Abstract
Background
Silent corticotroph adenomas (SCAs) are characterized by strong ACTH immunostaining without clinical manifestations of hypercortisolism. Patients with SCAs often present with mechanical symptoms related to tumor growth. This study investigates the hypothalamic pituitary adrenal axis (HPA) characteristics after adenomectomy in patients with SCAs.
Methods
Biochemical parameters of HPA function were monitored frequently after surgical resection of non-functioning macroadenomas. Levels of ACTH, cortisol, DHEA and DHEA-S were measured frequently for 48 h after adenomectomy. HPA data of patients with SCAs (n = 38) were compared to others (Controls) with non-secreting, ACTH-negative immunostaining adenomas of similar age and gender distribution (n = 182) who had adenomectomy.
Result
Plasma ACTH increased (P < 0.0001) equally in patients with SCA and controls reaching a peak at 3 h (238 ± 123 vs. 233 ± 96 ng/L, respectively) after extubation declining thereafter to baseline values 24–36 h. Similarly, serum cortisol levels increased (P < 0.0001) equally in both groups reaching a maximum at 7 h (36.8 ± 13.9 vs. 39.3 ± 13.3 ug/dL). Serum DHEA also increased (P < 0.001) equally in both groups in parallel to the rise in serum cortisol. Serum DHEA-S levels similarly increased (P < 0.001) from their respective baseline (105.9 ± 67.5 and 106.5 ± 58.7 ug/dL) reaching their peak (154.5 ± 69.5 and 153.5 ± 68.6 ug/dL; respectively) at 15 h after extubation. None of the patients acquired any hormone deficits.
Conclusions
Under the maximal stimulation of the peri-operative stress, HPA function in patients with SCA behaved in an identical manner to others with ACTH-negative macroadenomas. Thus, despite the strong ACTH-positive immunostaining of these tumors, SCAs are truly non- functional.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The frequency of atypical pituitary adenomas is reported to vary from 2.7 to 15% [1, 2]. The World Health Organization (WHO) classification system for pituitary tumors has outlined characteristics that aid in identifying such tumors such as invasive growth, an elevated mitotic index, a Ki-67 labelling index >3% and nuclear staining for the cellular tumor antigen p53 [3]. Many of these atypical tumors have been found to be silent corticotroph adenomas (SCAs) [1]. A case of SCA was first diagnosed in 1978 and reported as a disease category by Hassoun et al. in 1979 [4]. It was then described in more detail by Horvath et al. in 1980 where 17 cases were discovered among 300 adenomectomy specimens [5]. The hallmark of these tumors is that they have positive immunoreactivity for ACTH but have no clinical manifestations or biochemical evidence of hormone excess suggestive of Cushing’s syndrome, such as central obesity, moon face, facial plethora, diabetes, hypertension and osteoporosis. Because these tumors lack a distinguishing clinical phenotype, SCAs are not detected unless they cause mass effects or are discovered serendipitously [6].
Though the lack of clinical manifestations of hypercortisolism in patients with these tumors is well documented in the literature, the pathogenesis of SCAs remain unclear. Various mechanisms for the clinical silence of these tumors have been postulated and include, but are not limited to: biological inactivity of ACTH, increase in intracellular disposal by lysosomes, defective packaging of ACTH into secretory granules due to an inadequately developed Golgi complex and failure of exocytosis of hormone from the cell membrane [6, 7]. As there is no characteristic phenotype, the diagnosis of SCA is typically made postoperatively. This therefore makes the evaluation of pre- and peri-operative hypothalamic-pituitary adrenal (HPA) function in such patients generally limited.
One of the unique features of the postoperative period is a robust activation of the HPA function [8,9,10,11,12]. We have conducted several investigations that allowed us to characterize the HPA function during the peri-operative period in a large number of patients with pituitary adenomas irrespective of the immunostaining [8,9,10,11,12]. With the ACTH positive immunostaining within cells of these tumors, we questioned whether the acute surgical stress causes any alterations in HPA function in patients with SCAs and whether their HPA function in the peri-operative period would be similar to those of ACTH-secreting tumors. The aim of this investigation is to examine the HPA axis function in patients found to have a SCAs during the first 48 h following adenomectomy since this would represent maximal HPA activation. In evaluating the HPA function after adenomectomy, we follow a detailed published protocol that is applied to all patients who have pituitary tumor resection [8,9,10,11,12]. This has allowed for the examination of the HPA axis in patients with SCA and compare that to those with immune-negative tumors.
Subjects and methods
Subjects
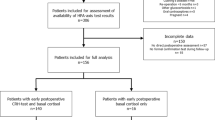
This was a prospective study on all patients with pituitary adenomas who had surgical resection of their adenomas over a 10 year period (2006–2015) and had normal HPA function before surgery. The data on a total of 220 patients with clinically non-functional macroadenomas who had surgical resection and also had normal HPA function were reviewed. All patients underwent complete evaluation of pituitary function preoperatively. Only data pertaining to the HPA axis are presented here. Tumor type was characterized on the basis of immune-histochemical staining of multiple sections of the resected adenoma. We excluded patients who had secreting adenoma including those with Cushing’s disease, acromegaly and prolactinomas. We also excluded patients who had pre or postoperative impairment in HPA function and those who have conditions or were taking medications that alter the interpretation of serum cortisol measurements [13].
Among the 220 patients with clinically non-functional tumors, we identified the SCA patients (n = 38) based on ACTH (moderate to strongly positive) immunoreactivity and the absence of preoperative clinical and laboratory features of hypercortisolism. The remaining patients (control group; n = 182) was comprised of subjects with clinically non-functional macroadenomas that had negative immunohistochemical staining for ACTH. However, adenomas in the control group had either totally negative immunostaining (n = 42) for any of the tested hormones (Prolactin, GH, FSH, LH and ACTH) or moderate staining for GH/prolactin (n = 35), FSH and/or LH (n = 64) and the remaining 41 had scattered cells that had positive immunostainig for multiple hormones. 11 of the 38 patients with SCA had scattered focal staining for other hormones including FSH, LH, GH and prolactin but had no clinical or biochemical abnormalities related to these hormones.
Endocrine evaluation of all subjects was done pre- and postoperatively at specified time points as described below [8,9,10,11,12]. Management of patients in the perioperative period followed the same standards and protocols established at our institution as previously described [8,9,10,11,12]. The primary objective of this study was to determine whether the biochemical response to stress in patients with SCAs would be different when placed under considerable physiological stress with regards to the ACTH-dependent steroids when compared to their immunonegative counterparts. The study was approved by the Institutional Review Board.
As stated earlier, the diagnosis of a SCA is typically made postoperatively upon immunohistochemical review in patients who did not exhibit clinical features of hypercortisolism. It would therefore be hard to prospectively perform detailed dynamic testing of HPA axis as one would expect in patients with clinical suspicion for hypercortisolism. In all patients however, preoperative testing is primarily focused on assessing pituitary function and ensuring adequate HPA function. We have, over the past 30 years followed an established protocol detailing perioperative management of all patients who underwent surgical resection of pituitary masses. This has allowed for obtaining consistent data at specific perioperative time points in patients who were subsequently shown to have a SCA and others who had an immunonegative tumors.
Thus, a total of 38 patients (18 women; 20 men, mean age 50.1 ± 13.7 years) were included in the analysis for the SCA group. The control group comprised 182 patients (89 women/93 men, mean age 51.8 ± 15.9 years) who had similar pre and postoperative data acquisition (Table 1).
Assessment of HPA function
HPA function was assessed in all patients as follows. Preoperative laboratory tests were obtained which included serum cortisol, ACTH, DHEAS, DHEA, prolactin, IGF-1, TSH, free thyroxine, FSH and LH in all patients, total and free testosterone in men and estradiol in women. Patients with a normal plasma ACTH concentration and a random serum cortisol of 12 µg/dL or greater were considered to have a normal HPA function [14, 15]. Patients with a random serum cortisol of >5 ug/dL and an age-appropriate serum DHEA-S levels were also considered to have a normal HPA function [14, 15]. Patients with a random serum cortisol of 5–11 ug/dL but had a low or low-normal serum DHEA-S levels had a 1 ug Cosyntropin test to assure normality of the HPA function [14, 15]. Post-operative laboratory evaluation included the aforementioned laboratory tests obtained at 2–4, 6–8, and every 6 h thereafter for 48 h [8,9,10,11,12]. The protocol stipulates that glucocorticoids are not administered before and during surgery to patients who have normal HPA function as previously defined [8,9,10,11,12].
Peri-operative HPA function was defined on the basis of repeated measurements of serum cortisol in response to the surgical stress. A normal response to surgery is defined on the basis of serum cortisol levels of >15 ug/dL on multiple occasions during the first 36 postoperative hours without having values below 5 ug/dL.
Laboratory analysis
Plasma ACTH concentrations were measured using solid-phase, two-site sequential chemiluminescent immunometric assay, using the Immulite 2000XPi (Siemens, Malvern, PA). The lower limit of quantitation for the assay is 5 ng/L. Intra-assay coefficients of variation for the lower and upper limits are 4.4 and 3.1%, respectively. Inter-assay coefficients of variation for the lower and upper assay ranges are 9.0 and 8.5%, respectively. The normal AM plasma ACTH in this assay was 5–46 ng/L. Serum cortisol measurements were made using the direct chemiluminescent immunoassay method, using Advia Centaur XP instrument (Siemens, Malvern, PA). The lower limit of quantitation for the assay is 0.2 ug/dL. Intra-assay coefficients of variation for the lower, mid and upper assay measurement ranges are 4.7, 3.9 and 2.3%, respectively. The inter-assay coefficients of variation for the lower, mid and upper limits are 6.1, 8.2 and 4.4%, respectively. The normal AM cortisol in this assay was 5–20 µg/dL. Serum DHEA levels were measured with HPLC-tandem mass spectroscopy method developed by Quest Diagnostics Nichols Institute (San Juan Capistrano, CA). The lower limit of detectability was 10 ng/dL. The intra-assay coefficients of variation at the lower, mid and upper limits are 13.5, 5.2 and 3.3% respectively. The interassay coefficients of variation at the lower, mid and upper and limits are 14.3, 7.7 and 7.1% respectively. The normal DHEA in this assay was 61–1636 ng/dL, depending on the age and gender of the subject. Serum DHEA-S levels were measured using solid-phase, chemiluminescent enzyme-labeled immunoassay, using the Immulite 2000XPi (Siemens, Malvern, PA). The lower limit of quantitation for the assay is 15 ug/dL. Intra-assay coefficients of variation at the lower, mid and upper limits are 7.4, 5.4 and 4.8%, respectively. The inter-assay coefficients of variation at the lower and upper limits are 8.5 and 7.9%, respectively. The normal DHEA-S in this assay was 28–290 µg/dL, depending on the age and gender of the subject.
Statistical analysis
Data are presented as mean ± standard deviation, unless stated otherwise. The perioperative data from the SCA as well as those with immunonegative tumors were first analyzed using the Kruskal–Wallis test, as a nonparametric alternative to the ANOVA test. Comparisons between groups were done using the Wilcoxon rank sum test for nonparametric measurements. Categorical data were compared using Chi square (χ2) and Fisher exact tests. Differences were considered significant when the two-sided P values were <0.05. All data analysis was made using the SPSS program (SPSS Inc., Chicago, IL).
Results
The Preoperative patients’ characteristics are shown in Table 1. As shown in Table, preoperative ACTH, cortisol, DHEA and DHEAS values in patients with SCAs and those of the Control group were identical and not shown to be statistically significant.
Peri-operative HPA function
The peri-operative data on all patients are illustrated in Figs. 1 and 2. Plasma ACTH as well as serum cortisol levels promptly increased shortly after extubation in both groups of patients. The response was identical in the two groups (Fig. 1). The rise in plasma ACTH was robust reaching a peak at around 2–4 h and persisting for nearly 24 h. This was followed by a gradually and robust increase in serum cortisol levels that reached a peak at 6–8 h after extubation and decreased gradually thereafter where it became close to baseline values at 48 h. A parallel rise in the serum level of another ACTH-dependent steroid, DHEA was observed to reach its peak at the same as that of serum cortisol (Fig. 2). The rise in serum DHEA was identical in both groups of patients.
Repeated measurements of plasma ACTH (top panel) and serum cortisol (lower panel) levels during the 48 postoperative hours obtained in patients with normal HPA function who had surgical resection of ACTH-negative (solid line) and ACTH-positive (dashed line) pituitary macroadenomas. There were no differences in plasma ACTH and serum cortisol levels between the two groups at any of the postoperative times these were determined
Repeated measurements of serum levels of DHEA (Top panel) and DHEA-S (lower panel) during the 48 postoperative hours obtained in patients with normal HPA function who had surgical resection of ACTH-negative (solid line) and ACTH-positive (dashed line) pituitary macroadenomas. There were no differences in serum DHEA levels between the two groups at any of the postoperative times these were determined. Serum DHEA levels reached a peak at 6–8 h and returned to baseline values 24 h after surgery in both groups of patients. The response paralleled that observed for the rise in serum cortisol depicted in Fig. 1. The peak DHEA-S response was noted at 15–18 h following which, the level declined t baseline values by 36–48 h
An additional response to the robust increase in ACTH secretion after surgery was the observed rise in serum DHEA-S as a result of increased DHEA secretion and sulfation. As expected, the observed peak in serum DHEA-S levels was not detected until 15–18 h after extubation with gradual return to preoperative baseline values by the 36th to the 48th postoperative hour (Fig. 2).
HPA function 3 months after surgery
HP function was normal and similar to preoperative data in all patients. That is none of the patients acquired new hormonal deficits.
Discussion
Although the lack of clinical manifestations of hypercortisolism is well appreciated in subjects with SCAs, the HPA axis response to stressful events in these patients has not been previously characterized. The current study provides such characterization. The presence of ACTH-positive immunostaining tumors in these patients did not influence HPA activation in response to the surgical stress. More specifically, the data presented herein demonstrate that under immense physiological stress, there was no difference with regards to ACTH and its three dependent steroids in the post-operative period between SCA subjects and their immunonegative counterparts with normal HPA axis function. The data reaffirm the complete absence of ACTH biologic effects in these tumors. The absence of Crooke’s hyaline changes histologically is consistent with the lack of excess glucocorticoid effects on these tumors.
The majority of SCA patients presented in the fifth and sixth decades of life, similar to most previous reports [16]. SCA affected both genders with a slightly higher prevalence in men, which is similar to three previous reports [16,17,18]. The hallmark of SCAs is that they have positive immunoreactivity for ACTH but demonstrate no biochemical evidence or clinical manifestations of cortisol excess. Because there is no specified clinical phenotype, these tumors are likely to go undetected unless mass effects occur because of their size (macroadenomas). A number of features, though nonspecific, characterize the presentation of patients with SCAs, namely high frequency of symptoms attributable to mass effects, particularly visual field defects and hypopituitarism [6]. The pathogenesis of SCAs is intriguing and not completely understood. Various mechanisms for the clinical silence of these tumors have been postulated and include, but are not limited to: biological inactivity of ACTH, increase in intracellular disposal by lysosomes, defective packaging of ACTH into secretory granules due to an inadequately developed Golgi complex and failure of exocytosis of hormone from the cell membrane [6, 7]. In a study by Gibson et al., it has been found that ACTH may be functionally inactive because of abnormal processing of POMC [19]. SCAs have also been found to have lower expressions of PC1/3, which is the enzyme that is responsible for cleaving POMC into ACTH [20, 21].
Currently, two distinct pathologic subtypes of SCAs are recognized [6]. Macroscopically, both SCA subtypes are generally macroadenomas with variable invasion or compression of parasellar structures. This is in stark contrast to functional ACTH adenomas, which are mostly found to be microadenomas at the time of clinical presentation due to the manifestations of hypercortisolemia. Type I SCA, which accounts for 68%, is histologically and ultrastructurally indistinguishable from classical Cushing’s adenoma with the exception of having the Crooke’s hyaline changes, which are pathognomonic for Cushing’s as a result of persistent hypercotisolemia. These type-1 tumors show strong ACTH expression by the majority of tumor cells. In contrast, the type II SCA has only patchy or faint ACTH-positivity by immunohistochemistry.
Medical treatment is not currently included in the therapeutic protocol of SCAs and surgery remains the main therapeutic approach. There have been attempts to identify predictors for recurrence but these were proven to be unsuccessful. Preoperative tumor invasiveness, cavernous sinus extension, tumor subtype, degree of ACTH immunoreactivity and Ki-67 index do not seem to be associated with increased risk of recurrence [17].
In summary, the current investigation shows that under the immense physiological stress of the postop period, the pattern and magnitude of HPA activation in patients with SCAs were similar to other patients who had surgical resection of tumors that had negative ACTH immunoreactivity. Specifically, there were no differences in plasma ACTH levels or in any of the three ACTH-dependent adrenal steroids when SCAs were compared to their immunonegative counterparts. The data reaffirm the complete absence of ACTH biologic effects in these tumors.
References
Zada G, Woodmansee WW, Ramkissoon S, Amadio J, Nose V, Laws ER Jr (2011) Atypical pituitary adenomas: incidence, clinical characteristics, and implications. J Neurosurg 114:336–344
Saeger W, Ludecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S (2007) Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Registry. Eur J Endocrinol 156:203–216
Di leva A, Rotondo F, Syro LV, Cusimano MD, Kovacs K (2014) Aggressive pituitary adenomas—diagnosis and emerging treatments. Nat Rev Endocrinol 10:423–435
Hassoun J, Charpin C, Jaquet P, Oliver C, Lissitky JC, Grisoli F, Toga M (1987) Analogies immunocytochemiques des adenomes hypohysaires de la maladie de Cushing et des adenomes “non functionnels” (adenomes chromophobes) de l’hypohpyse. Ann Endocrinol (Paris) 40:559
Horvath E, Kovacs K, Killinger DW, Smyth HS, Platts ME, Singer W (1980) Silent corticotropic adenomas of the human pituitary gland. a histological, immunocytologic and ultrastructural study. Am J Pathol 98:617–636
Scheithauer, B, Jaap A, Horvath E, Kovacs K, Lloyd R, Meyer F, Laws E, Young W Jr (2000) Clinically silent corticotroph tumors of the pituitary gland. Neurosurgery 47:723–729
Jahangiri A, Wagner J, Pekmezci M, Hiniker A, Chang E, Kunwar S, Blevins L, Aghi M (2013) A comprehensive long-term retrospective analysis of silent corticotrophic adenomas versus hormone-negative adenomas. Neurosurgery 73:8–17
Arafah BM, Kailani SH, Nekl KE, Gold RS, Selman WR (1994) Immediate recovery of pituitary function following transsphenoidal resection of pituitary macroadenoma. J Clin Endocrinol Metab 79:348–354
Hamrahian A, El-Malawany NK, Arafah BM (1999) Evaluation and management of pituitary-adrenal function after pituitary surgery. Endocrinologist 9:16–24
Abdelmannan D, Selman WR, Arafah BM (2010) Peri-operative management of cushing’s disease. Rev Endocr Metab Disord 11:127–134
Abdelmannan D, Selman WR, Arafah BM (2013) Recurrences of ACTH-secreting adenomas after pituitary adenomectomy can be accurately predicted by peri-operative measurements of plasma ACTH levels. J Clin Endocrinol Metab 98:1458–1465
El-Asmar, El-Sibai K, Al-Aridi R, Selman WR, Arafah BM (2016) Post operatively sellar hematoma after pituitary surgery: clinical and biochemical characteristics. Eur J Endocrinol 174:573–582
Bansal V, El Asmar N, Selman WR, Arafah BM (2015) Pitfalls in the diagnosis and management of Cushing’s syndrome. Neurosurg Focus 38:1–11
Al-Aridi R, Abdelmannan D, Arafah BM (2011) Biochemical diagnosis of adrenal insufficiency: the added value of DHEA-S measurements. Endocr Pract 17:261–270
Sayyed Kassem L, El Sibai K, Chaiban J Abdelmannan D, Arafah BM (2012) Measurements of serum DHEA and DHEA-S levels improve the accuracy of low dose cosyntropin test in the diagnosis of central adrenal insufficiency. J Clin Endocrinol Metab 97:3655–3663
Ioachimescu A, Eiland L, Chhabra V, Mastrogianakis G, Schniederjan M, Brat B, Pileggi A, Oyesiku N (2012) Silent corticotroph adenomas: emory university cohort and comparison with ACTH-negative nonfunctioning pituitary adenomas. Neurosurgery 71:296–303
Bradley KJ, Wass JA, Turner HE (2003) Non functioning pituitary adenomas with positive immunoreactivity for ACTH behave more aggressively than ACTH immunonegative tumours but do not recur more frequently. Clin Endocrinol 58:59–64
Baldeweg SE, Pollock JR, Powell M, Ahlquist J (2005) A spectrum of behavior in silent corticotroph pituitary adenomas. Br J Neurosurg 19(1):38–42
Gibson S, Ray DW, Crosby SR, Dornan TL, Jennings AM, Bevan JS, Davis JR, White A (1996) Impaired processing of proopiomelanocortin in cotricotroph macroadenomas. J Clin Endocrinol Metab 81(2):497–502
Ohta S, Nishizawa S, Oki Y, Yokoyama T, Namba H (2002) Significance of absent prohormone convertase 1/3 in inducing clinically silent corticotroph pituitary adenoma of subtype I—immunohistochemical study. Pituitary 5(4):221–223
Tateno T, Izumiyama H, Doi M, Akashi T, Ohno K, Hirata Y (2007) Defective expression of prohormone convertase 1/3 in silent corticotroph adenoma. Endocr J 54(5):777–782
Funding
This work was funded by local/departmental grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Cheres, A.F., ElAsmar, N., Rajpal, A. et al. Perioperative hypothalamic pituitary adrenal function in patients with silent corticotroph adenomas. Pituitary 20, 471–476 (2017). https://doi.org/10.1007/s11102-017-0809-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-017-0809-7