Abstract
Background
Surgical procedures are associated with activation of the hypothalamic-pituitary-adrenal axis (HPA). Studies examining HPA dynamics peri-operatively are limited and the modulating influence of peri-operatively administered glucocorticoids on that is not well established. This investigation examined alterations in HPA function and the impact of dexamethasone (DEX) administration during the peri-operative period.
Methods
We examined HPA function in 297 patients with normal function who had surgical procedures including pituitary mass resection (n = 191), craniotomy (n = 17) and other thoracic/ abdominal/ pelvic surgeries (n = 89). HPA function was assessed by frequent measurements of parameters defining adrenal function: ACTH, cortisol, DHEA and DHEA-S levels for 48 h. DEX was administered as a single dose (2–10 mg) to 30 and as multiple doses (12–36 mg) to 21 patients. The data of DEX-treated subjects within each group were similar and were combined together.
Results
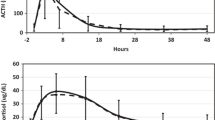
Pre-operative data were similar for patients having different surgical procedures. Without DEX exposure, ACTH increased to 225 ± 100 ng/L at 2–4 h and gradually declined to baseline values by 36 h while cortisol levels peaked (39.2 ± 13.2 ug/dL) at 6–8 h declining gradually thereafter. Cortisol rise was paralleled by an equimolar increase in DHEA and a subsequent increase in DHEA-S levels. Single doses of DEX did not influence ACTH or cortisol secretion but suppressed the expected rise in DHEA and DHEA-S levels. Multiple doses of DEX suppressed ACTH and cortisol after the 15th postoperative hour and completely blocked the expected rise in DHEA and DHEA-S levels.
Conclusions
The data provide a detailed overview of HPA function in a large number of subjects who had major surgical procedures. Single and large doses of DEX did not suppress ACTH or cortisol secretion but suppressed adrenal androgen secretion. It took multiple doses of DEX to partially suppress ACTH and cortisol secretion in the peri-operative period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Activation of the hypothalamic pituitary adrenal axis is one of several important responses to stressful events and critical illnesses [1]. Numerous studies have documented activation of the hypothalamic-pituitary-adrenal (HPA) axis during acute and chronic stressful events such as in patients undergoing surgery [2,3,4,5,6,7,8,9,10,11,12], those with sepsis [12, 13], trauma [13], burns [14] and others with different critical illnesses [12]. The need for glucocorticoids during any stressful event such as a surgical procedure is due, in part, to the known effects of these steroids on several components of the host response to the stress of surgery. In this respect, glucocorticoids help support and stimulate cardiovascular response to stress. Additionally, glucocorticoids support many of the components of the inflammatory response to tissue injury occurring during surgery.
Several studies investigated the HPA axis during and after minor as well as major surgical procedures. Published data on adrenal function and activity were reported in patients with normal HPA function who had various surgical procedures [3] including abdominal surgery [7, 9], open heart, coronary artery bypass graftDEX surgery [11, 15], and pituitary adenomectomy [6, 16]. Most of these studies included data on serum cortisol levels that were infrequently measured at various times after surgery. There are only a few studies that included small number of patients where repeated measurements of hormones related to HPA function were performed after surgery [4,5,6, 10, 11, 16].
It is not uncommon for patients undergoing any major surgical procedure to receive exogenous glucocorticoids during the perioperative period. In some instances, anesthesiologists administer dexamethasone (DEX) to manage bronchospasm or to prevent or minimize nausea. It is also common for patients who are having craniotomy for resection of brain lesions to receive large doses of DEX during and after surgery to prevent or minimize the chance for brain edema. Since the doses of DEX administered in such instances are often large enough to have a suppressive effect on hypothalamic pituitary adrenal function, we questioned whether this would result in any impairment in function that could potentially put such patients at risk during the peri-operative period. Preliminary data in five patients who had craniotomy for resection of meningiomas showed that dexamethasone administration did not suppress plasma ACTH or serum cortisol levels [12].
The primary goal of the current investigation was to characterize the details of the HPA response to various surgical procedures. In conducting the study, we recognized that some patients enrolled in the study could, at the discretion of their anesthesiologist/ surgeon receive dexamethasone for a variety of reasons. That provided us with the unique opportunity to examine the impact of DEX administration before, during and/ or after surgical procedure in a large number of patients who have normal HPA function and who had a variety of surgical procedures. In addition to determining the impact of DEX administration on adrenocorticotropic hormone (ACTH) and cortisol secretion we also examined the acute effects of this treatment on other markers of ACTH function; namely adrenal androgen secretion. We postulated that activation of the HPA axis as a consequence of the stress of the peri-operative period is quite strong that it can’t be suppressed by the concomitant administration of large single doses of DEX. We also postulated that in that setting, the HPA function can be suppressed with repeated DEX administration.
Patients and methods
This is a prospective observational study conducted over an 8-year period [2008–2016] that is aimed at assessing HPA function in the peri-operative period. The study was approved by the Institutional Research Board and informed consent was obtained from participants.
Patients enrolled in the study included 191 consecutive adults with pituitary masses of >1 cm (adenomas or Rathke’s Cleft Cyst), who had transsphenoidal surgery,17 who had craniotomies for resection of meningiomas and 89 who had general major surgical procedures. The latter included hip or knee replacement (n = 27), abdominal/ pelvic surgery (n = 28) and thoracic / vascular procedures (n = 34). Patients who presented with pituitary tumor apoplexy syndrome were excluded. Patients with pituitary adenomas and those who had a craniotomy for removal of a meningiomas were all documented to have normal pituitary adrenal function as described earlier [17,18,19]. The surgical procedure in all patients was performed under general anesthesia. None of the patients received etomidate, a known inhibitor of 11-β hydroxylase, before intubation. Peri-operatively all patients received the following medications: lidocaine, midazolam, ondansetron, rocuranium, and propofol and in addition some received fentanyl. Some patients also received IV antibiotics before or during the surgical procedure. The primary IV fluid used throughout all procedures was lactated Ringers solution. The clinical characteristics of all groups of patients are shown in Table 1.
While patients with pituitary masses and meningiomas had thorough endocrine evaluation prior to surgery, the remainder of the patients had baseline studies just before surgery if they were deemed eligible for the study and after consenting to participate. None of the patients included in the study were known to have adrenal dysfunction nor were they on any form of glucocorticoids during the preceding 6 months. Exclusion criteria included intake of any medication that is known to influence the function or the measurements of any of the components of the HPA axis [20].
The managing team independently made the decision to administer DEX to 51 of the study group. The research team had no role in the decision to administer DEX to the 51 patients and became aware of it after the drug was administered. In all subjects who received dexamethasone, the drug was administered either an hour before induction of anesthesia, intra-operatively or within an hour of extubation. Different doses of DEX were administered, ranging from 2 to 36 mg during the first 24 peri-operative hours. Of the 51 patients who received DEX, 30 received single doses of 2–10 mg IV while the remaining 21 received multiple doses ≥ 10 mg during the first 24 perioperative hours (Table 1).The rationale provided by the managing team for administering DEX varied from its anti-emetic effects to bronchospasm during intubation to management of inflammatory diseases such as bronchospasm, inflammatory bowel disease, and prevention of brain edema after craniotomy.
Standard peri-operative management protocol
Pre and peri-operative clinical and biochemical data were similarly collected and analyzed in all patients. In this study, we followed the same protocol in all patients irrespective of the nature of the surgical procedure. The details of the protocol followed have been published and are presented briefly here.
Clinical monitoring
Pituitary surgery/craniotomy
Following pituitary surgery, patients are monitored carefully by the endocrine /pituitary service, the neurosurgical team along with highly specialized experienced nurses either in the neurological ICU or a step-down unit. A similar protocol is followed in patients who had craniotomies. Monitoring parameters in these patients include but are not limited to cardiovascular/ respiratory evaluation along with assessment of headaches intensity, need for pain medications, mental status, neurological and/ or visual symptoms, fluid balance, and urine flow rate. Various degrees of headaches are expected in the majority of patients and most require pain control during the first 24 h.
Other surgical procedures
Similarly, patients who had other surgical procedures were monitored as clinically indicated for their respective procedure and in addition had biochemical assessment that was identical to the protocol followed for patients with pituitary surgery.
Biochemical monitoring
Biochemical monitoring is carried out in parallel with that of clinical assessment described above. The protocol stipulates that glucocorticoids are not administered before and during surgery to patients who had normal HPA function as recently defined [17]. Instead, patients were frequently evaluated clinically and biochemically for evidence for adrenal insufficiency [17,18,19]. Blood samples for the determination of plasma ACTH, serum cortisol, dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEA-S) levels were drawn before surgery and at 2–4, 6–8, and every 6 h thereafter for 48 h after surgery. Blood samples were drawn within 1–2 h of the specified time window. These time-intervals were used based on earlier studies where hourly samples were obtained in the peri-operative period demonstrating the peak rise in ACTH to be at 2–3 h while that of cortisol was at 6–8 h [6]. Similar peak responses were reported by other investigators [8, 10, 11].
A serum cortisol of ≥15 ug/dL on multiple occasions during the first 36 post-operative hours is considered a normal post-operative HPA response [16, 18,19,20]. Serum cortisol levels are usually available within 2–3 h which would allow the treating physician to make a decision as to whether glucocorticoids should be administered. The protocol stipulates that glucocorticoids should be administered when the serum cortisol is < 10 ug/dL on more than one occasion during the first 24 post-operative hours and/ or when clinical suspicion for adrenal insufficiency is considered likely. The latter patients are excluded from the analysis. In addition to the above routine measurements we examined changes in patients’ electrolytes at least twice daily for the first 2 post-operative days.
In a subset of patients who did not receive dexamethasone (n = 11), we examined changes in plasma ACTH, cortisol, and adrenal androgens every 20 min for up to 2 h during the surgical procedure.
Laboratory assays
Plasma ACTH concentrations were measured using solid-phase two-site sequential chemiluminescent immuno-metric assay (Immulite 2000 XPi) supplied by Siemen (Malvern, PA). The lower limit of detectability in the assay was 5 ng/L while the intra- and inter-assay coefficients of variation determined at different ranges in the assays varied from 3.1 and 9%. The assay utilized two specific antibodies that detect the entire ACTH molecule. Testing by the manufacturer indicated that there was no cross reactivity in this assay system with the ACTH fragment molecules or with ACTH molecule precursors such as α MSH and pro-opiomelanocortin. The normal AM plasma ACTH in this assay was 8–46 ng/L. Measurements of serum total cortisol were performed using a chemiluminescence immunoassay technique using Advia Centaur XP instrument (Siemen, PA). The lower limit of detectability was 0.2 µg/dl, and the inter-assay and the intra-assay coefficients of variation determined at different ranges of concentrations varied from 4.4 to 8.2% and from 2.3 to 4.7%, respectively. Serum DHEA levels were determined by HPLC-tandom mass spectroscopy method developed by Quest Diagnostics Nichols Institute (San Juan Capistrano, CA). The inter and intra assay CV determined at concentrations relevant to our study population (2–10 ng/ml) were 3.3 and 7.1 %, respectively. Serum DHEA-S levels were measured utilizing a solid phase chemiluminescent enzyme-labeled immunoassay using Immulite 2000XPi (Siemen, PA). The lower level of detectability for the assay is 15 ug/dL and the intra and inter-assay coefficient of variation determined at various concentrations ranged from 4.8 to 7.4% and 7.9 to 8.5%, respectively.
Statistical analysis
Data are presented as mean ± standard deviation, unless stated otherwise. The perioperative data on patients who had different surgical procedures were first analyzed and compared separately to each other. That is, we evaluated baseline data and the HPA responses to surgery for each of the different surgical groups. We then compared within each group, patients who received DEX VS those who did not. Initial analysis of the data showed that the impact of single doses of DEX were somewhat different from that of multiple doses. We therefore analyzed those separately. Therefore, the data on patients who received single doses of DEX (n = 30) were compared to that obtained in subjects who had multiple doses of the drug (n = 21) as well as that of others who were not exposed to any glucocorticoids during the peri-operative period (n = 246). Data were first analyzed using the Kruskal–Wallis test, as a non-parametric alternative to analysis of variance test and then comparisons between groups were done using the Wilcoxon Rank Sum test for non-parametric measurements. Categorical data were compared using Chi square and Fisher exact tests. Differences were considered significant when the two-sided P-values were less than 0.05. We used the Bonferroni’s correction factor when comparing multiple groups. All data analysis was made using the SPSS program.
Results
Pre-and intra-operative HPA function
Data on the preoperative HPA function in the three groups of patients who had different surgical procedures are illustrated in Table 1. As shown in the table, the three groups of patients had almost identical baseline HPA function as demonstrated by the similarities in plasma ACTH as well as the serum concentrations of cortisol, DHEA, and DHEA-S. Likewise, baseline levels of ACTH, cortisol, DHEA, and DHEA-S were similar in patients who had different general surgical procedures: knee/hip vs. abdomen/ pelvis vs. thoracic/vascular surgeries. Intraoperative data on the 11 patients who did not receive DEX showed minimal and statistically insignificant rise in plasma ACTH, serum cortisol, and DHEA or DHEA-S levels for up to 2 h of measurements (data not shown).
Peri-operative ACTH and cortisol levels
Patients without DEX exposure
The post-operative data on all patients in patients who had different surgical procedures are shown in Table 2. As shown in the Table, plasma ACTH and serum cortisol levels were almost identical for patients who had different surgical procedures up to the 48th postoperative hour. The data on these patients after the 12th–15th postoeptive hour are not shown in the Table to minimize redundancy. We therefore combined the data on all patients as illustrated in Figs. 1 and 2. Plasma ACTH increased to very high levels at 2–4 h after surgery (225 ± 100 ng/L) and declined gradually thereafter reaching baseline values at the 36th post-operative hour. Serum cortisol increased promptly reaching a mean peak level of 39.2 ± 13.2 ug/dL at 6–8 h declining gradually thereafter so that it is still slightly but significantly (P = 0.02) higher at the 48th postoperative hour than the pre-operative level. Also observed was a rise in serum DHEA levels that paralleled the increase in serum cortisol and occurred 3–4 h after the peak increase in plasma ACTH. That is, the peak rise in serum DHEA occurred at the same time the maximal cortisol response was noted (Figs. 1 and 2). The rise in serum DHEA was equimolar to the increase in serum cortisol such that the molar ratio of cortisol/ DHEA remain unchanged throughout the 48 postoperative hours although a minimal and statistically insignificant increase in the molar ratios was noted after the 24th postoperative hour. Postoperatively, serum DHEA-S increased reaching a peak at nearly 18 h after surgery (P < 0.001) following which the levels declined to baseline values by the 48th post-operative hour (Fig. 2).
Repeated measurements of plasma ACTH (Top panel) and serum cortisol levels (lower panel) during the 48 postoperative hours obtained in patients with normal HPA function (solid continuous line) who did not receive any peri-operative glucocorticoids, those who received a single dose (2–10 mg) of dexamethasone (DEX; solid interrupted line) and others who received multiple doses of DEX (dotted line). Measurements were obtained before, and immediately after surgery for 48 h. Patients who had received a single dose of DEX had ACTH and cortisol levels that were almost identical to those who did not receive any glucocorticoids (P values > 0.1 at all time-points. Patients who received multiple doses of DEX had similar ACTH and cortisol levels to those in the other two groups before surgery and at 2–4 and 6–8 h. However, at and beyond the 18th postoperative hour, plasma ACTH levels decreased (P < 0.01 as compared to the other two groups). With multiple doses of DEX, serum cortisol levels were lower (P < 0.01) at the 24th postoperative hour and beyond (P < 0.001)
Repeated measurements of serum androgen levels (DHEA and DHEA-S during the 48 postoperative hours obtained in patients with normal HPA function (solid continuous line) who did not receive any peri-operative glucocorticoids, those who received a single dose (2–10 mg) of dexamethasone (DEX; solid interrupted line) and others who received multiple doses of DEX (dotted line). Measurements were obtained before, and immediately after surgery for 48 h. Patients who had received a single dose of DEX had minimal and statistically insignificant peak rise in serum DHEA (P = 0.062) and DHEA-S levels (P = 0.08) despite having a similar rise in plasma ACTH as shown in Fig. 1. Serum DHEA levels in patients who received single doses of DEX declined further after the 24th postoperative hour becoming even lower (<0.01) than the patients’ own baseline levels (Fig. 2). Similar findings were observed in serum DHEA-S levels (Fig. 2). That is, there was no increase in serum DHEA-S levels after surgery and the actual levels at 24 h and beyond were lower (P < 0.01–<0.001) than pre-operative levels Instead of the expected rise in serum DHEA and DHEA-S, patients who received multiple doses of DEX had a decrease in serum DHEA and DHEA-S levels noted at the 24th postoperative hours and beyond (P < 0.01 for both)
Dexamethasone administration
A total of 51/ 297 patient received DEX during the peri-operative period (Table 1). While 30 of the latter 51 patients received single doses of 2–10 (8.1 ± 3.1; median: 10 mg) the remaining 21 received multiple doses during the first 24 post-operative hours totaling 12–36 (22 ± 5) mg of DEX. Patients who received multiple doses of DEX were on such therapy for at least 36 of the 48 post-operative hours where HPA assessments were determined. There were no differences in the pre-operative (baseline) levels of ACTH, cortisol, DHEA, and DHEA-S between patients who received DEX and those who did not. This was true of patients with pituitary masses as well as those who had other surgical procedures. Thus, data on all patients were categorized on the basis of DEX administration.
Influence of single doses of DEX on HPA function
A total of 21 of the 30 patients who received only single doses of DEX peri-operatively had pituitary surgery while 8/30 had other general surgical procedures and only 1/30 had a craniotomy. DEX effects on ACTH, cortisol, DHEA, and DHEA-S in patients who had pituitary surgery were similar and almost identical to those who had other types of surgery. Since both groups had similar baseline data as well as identical responses to DEX we combined their data to illustrate that effect.
Despite their relatively large amounts (2–10 mg) single doses of DEX administered in the peri-operative period had no effects on measured ACTH and cortisol levels as their values promptly increased and were similar to those observed in patients who did not receive any exogenous glucocorticoids throughout the first post-operative 48 h (Fig. 1). Even with the administration of single doses of DEX, the peak rise in serum DHEA levels at the 6th to 8th post-operative hours (Fig. 2) was blunted (P = 0.062 as compared to baseline values) despite the fact that these patients had increases in plasma ACTH that were quantitatively similar (Fig. 1) to those who did not receive glucocorticoids. Serum DHEA levels in patients who received single doses of DEX declined further after the 24th postoperative hour becoming even lower (<0.01) than the patients’ own baseline levels (Fig. 2). Similar findings were observed in serum DHEA-S levels (Fig. 2). That is, there was no increase in serum DHEA-S levels after surgery and the actual levels at 24 h and beyond were lower (P < 0.01–<0.001) than pre-operative levels (Fig. 2).
Influence of multiple doses of DEX on HPA function
Plasma ACTH levels were similar to those observed in subjects who had no DEX exposure or others who received a single dose of the drug until the 18th post-operative hour when the level was significantly (P < 0.01)lower and remained lower (P < 0.001) throughout the remainder of the post-operative period. Serum cortisol levels were also similar in all patients until the 24th post-operative hour when it was noted to be significantly lower (P < 0.001) in patients who received multiple doses of DEX as compared to others who received either a single dose or none at all (Fig. 1). Serum cortisol levels decreased further (P < 0.001) after the 24th postoperative hour. Multiple doses of DEX completely inhibited the expected rise in serum DHEA and DHEA-S levels (Fig. 2). In addition, serum levels of DHEA and DHEA-S were lower (P < 0.001) than the respective baseline values after the 24th post-operative hour. It was difficult to assess potential differences in the effects of multiple doses of DEX among various surgical groups as the majority of such patients had craniotomies and only three had general surgical procedures.
Follow up studies
HPA function tests were repeated in all patients who had pituitary surgery as well as in 13/17 patients who had meningiomas at 6–12 weeks after surgery. All had normal HPA function as determined by the absence of any related symptoms, a normal plasma ACTH, a normal serum DHEAS and cortisol (≥12 ug/dL) and /or an appropriate response to 1 ug of Cosyntropin as described earlier [12,13,14]. None of the patients who had other surgical procedures had symptoms related to adrenal dysfunction during the remainder of their hospitalization and therefore repeat testing of HPA function after discharge was not conducted.
Discussion
The current investigation conducted in a large number of patients with normal adrenal function and who had various surgical procedures characterized the hormonal pattern of HPA activation after major surgical procedures. The data demonstrate that the stress of surgery was associated with a robust stimulation of ACTH secretion that was followed by the synchronous and parallel increase in two ACTH-dependent steroids; namely cortisol and DHEA with a subsequent rise of the latter’s sulfated conjugate (DHEA-S). The peak rise in plasma ACTH was noted 2–4 h after extubation while the maximal increase in serum cortisol and DHEA levels was observed at 6–8 h. These findings are consistent with data reported by other investigators in patients undergoing other surgical procedures [4, 8, 10, 11]. The increase in serum DHEA-S levels was noted a few hour later where it peaked at approximately 15 to 18 h. Although we examined the intraoperative changes in only a small number of patients, we did not observe any significant changes in any of these parameters during the surgical procedure. This confirms previous observations by other investigators where no major alterations in HPA function were detected intra-operatively [5]. The study is unique in that it included subjects who had different types of major surgical procedures and in that it demonstrated similar if not identical pattern of HPA activation peri-operatively in such patients. Thus, the stressful events after extubation [4, 5] rather than the anesthetics or the type of surgery contributed to HPA activation peri-operatively. It is important to point out that the robust HPA activation peri-operatively was observed despite the use of medications such as narcotics that are known to blunt HPA function [21].
It was quite interesting to note that similar HPA responses to different surgical procedures were reported by other investigators [2,3,4,5,6,7,8,9,10,11]. Only few of these studies included detailed and frequent measurements of HPA function parameters during the peri-operative period [4, 5, 10, 11, 16, 19]. Our data are consistent with an earlier study by Udelsman and colleagues that was conducted in 11 patients who had thyroid or parathyroid surgeries [4] which demonstrated that the maximal rise in ACTH was noted at 2–3 h. A study by Hall et al [10] conducted in patients who had knee or hip arthroplasty showed that the magnitude and timing of the rise in serum cortisol after surgery were almost identical to those observed in our study. Similarly, a study by Gibbison and colleagues [11] that included 20 patients who had cardiac surgery also showed patterns of ACTH and cortisol levels that were similar to those observed in our study.
It is interesting to note that in subjects who were not exposed to any exogenous glucocorticoids, the postoperative rise in ACTH resulted in an equimolar and parallel increases in the two ACTH-dependent steroids: Cortisol and DHEA. This feature is reminiscent of the reported equimolar responses in cortisol and DHEA after Cosyntropin stimulation in subjects with normal HPA function [22]. In the latter study, we demonstrated that the administration of Cosyntropin resulted in parallel and equimolar increases in both cortisol and DHEA such that the molar ratio of the two steroids remained unchanged during Cosyntropin stimulation [22]. Thus, the robust increase in endogenous ACTH secretion or the administration of an ACTH analogue (Cosyntropin) stimulated the release of cortisol and DHEA in equal concentrations. To our knowledge, the data presented herein are the first demonstration of this feature and represent a new approach in the assessment of adrenal function in different settings. The latter approach showed its advantage in improving our ability to diagnose adrenal insufficiency [22]. Moreover, it was of interest to note that the mean and median peak rise in serum DHEA levels in response to surgical stress (7.8 and 8.0 ng/ml) were very similar to those observed in other subjects [22] with established normal HPA function (8.4 and 8.0 ng/ml, respectively) after Cosyntropin stimulation. However, the rise in serum cortisol in response to surgery was more than that observed with Cosyntropin stimulation (39.2 and 40.0 vs. 27.7 and 28.1 ug/dL for the mean and median respectively). It is possible that the prolonged endogenous release of ACTH after surgical stress contributed to the higher and more persistent rise in serum cortisol levels in that setting.
The current investigation provides detailed characterization of the alterations in HPA function that are associated with DEX administration during the peri-operative period. We examined that feature in subjects given relatively large doses of DEX in order to truly appreciate the degree of HPA activation during the perioperative period. Single doses (2–10 mg) of DEX did not influence ACTH or cortisol levels but blunted the expected increase in DHEA secretion peri-operatively. Moreover, when multiple large doses of DEX were administered peri-operatively the secretion of ACTH and subsequently cortisol were partially blocked and a more impressive suppression of adrenal androgen secretion was noted. The data also showed that with single or multiple doses of DEX, the expected increase in serum DHEA and its sulfated conjugate were both blocked and as a result the molar ratio of cortisol / DHEA increased significantly in the perioperative period. One limitation to our study is the fact that DEX administration was not randomized but given based on the discretion of the managing team. This may have created an un-intentional bias that could not be avoided by the research team. Although we did not observe low serum cortisol levels in any of the patients who had one injection of DEX, it is worth emphasizing that the number of such subjects is relatively small and therefor it is possible that some patients could end up with a low serum cortisol level after a single dose of DEX. It follows that one need to be careful in interpreting low serum cortisol levels in the latter setting.
Our study is the first to demonstrate the discordance in the adrenal steroid response to DEX during the peri-operative period. The effects of dexamethasone on DHEA secretion were evident within hours of surgery and occurred despite an adequate rise in plasma ACTH. This would suggest that this potent glucocorticoid seem to inhibit DHEA synthesis much more efficiently and effectively than it does for cortisol. It is well known that after prolonged exposure to glucocorticoids, recovery of cortisol synthesis can occur despite persistence of continued adrenal androgen deficiency [23,24,25]. The latter feature is consistent with our findings.
The ability to maintain cortisol secretion while at the same time blocking DHEA release raises an important and challenging physiological question. Our study was not designed to address that question. It is not completely clear why DEX administration resulted in blocking adrenal androgen release even though these patients had robust rise in plasma ACTH that was similar in magnitude to that noted in those who did not receive any glucocorticoids. Although there are no obvious answers to address this question, one can attempt to postulate some. It is conceivable that DEX exerted direct effects on the zona reticularis function, possibly through modulation of some enzyme activity. A recent elegant study by Topor and colleagues [26] conducted on human adrenal cells in-vitro showed that cortisol stimulated DHEA synthesis through inhibition of 3-β hydroxysteroid dehydrogenase-type II enzyme. In contrast, the same study found that dexamethasone which is a potent mediator of glucocorticoid transcription did not stimulate but instead inhibited DHEA synthesis [26]. Earlier studies suggested that dexamethasone may act directly on steroidogenesis by inhibiting or suppressing transcription of the human gene for P450c17 [27, 28]. Additional studies are needed to fully elucidate the exact mechanisms involved and the potential enzymatic effects of DEX.
The study provides an extensive overview of the expected serum cortisol levels during the first 48 postoperative hours. The large number of patients involved and the various types of surgeries conducted offer a realistic view of variations in serum cortisol levels in the postoperative period. Such data can be helpful in designing appropriate doses for patients with adrenal insufficiency who would need glucocorticoid coverage during and after major surgical procedures. There are currently no data-supported guideline addressing the doses to be used in such patients. Finally, it is imperative to emphasize that one needs to avoid any attempt to investigate potential causes of hypercortisolism during that time frame. The current data indicate that such an evaluation during the peri-operative period will not only be inaccurate but also misleading [20]. Likewise, it would be prudent to consider the potential suppressive effects of DEX on HPA function even when a single dose of the drug is given peri-operatively and therefore one should avoid extensive testing for adrenal dysfunction in that setting.
In summary, the data provide detailed characterization of HPA function in a large number of patients with normal HPA function during the peri-operative period. The latter data can be useful in designing studies on glucocorticoid dose requirements in patients with adrenal insufficiency during major surgical procedures. The data also demonstrate that HPA function is strongly activated during the peri-operative period to the extent that glucocorticoid secretion is not suppressible with even large doses of dexamethasone. In contrast, DEX administration during the peri-operative period suppressed adrenal androgen production despite a robust increase in ACTH secretion. The discordance in DEX effects on glucocorticoid and adrenal androgen secretion suggests that it likely modulates adrenal enzyme function. The peri-operative administration of dexamethasone should not imply the need for any therapeutic intervention unless adverse effects are encountered and the potential suppression of HPA function with prolonged use.
References
K.E. Habib, P.W. Gold, G.P. Chrousos, Neuroendocrinology of stress. Endocrinol. Metab. Clin. 30, 695–728 (2001)
R. Udelsman, J. Ramp, W.T. Gallucci, A. Gordon, E. Lipford, J. Norton, D.L. Loriaux, G.P. Chrousos, Adaptation during surgical stress. A reevaluation of the role of glucocorticoids. J. Clin. Invest. 77(4), 1377–1381 (1986)
B. Chernow, H.R. Alexander, R.C. Smallridge, W.R. Thompson, D. Cook, D. Beardsley, M.P. Fink, R. Lake, J.R. Fletcher, Hormonal responses to graded surgical stress. Arch. Intern. Med. 147, 1273–1278 (1987)
R. Udelsman, J.A. Norton, S.E. Jelenich, D.S. Goldstein, W.M. Linehan, D.L. Loriaux, G.P. Chrousos, Responses of the hypothalamic-pituitary-adrenal and renin-angiotensin axes and the sympathetic system during controlled surgical and anesthetic stress. J. Clin. Endocrinol. Metab. 64, 986–994 (1987)
R. Udelsman, D.S. Goldstein, D.L. Loriaux, G.P. Chrousos, Catecholamine-glucose interactions during surgical stress. J. Surg. Res. 43, 539–545 (1987)
W.M. Hout, B.M. Arafah, R. Salazar, W.R. Selman, Evaluation of the hypothalamic- pituitary- adrenal axis immediately after pituitary adenomectomy: Is perioperative steroid therapy necessary? J. Clin. Endocrinol. Metab. 66, 1208–1212 (1988)
R.A. Donald, E.G. Perry, G.A. Wittert, M. Chapman, J.H. Livesey, M.J. Ellis, M.J. Evans, T. Yandle, T.A. Espiner, The plasma ACTH, AVP, CRH and catecholamine responses to conventional and laparoscopic cholecystectomy. Clin. Endocrinol. 38(6), 609–615 (1993)
P. Khilnani, R. Munoz, M. Salem, C. Gelb, I.D. Todres, B. Chernow, Hormonal responses to surgical stress in children. J. Ped. Surg. 28, 1–4 (1993)
Y. Naito, S. Tamai, K. Shingu, Responses of plasma ACTH, cortisol and cytokines during and after upper abdominal surgery. Anesthesiology. 77, 426–431 (1993)
Gh Hall, D. Peerbhoy, A. Shenkin, C.J.R. Parker, P. Salmon, Hip and knee arthroplasty: a comparison and endocrine, metabolic and inflammatory responses. Clin. Sci. 98, 71–79 (2000)
B. Gibbison, F. Spiga, J.J. Walker, G.M. Russell, K. Stevenson, Y. Kershaw, Z. Zhao, D. Henley, G. Angelini, S.L. Lightman, Dynamic pituitary-adrenal interactions in response to cardiac surgery. Crit. Care. Med. 43, 791–800 (2015)
B.M. Arafah, Hypothalamic pituitary adrenal function during critical illness: a critical review and reappraisal. J. Clin. Endocrinol. Metab. 91, 3725–3745 (2006)
A. Beishuzen, L.G. Thijs, I. Vermes, Patterns of corticosteroid-binding globulin and the free cortisol index during septic shock and mutitrauma. Intensive Care Med. 27, 1584–1591 (2001)
G.M. Vaughn, R.A. Becker, J.P. Allen, Cortisol and corticotrophin in burned patients. J. Trauma Acute Care Surg. 221, 263–273 (1982)
I.E. Widmer, J.J. Puder, C. Konig, H. Pargger, R.H. Zerkowski, J. Girard, B. Muller, Cortisol response in relation to the severity of stress and illness. J. Clin. Endocrinol. Metab. 90, 4579–4586 (2005)
B.M. Arafah, S.H. Kailani, K.E. Nekl, R.S. Gold, W.R. Selman, Immediate recovery of pituitary function after transsphenoidal resection of pituitary macroadenomas. J. Clin. Endocrinol. Metab. 79, 348–354 (1994)
R. Al-Aridi, D. Abdelmannan, B.M. Arafah, Biochemical diagnosis of adrenal insufficiency: The added value of dehydroepiandrosterone sulfate (DHEA-S) measurements. Endocr. Pract. 17, 261–270 (2011)
J. Chaiban, D. Abdelmannan, M. Cohen, W.R. Selman, B.M. Arafah, Rathke’s cyst apoplexy: A newly characterized distinct clinical entity. J. Neurosurg. 114, 318–324 (2011)
N. El Asmar, K. El-Sibai, R. Al-Aridi, W.R. Selman, B.M. Arafah, Postoperative Sellar Hematomas after pituitary surgery: Clinical and biochemical characteristic. Eur. J. Endocrinol. 174, 573–582 (2016)
V. Bansal, N. El Asmar, W.R. Selman, B.M. Arafah, Pitfalls in the diagnosis and management of Cushing’s syndrome. Neurol. Focus 38, 1–11 (2015)
K.E. Schimke, P. Greminger, M. Bandle, Secondary adrenal insufficiency due to opiate therapy: another differential diagnosis worth consideration. Exp. Clin. Endocrinol. Diabetes 117, 649–651 (2009)
L. Sayyed Kassem, K. El Sibai, J. Chaiban, D. Abdelmannan, B.M. Arafah, Measurements of serum DHEA and DHEA-sulphate levels improve the accuracy of the low dose cosyntropin test in the diagnosis of central adrenal insufficiency. J. Clin. Endocrinol. Metab. 97, 3655–3663 (2012)
G.B. Cutler, E.S. Davis, R.E. Johnsonbaugh, D.L. Loriaux, Dissociation of cortisol and adrenal androgen secretion in patients with secondary adrenal insufficiency. J. Clin. Endocrinol. Metab. 49, 604–609 (1979)
A.C. Watson, R.L. Rosenfield, Recovery from glucocorticoid inhibition of the responses to corticotrophin-releasing hormone. Clin. Endocrinol. 28, 471–477 (1988)
D.F. Brigell, V.S. Fang, R.L. Rosenfield, Recovery of responses to ovine corticotropin releasing hormone after a short course of glucocorticoid. J. Clin. Endocrinol. Metab. 74, 1036–1039 (1992)
L.S. Topor, M. Asai, J. Dunn, J.A. Majzoub, Cortisol stimulates secretion of dehydroepiandrosterone in human adrenocortical cells through inhibition of 3 β- HSD2. J. Clin. Endocrinol. Metab. 96(1), E31–E39 (2011)
G.C. Byrne, Y.S. Perry, J.S.D. Winter, Steroid inhibitory effects upon human adrenal 3β hydroxysteroid dehydrogenase activity. J. Clin. Endocrinol. Metab. 62, 413–418 (1986)
T.C. Lee, W.L. Miller, R.J. Auchus, Medroxyprogesterone acetate and dexamethasone are competitive inhibitors of different human steroidogenic enzymes. J. Clin. Endocrinol. Metab. 84, 2104–2110 (1999)
Funding
This work was supported by a local departmental grant to the Division of Endocrinology
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
El-Sibai, K., Rajpal, A., Al-Aridi, R. et al. The impact of peri-operative dexamethasone administration on the normal hypothalamic pituitary adrenal response to major surgical procedures. Endocrine 58, 134–142 (2017). https://doi.org/10.1007/s12020-017-1398-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1398-y