 36, 37, 43] and eight with oral drugs [19, 23, 24, 28, 29, 32, 35, 38]. The mean duration of these trials was 64 weeks (range 16–156 weeks). All of these trials were supported by companies; other characteristics of these trials are shown in Table 1.
In a pooled analysis of 26 RCTs, use of once-weekly GLP-1RAs did not result in an increase in tumour risk (RR 1.02; 95 % CI 0.74–1.41; p = 0.91) (Fig. 2) when compared with the use of other therapies [18–43]. Similarly, in the subgroup analysis according to the type of once-weekly GLP-1RAs, use of albiglutide [17–26], exenatide-ER [27–32] and dulaglutide [33–43] did not increase the risk for tumours (RR 0.81; 95 % CI 0.51–1.27; p = 0.63 for albiglutide; RR 1.54; 95 % CI 0.54–4.40; p = 0.72 for exenatide-LAR; RR 1.26; 95 % CI 0.75–2.12; p = 0.83 for dulaglutide). As the duration of trials might influence the pooled results, we performed a subgroup analysis for trials with a duration of ≥52 weeks. Seven trials that evaluated albiglutide and another seven trials that evaluated dulaglutide were included in this analysis. Once-weekly GLP-1RAs did not increase tumour risk in 52 weeks (RR 0.93; 95 % CI 0.65–1.33; p = 0.81) [18, 19, 22–26, 35–39, 42, 43]. No heterogeneity (I 2 = 0 %) or publication biases were noted (p = 0.110 for total, p = 0.169 for 52 weeks, p = 0.484 for albiglutide, p = 0.635 for exenatide-ER and p = 0.538 for dulaglutide) in these analyses. All of these results were robust in the sensitivity analysis and were not affected by any single study. A funnel plot for the total analysis is shown in Fig. 3.
We also performed subgroup analyses according to the type of control groups and tumour location. Once-weekly GLP-1RAs did not increase the tumour risk compared with placebo (RR 0.72; 95 % CI 0.37–1.39; p = 0.98) [18, 20, 23, 24, 26, 28, 40, 41], exenatide and liraglutide (RR 1.73; 95 % CI 0.72–4.15; p = 0.71) [20, 21, 27, 30, 33, 39, 42], insulin (RR 1.38; 95 % CI 0.76–2.52; p = 0.76) [22, 25, 31, 34, 36, 37, 43] and oral antidiabetic drugs (RR 0.73; 95 % CI 0.44–1.22; p = 0.70) (Fig. 4) [19, 23, 24, 28, 29, 32, 35, 38]. No heterogeneity (I 2 = 0 %) or publication biases were noted (p = 0.837 for placebo; p = 0.392 for exenatide and liraglutide; p = 0.523 for insulin; p = 0.561 for oral antidiabetic drugs) in these analyses. All these results were robust in the sensitivity analysis and were not affected by any single study. The results of total and subgroup analyses using the random-effects model are shown in Supplementary Table 1.
Additionally, once-weekly GLP-1RAs did not increase the tumour risk in any tissue (Table 2). A total of five cases of pancreatic cancer were reported: two cases of metastatic pancreatic carcinoma, one treated with albiglutide [18] and the other with liraglutide [39]; one case of pancreatic carcinoma treated with dulaglutide [42]; one case of adenocarcinoma pancreas treated with oral drugs [23]; and one case of benign pancreatic neoplasm treated with albiglutide [19]. No heterogeneity (I 2 = 0) was noted in these analyses. All of these results were robust and were not affected by any single study. Significant publication biases existed only in the analyses of breast and lung tumours (p = 0.07 and p = 0.06, respectively). However, no trimming was performed in the ‘trim and fill’ analysis and the data remained unchanged, which suggests that publication bias might not affect these results significantly.
4 Discussion
Although GLP-1RAs are a novel class of antihyperglycaemic agents, their safety with respect to tumour risk has attracted a high level of concern in the past 5 years. In 2011, the FDA examined the adverse events database of studies that investigated these treatments [44, 45]. This report indicated that GLP-1RAs increase the risk for pancreatitis and raised caution about the potential long-term actions of these drugs in promoting pancreatic cancer. However, the FDA report had many issues. The FDA Adverse Event Reporting System database does not provide information regarding obesity, smoking habits, alcohol consumption or chronic pancreatitis, which are well-established additional risk factors for pancreatic cancer. At the same time, many fundamental studies had been performed to evaluate the safety of GLP-1RAs with respect to the risk of pancreatic, thyroid, prostate, colon and breast cancer [46–51]. However, there was neither firm evidence in favour of this hypothesis nor evidence strong enough to rule out the possibility of increased risk based on the results available at present.
Our systematic review also has many limitations. First, because the description of neoplasms in some trials was not sufficiently detailed and we included all types of tumours, namely benign, malignant and unspecified neoplasms. Second, it was unclear whether the neoplasms were present before treatment and we could not determine if there was a link between the neoplasms and treatment. Third, the duration of these randomized controlled trials was short for evaluating cancer risk, especially that of trials that evaluated exenatide-ER, with most being conducted for about 6 months. Besides, even though a total of 16,090 patients were included in our analysis, this sample size is still insufficient to evaluate the risk of site-specific cancer (e.g. pancreas or thyroid). Finally, only 149 tumour cases were reported in both the GLP-1RAs and control group and the small number of tumour cases decreased the reliability of our final conclusion.
To our knowledge, this is the first article that systematically evaluated the clinical safety of once-weekly GLP-1RAs with respect to tumour risk in type 2 diabetes patients. Our study showed that once-weekly GLP-1RAs did not increase tumour risk compared with other treatments and this result was independent of the type of once-weekly GLP-1RA administered and the treatment duration. Subgroup analyses performed according to the tumour tissue and types of control groups also revealed similar results.
5 Conclusion
We can conclude that compared with other treatments, once-weekly GLP-1RAs do not increase the risk of tumour occurrence and this is independent of the type of GLP-1RAs and treatment duration. However, there were limitations to our study, and further larger sample, long-term clinical trials should be conducted in future to confirm our results.
References
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57.
Kolterman OG, Kim DD, Shen L, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm. 2005;62:173–81.
Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept. 2004;117:77–88.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39.
Holst JJ, Deacon CF, Vilsboll T, et al. Glucagon-like peptide-1, glucose homeostasis and diabetes. Trends Mol Med. 2008;14:161–8.
Nauck MA, Vilsboll T, Gallwitz B, et al. Incretin-based therapies: viewpoints on the way to consensus. Diabetes Care. 2009;32(Suppl 2):S223–31.
Drucker DJ, Buse JB, Taylor Kendall DM, et al. Duration-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, openlabel, non-inferiority study. Lancet. 2008;372(9645):1240–50. doi:10.1016/S0140-6736(08)61206-4.
Buse JB, Rosenstock J, Sesti G, et al. LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomized, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39–47. doi:10.1016/S0140-6736(09)60659-0.
Liu FP, Dong JJ, Yang Q, et al. Glucagon-like peptide 1 receptor agonist therapy is more efficacious than insulin glargine for poorly controlled type 2 diabetes: a systematic review and meta-analysis. J Diabetes. 2015;7:322–8.
Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273(5 Pt 1):E981–8.
Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–4.
Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, an insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239–46.
Bydureon package insert. http://www.azpicentral.com/bydureon/pi_bydureon.pdf#page=1. Accessed 23 Apr 2015.
Tanzeum package insert. https://gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Tanzeum/pdf/TANZEUM-PI-MG-IFU-COMBINED.PDF. Accessed 3 May 2015.
Trulicity package insert. http://pi.lilly.com/us/trulicity-uspi.pdf. Accessed 3 May 2015.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
GlaxoSmithKline. Safety and efficacy study of albiglutide in type 2 diabetes. https://clinicaltrials.gov/ct2/show/NCT00849017?term=NCT00849017&rank=1. Accessed 25 Oct 2015.
GlaxoSmithKline. A study of the efficacy and safety of albiglutide in subjects with type 2 diabetes with renal impairment. https://clinicaltrials.gov/ct2/show/NCT01098539?term=01098539&rank=1. Accessed 25 Oct 2015.
GlaxoSmithKline. A monotherapy study to evaluate the efficacy and safety of 2 dose levels of albiglutide in Japanese subjects with type 2 diabetes mellitus. https://clinicaltrials.gov/ct2/show/NCT01733758?term=01733758&rank=1. Accessed 25 Oct 2015.
GlaxoSmithKline. A study to determine the efficacy and safety of albiglutide as compared with liraglutide. https://clinicaltrials.gov/ct2/show/NCT01128894?term=01128894&rank=1. Accessed 25 Oct 2015.
GlaxoSmithKline. A study to determine the safety and efficacy of albiglutide in patients with type 2 diabetes. https://clinicaltrials.gov/ct2/show/NCT00838916?term=00838916&rank=1. Accessed 25 Oct 2015.
GlaxoSmithKline. A study to determine the safety and efficacy of albiglutide in subjects with type 2 diabetes. https://clinicaltrials.gov/ct2/show/NCT00839527?term=00839527&rank=1. Accessed 25 Oct 2015.
GlaxoSmithKline. Efficacy and safety of albiglutide in treatment of type 2 diabetes. https://clinicaltrials.gov/ct2/show/NCT00838903?term=00838903&rank=1. Accessed 25 Oct 2015.
GlaxoSmithKline. A study to determine the safety and efficacy of albiglutide administered in combination with insulin glargine. https://clinicaltrials.gov/ct2/show/NCT00976391?term=00976391&rank=1. Accessed 25 Oct 2015.
GlaxoSmithKline. Safety and efficacy of albiglutide in type 2 diabetes. https://clinicaltrials.gov/ct2/show/NCT00849056?term=00849056&rank=1. Accessed 25 Oct 2015.
AstraZeneca. Effects of exenatide long-acting release on glucose control and safety in subjects with type 2 diabetes mellitus (DURATION-1). https://clinicaltrials.gov/ct2/show/NCT00308139?term=00308139&rank=1. Accessed 25 Oct 2015.
AstraZeneca. Comparison study of the glycemic effects, safety, and tolerability of exenatide once weekly suspension to sitagliptin and placebo in subjects with type 2 diabetes mellitus (DURATION-NEO-2). https://clinicaltrials.gov/ct2/show/NCT01652729?term=NCT01652729&rank=1. Accessed 25 Oct 2015.
AstraZeneca. Safety and efficacy of exenatide once weekly injection versus metformin, dipeptidyl peptidase-4 inhibitor, or thiazolidinedione as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4). https://clinicaltrials.gov/ct2/show/NCT00676338?term=00676338&rank=1. Accessed 25 Oct 2015.
AstraZeneca. Safety and efficacy of exenatide once weekly versus liraglutide in subjects with type 2 diabetes. https://clinicaltrials.gov/ct2/show/NCT01029886?term=01029886&rank=1. Accessed 25 Oct 2015.
AstraZeneca. Efficacy of once-weekly exenatide versus once or twice daily insulin detemir in patients with type 2 diabetes. https://clinicaltrials.gov/ct2/show/NCT01003184?term=01003184&rank=1. Accessed 25 Oct 2015.
AstraZeneca. A study to compare the glycemic effects, safety, and tolerability of exenatide once weekly to those of sitagliptin and pioglitazone,in subjects with type 2 diabetes treated with metformin (DURATION-2). https://clinicaltrials.gov/ct2/show/NCT00637273?term=00637273&rank=1. Accessed 25 Oct 2015.
Eli Lilly and Company. A study comparing the effect of dulaglutide with liraglutide in type 2 diabetes (AWARD-6). https://clinicaltrials.gov/ct2/show/NCT01624259?term=01624259&rank=1. Accessed 25 Oct 2015.
Eli Lilly and Company. A study of dulaglutide in Japanese participants with type 2 diabetes mellitus. https://clinicaltrials.gov/ct2/show/NCT01584232?term=01584232&rank=1. Accessed 25 Oct 2015.
Eli Lilly and Company. A study of LY2189265 compared to sitagliptin in participants with type 2 diabetes mellitus on metformin. https://clinicaltrials.gov/ct2/show/NCT00734474?term=00734474&rank=1. Accessed 25 Oct 2015.
Eli Lilly and Company. A study in participants with type 2 diabetes mellitus (AWARD-2). https://clinicaltrials.gov/ct2/show/NCT01075282?term=01075282&rank=1. Accessed 25 Oct 2015.
Eli Lilly and Company. A study in participants with type 2 diabetes mellitus (AWARD-4). https://clinicaltrials.gov/ct2/show/NCT01191268?term=01191268&rank=1. Accessed 25 Oct 2015.
Eli Lilly and Company. A study in participants with type 2 diabetes mellitus (AWARD-3). https://clinicaltrials.gov/ct2/show/NCT01126580?term=01126580&rank=1. Accessed 25 Oct 2015.
Eli Lilly and Company. A study of LY2189265 in Japanese participants with type 2 diabetes mellitus. https://clinicaltrials.gov/ct2/show/NCT01558271?term=01558271&rank=1. Accessed 25 Oct 2015.
Eli Lilly and Company. A study of the effect of LY2189265 on blood pressure and heart rate in type 2 diabetes. https://clinicaltrials.gov/ct2/show/NCT01149421?term=01149421&rank=1. Accessed 25 Oct 2015.
Eli Lilly and Company. A study of dose titration of LY2189265 in overweight participants with type 2 diabetes mellitus (EGO). https://clinicaltrials.gov/ct2/show/NCT00630825?term=00630825&rank=1. Accessed 25 Oct 2015.
Eli Lilly and Company. A study in participants with type 2 diabetes mellitus (AWARD-1). https://clinicaltrials.gov/ct2/show/NCT01064687?term=01064687&rank=1. Accessed 25 Oct 2015.
Eli Lilly and Company. A study comparing the effects and safety of dulaglutide with insulin glargine in type 2 diabetes mellitus. https://clinicaltrials.gov/ct2/show/NCT01648582?term=NCT01648582&rank=1. Accessed 25 Oct 2015.
U.S. Department of Food and Drug Administration. Adverse Event Reporting System [Internet], 2011. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/default.htm. Accessed 31 Mar 2014.
Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150–6.
Butler PC, Matveyenko AV, Dry S, et al. Glucagon-like peptide-1 therapy and the exocrine pancreas: innocent bystander or friendly fire? Diabetologia. 2010;53:1–6.
Gier B, Matveyenko AV, Kirakossian D, et al. Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the Kras(G12D)mouse model. Diabetes. 2012;61:1250–62.
Bjerre Knudsen L, Madsen LW, et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473–86.
Nomiyama T, Kawanami T, Irie S, et al. Exendin-4, a GLP-1 receptor agonist, attenuates prostate cancer growth. Diabetes. 2014;63(11):3891–905.
Koehler JA, Kain T, Drucker DJ. Glucagon-like peptide-1 receptor activation inhibits growth and augments apoptosis in murine CT26 colon cancer cells. Endocrinology. 2011;152(9):3362–72.
Ligumsky H, Wolf I, Israeli S, et al. The peptide-hormone glucagon-like peptide-1 activates cAMP and inhibits growth of breast cancer cells. Breast Cancer Res Treat. 2012;132:449–61.
Acknowledgments
Fupeng Liu would like to acknowledge the support from The China Scholarship Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study received no external funding.
Conflict of interest
XG, QY, JD, LL, WZ and FL have no financial conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, X., Yang, Q., Dong, J. et al. Tumour Risk with Once-Weekly Glucagon-Like Peptide-1 Receptor Agonists in Type 2 Diabetes Mellitus Patients: A Systematic Review. Clin Drug Investig 36, 433–441 (2016). https://doi.org/10.1007/s40261-016-0389-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0389-8