Abstract
Purpose
During follow-up of acromegaly patients, there is a discordance rate of 30% between the measurements of growth hormone and insulin-like growth factor-1 levels. Further tests are required to determine disease activity in patients with discordant results. This study was planned to investigate an association of serum levels of matrix metalloproteinase-2, matrix metalloproteinase-9, and cathepsin B with disease activity in acromegaly patients.
Methods
In this study, 64 acromegaly patients followed in our clinic were divided into two groups according to the 2010 consensus criteria for cure of acromegaly as patients with active disease (n = 24) and patients with controlled disease (n = 40). Serum matrix metalloproteinase-2, matrix metalloproteinase-9, and cathepsin B levels were measured by the enzyme-linked immunosorbent assay method.
Results
The mean serum matrix metalloproteinase-2 level was significantly higher in the active acromegaly patients than in the controlled acromegaly patients (150.1 ± 54.5 ng/mL vs. 100.2 ± 44.6 ng/mL; p < 0.0001). There was no significant difference between the active and controlled acromegaly patients regarding serum matrix metalloproteinase-9 and cathepsin B levels (p = 0.205 and p = 0.598, respectively). Serum matrix metalloproteinase-2 levels of 118.3 ng/mL and higher had a sensitivity of 75% and a specificity of 77.5% in determining active disease. The risk of active acromegaly was 3.3 fold higher in the patients with a matrix metalloproteinase-2 level of >118.3 ng/mL than in the patients with a matrix metalloproteinase-2 level of <118.3 ng/mL.
Conclusions
In this study, serum matrix metalloproteinase-2 level is increased in the active acromegaly patients and a threshold value in determining active disease was defined for serum matrix metalloproteinase-2 level. This study is the first to compare acromegaly patients having active or controlled disease in terms of matrix metalloproteinase-2 and matrix metalloproteinase-9 levels. The results need to be confirmed by a study that will be conducted in a larger patient group also including a healthy control group to demonstrate the value of this novel marker in disease activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acromegaly is a progressive chronic disease that develops due to increase in the level of growth hormone (GH) and insulin-like growth factor-1 (IGF-1) due to GH-secreting pituitary adenoma in more than 95% of the patients. Clinical signs and symptoms in acromegaly may result from the mass effect of the tumor; moreover, somatic, and metabolic complications involving many systems in the body can also be observed due to increased levels of GH and IGF-1 [1]. Treatment of acromegaly includes transsphenoidal surgery, medical treatment, and radiotherapy; biochemical response to treatment is assessed by measurements of GH and IGF-1 levels [2]. However, in acromegaly patients, discordance rate of 8.6 and 28.6% have been reported between GH and IGF-1 measurements after surgery and during octreotide therapy, respectively [3]. Based on the measurements of GH and IGF-1 levels by radioimmunoassay method after treatment, patients having a basal GH level of ≤2.5 ng/mL and an IGF-1 level within the normal ranges for age and gender are expected to have a normal life expectancy [4].
Normal pituitary tissue and pituitary adenomas contain extracellular matrix (ECM) components. It is thought that degradation and remodeling of ECM play a role in the growth of pituitary adenomas [5]. Studies have demonstrated that ECM and basal membranes, which are the natural barriers, are degraded during invasion and metastasis processes of cancer. Serine proteases, cathepsins, and matrix metalloproteinases (MMPs), which are called as proteases and released from tumor cells, play a role in the degradation of these barriers [6]. MMPs, also known as matrixins, are the proteases of endopeptidase family which contain zinc, are active in neutral environment, have the ability to degrade all components of basal membrane, and act in various physiological and pathological processes [7]. MMP-2 and MMP-9 are the members of the gelatinase subgroup of MMPs. They contain three fibronectin type II repeats in their catalytic region, which enable them to bind to gelatin and collagen with high affinity and enhance their proteolytic activities [8]. Cathepsin B is a member of cysteine cathepsin family, is active and stable in acidic cell structures such as lysosome and endosome, and has the ability to effectively degrade a great variety of substrates [9]. In the light of this information, studies investigating MMP expression and invasion of pituitary adenomas have been conducted. Among these, while there are studies demonstrating increased MMP-2 and MMP-9 expressions in invasive pituitary adenomas [10, 11], there are also studies not suggesting a relation between expression of these proteases and cathepsin B and the invasion of pituitary adenomas [12, 13]. In addition to invasion, studies have also investigated the MMP expression and functional status of pituitary adenomas. Postoperative immunohistochemical staining of the anterior pituitary cells has revealed lower MMP-2 and MMP-9 expressions in non-functional adenomas than in functional adenomas [14]. Páez Pereda et al. [15] demonstrated that MMPs secreted by the pituitary cells controlled pituitary cell proliferation and hormone secretion by leading to release of growth factors from the ECM. Daroszewski et al. [16] reported significantly higher cathepsin B and cysteine protease inhibitor activities in acromegaly patients than in healthy controls.
In the present study, we aimed to investigate serum MMP-2, MMP-9, and cathepsin B levels in patients with active and controlled acromegaly, correlation with serum GH and IGF-1 levels and usefulness of these markers in identifying active disease.
Materials and methods
Patients who were diagnosed with acromegaly and followed-up in the Hypophysis Diseases Polyclinic of Kocaeli University Faculty of Medicine, Department of Internal Medicine, Endocrinology and Metabolism Division were included in the present study. Newly diagnosed patients or patients who were in the postoperative 3-month period were excluded. Sixty-four patients (34 males and 30 females) included in the study were invited to the polyclinic by phone calls between January 2011 and April 2011. The present study was approved by the Research Ethics Committee of Kocaeli University (date: March 04, 2011) and informed consents of all patients were obtained.
The disease activity and control status of the patients were determined in accordance with the criteria published in 2010 by the Acromegaly Consensus Group [17]. According to these criteria, controlled disease was defined as follows: (1) age-adjusted IGF-1 level being within the normal ranges and the lowest GH level being below 0.4 µg/L during oral glucose tolerance test (OGTT) performed with 75 g glucose for the drug-free patients or for the operated patients after 3 months of surgery and (2) a basal GH being <1 µg/L and age-adjusted IGF-1 level being within the normal ranges for the patients receiving somatostatin analogs and/or dopamine agonists. In two patients treated with the combination of a somatostatin analog and pegvisomant, the disease was considered to be under control if the age-adjusted IGF-1 level was within the normal ranges. Based on these criteria, acromegaly was considered to be active in 24 patients and to be controlled in 40 patients.
Past medical files of all patients were reviewed and data regarding size of adenoma prior to treatment, time elapsed between the onset of clinical symptoms and diagnosis, treatment modalities, pre-treatment and post-treatment pituitary insufficiency, and comorbidities were obtained. Comorbidities were determined by blood pressure monitoring, OGTT with 75 g glucose, echocardiography, thyroid, and abdominal ultrasonography, colonoscopy, mammography in females, and prostate gland examination in males, which are routinely performed in every acromegaly patient. The patients with pituitary hormone deficiency continued to receive their appropriate replacement therapy regularly.
Blood samples were collected from the patients after an 8-h fasting period on the day of admission. The obtained blood samples were transferred to the central laboratory for the measurements of GH and IGF-1 levels. The blood samples collected for the measurements of MMP-2, MMP-9, and cathepsin B levels were centrifuged and serum samples were then stored at –80 °C to be analyzed later. An OGTT with 75 g glucose was performed in the drug-free patients or in the operated patients after 3 months of surgery and blood samples were then collected at the 0th, 30th, 60th, 90th, and 120th min for the measurement of GH level.
The chemiluminescence immunoassay method (IMMULITE® 2000 immunoassay system; Siemens, United Kingdom) was used for the measurement of GH level. Recombinant GH IS 98/574, which is the international standard recommended by the World Health Organization (WHO), was used as the calibrator. In this method, the analytical sensitivity was 0.01 ng/mL and the measurement range was 0.05–40 ng/mL for GH. IGF-1 was measured by immunoenzymatic assay (IEMA, Immunodiagnostic Laboratories, Boldon, United Kingdom). In this method, IS 87/518, which is recommended by the WHO as the second international calibrator, was used. The sensitivity of IGF-1 measurement was 3.1 µg/L. The level of IGF-1 was interpreted based on the age-adjusted reference values.
Enzyme-linked immunosorbent assay (ELISA; Ray Biotech, Inc. Norcross, USA) was used for the measurements of MMP-2 and MMP-9 levels. The sensitivity, intra-assay coefficient of variation, and inter-assay coefficient of variation values for MMP-2 measurement were <3.5 ng/mL, <10, and <12%, respectively. The sensitivity, intra-assay coefficient of variation, and inter-assay coefficient of variation values for MMP-9 measurement were <10 pg/mL, <10, and <12%, respectively. Cathepsin B level was also measured by the ELISA method (Cusabio Biotech Co., Ltd. China). The sensitivity and measurement range for cathepsin B measurement were <0.08 ng/mL and 0.31–20 ng/mL, respectively.
Statistical analysis
Data analyses of the study were performed using the NCSS (Number Cruncher Statistical System) 2007 Statistical Software (Utah, USA) and the Predictive Analytics Software (PASW; SPSS Inc., Chicago, IL, USA) version 18.0 for Windows. In addition to the descriptive statistical methods (mean, standard deviation) used for the evaluation of data, independent t-test was used for the comparisons between the groups and chi-square test and Fisher’s exact test were used for the comparison of qualitative data. The relation between numerical variables was determined by Pearson’s correlation analysis. The area under the receiver operating characteristics (ROC) curve, sensitivity, specificity, negative predictive value, positive predictive value, and relative ratio of the variables were calculated to identify the cut-off value. The results were evaluated at a significance level of p < 0.05 and within 95% confidence interval (CI).
Results
The general characteristics of acromegaly patients with active and controlled disease are demonstrated in Table 1. No significant difference was determined between the acromegaly patients with active and controlled disease in terms of age and gender distribution. While pre-treatment adenoma size was significantly higher in the patients with active acromegaly than in the patients with controlled acromegaly (p = 0.002), no significant difference was determined between these patients in terms of presence of macroadenoma (p = 0.14). Transsphenoidal surgery was performed at least for once in 58 patients (one time in 44 patients, two times in 9 patients, and three times in 5 patients), whereas 6 patients were followed-up with primary medical therapy. No difference was determined between the patients with active and controlled disease in terms of the number of surgical interventions. In the active acromegaly group, a total of 11 patients were under follow-up with medical therapy (somatostatin analog in 6 patients, somatostatin analog + cabergoline combination in 4 patients, and somatostatin analog + pegvisomant combination in 1 patient). In the controlled acromegaly patients, a total of 9 patients were under follow-up with medical therapy (somatostatin analog in 8 patients and somatostatin analog + pegvisomant combination in 1 patient). The rate of receiving postoperative medical therapy was higher in the acromegaly patients with active disease (p = 0.048). Four patients received conventional radiotherapy and 8 patients received gamma-knife therapy. There was no significant difference between the patients with active and controlled disease regarding radiotherapy. No significant difference between the patients with active and controlled disease was noted in terms of the presence of hypertension, diabetes mellitus, heart failure, and cancer.
The mean basal GH, IGF-1, and MMP-2 levels were significantly higher in the active acromegaly patients than in the controlled acromegaly patients (p = 0.0001). No significant difference was determined between the patients with active and controlled disease in terms of cathepsin B and MMP-9 levels (Table 2).
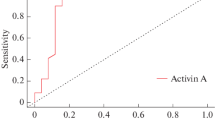
A ROC curve was plotted to determine the cut-off value for MMP-2 in the differential diagnosis of active and controlled acromegaly (Table 3, Fig. 1). The area under the ROC curve for MMP-2 was found to be 0.780 ± 0.063 (95% CI: 0.659–0.874). The sensitivity, specificity, positive predictive value and negative predictive value were 75, 77.5, 66.7, and 83.8%, respectively, for the cut-off value of 118.36 ng/mL. The risk of the disease being active was found to be 3.3 times higher for a patient with a MMP-2 level of >118.36 ng/mL than for a patient with a MMP-2 level of <118.36 ng/mL. It was demonstrated that IGF-1 and GH levels were statistically superior to MMP-2 level in the differential diagnosis of active and controlled disease (p = 0.007 and p = 0.001).
The significant positive relation of MMP-2 level with GH and IGF-1 levels (r = 0.463, p < 0.01 and r = 0.461, p < 0.01; Fig. 2) that was determined when all patients were taken into account could not be demonstrated for the active group alone (r = 0.183, p = 0.391 and r = 0.371, p = 0.075; Fig. 3).
Discussion
In this study, the acromegaly patients followed in our clinic were divided into two groups as active and controlled disease. Serum levels of MMP-2, MMP-9, and cathepsin B, which are considered to play a role in the development of pituitary adenoma and hormone secretion, were compared between these two groups. The mean serum MMP-2 level was significantly higher in the active acromegaly patients than in the controlled acromegaly patients, whereas no significant difference was determined between two groups in terms of serum MMP-9 and cathepsin B levels. In addition to the studies investigating the relation between expression of proteases in the pituitary tissue and invasion of pituitary adenomas, there are also studies in the literature which investigates the expression of proteases and functionality of pituitary adenomas. In a study including 84 patients, of whom pituitary tissues were examined for protease expression after being resected by transsphenoidal surgery, expressions of MMP-2, MMP-9, and tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) were found to be increased in the adrenocorticotropic hormone (ACTH)-secreting adenomas as compared to the other adenomas and MMP-2 and MMP-9 expressions were significantly lower in the non-functional adenomas as compared to all other adenomas [14]. On the other hand, Liu et al. [11] failed to demonstrate any difference between GH, prolactin (PRL) and ACTH-secreting adenomas and non-functional adenomas in terms of MMP expression. Another study reported that MMP activity plays a role in tumor growth and hormone secretion by releasing the growth factors bonded to the ECM in pituitary cells [15]. Yoshimura et al. [18] investigated the expressions of cathepsin B and its inhibitor cystatin C in the normal pituitary gland and in prolactinomas and found the rate of cathepsin B expression to be 5% and the rate of cystatin C expression to be 70% in the GH-secreting and PRL-secreting cells of the normal pituitary gland, whereas they demonstrated strong cathepsin B expression but weak cystatin C expression in prolactinomas. Accordingly, they thought that cathepsin B might play a role in PRL secretion particularly in prolactinomas. Considering the studies, the relation between protease expression and adenoma function in the pituitary tissue could not been demonstrated in all studies. The major reason for this difference between the studies is the lack of standardization for immunostaining of proteases and their evaluation in the pituitary tissue. Moreover, to the best of our knowledge, no study until today has compared serum MMP-2, MMP-9 levels between active and controlled acromegaly patients. In the present study, significantly higher serum MMP-2 level in the active acromegaly patients than in the controlled acromegaly patients suggested that MMP-2 might play a role in GH secretion.
Daroszewski et al. [16] demonstrated significantly higher serum cathepsin B and cysteine peptidase inhibitor activities in the acromegaly patients than in the healthy control subjects; however, they failed to demonstrate a correlation of serum cathepsin B activity with serum GH and IGF-1 levels. The results of our study were not consistent with the study by Daroszewski et al. [16] that suggested increased cathepsin B activity in acromegaly patients. One of the reasons for this inconsistency might be the fact that acromegaly patients were compared with healthy controls in the study by Daroszewski et al. [16], whereas this study compared the active and controlled acromegaly patients. Moreover, the two studies were different from each other also in terms of the method used to measure cathepsin B. The serum cathepsin B level was measured by the ELISA method in this study, whereas Daroszewski et al. [16] used Barrett method to measure the serum cathepsin B activity. In the review by Berdowska [19] on cathepsins, it was stated that ELISA method was able to measure all forms of the enzyme, whereas enzymatic methods could measure the level of enzyme activity. Accordingly, Berdowska [19] reported that enzymatic methods were less reliable as they yielded lesser protease levels than the actual levels due to interference between the specificity of substrates, unstable enzyme activity, and interaction with endogenous inhibitors. In the study by Paisley et al. [20], serum MMP-2 and MMP-9 levels were measured before and after pegvisomant treatment in 20 acromegaly patients with active disease and these levels were with those of healthy control group. While pre-treatment serum MMP-2 level was significantly higher in the active acromegaly group than in the control group, no difference was found between the groups in terms of serum MMP-9 level [20]. Serum MMP-2 level and IGF-1 level were observed to be decreased after pegvisomant treatment; however, MMP-9 level remained unchanged [20]. Moreover, a significantly positive correlation was found between MMP-2 and IGF-1 levels, whereas no correlation was determined between MMP-9 and IGF-1 [20]. The results of our study were consistent with the results of the study by Paisley et al. [20]. Although both studies demonstrated that serum MMP-2 level was significantly increased in the active acromegaly patients as compared to the controlled groups, the difference between the control groups of these two studies should be kept in mind. Moreover, although serum MMP-2 level was found to be significantly and positively correlated with GH and IGF-1 levels when the whole study population of the this study were taken into account, such a correlation could not be demonstrated when only the active patients were taken into consideration. When the statistical analyses were performed for the correlation between the MMP-2 and IGF-1 levels in the active acromegaly group, a p value of 0.075 was achieved. This p value can be accepted significant with a weak correlation (r = 0.371) due to Type 2 error caused by low number of patients in the active acromegaly group. The statistical analyses performed for the correlation between MMP-2 and IGF-1 resulted in a significant p value of 0.013 and a weak correlation (r = 0.390); this can be the reason for the correlation between the GH and MMP-2 levels in the whole acromegaly group. Neither of the two studies could find a significant difference between the active acromegaly patients and control groups in terms of MMP-9 concentration although MMP-9 is also a member of the gelatinase class of MMPs as is MMP-2. In the light of these findings, MMP-2 might have a role in hormone secretion in acromegaly patients. Increased MMP-2 level in active acromegaly patients may be attributed to the complications such as hypertension, diabetes mellitus, cardiovascular disease, malignancy encountered during the course of acromegaly. In this study, however, no significant difference between the active and controlled acromegaly patients was determined in terms of these comorbidities.
Levels of GH and IGF-1 should be evaluated together in monitoring biochemical response to treatment in acromegaly patients. In a meta-analysis comprising 7071 patients from recently published 39 studies, the rate of discordance between GH and IGF-1 levels was reported as 25.7%. The predominant format (15.3%) of this discordance was demonstrated to be increased IGF-1 level and normal GH level. The rate of discordance was found to be higher in the patients who were treated with somatostatin analogs and in whom ultrasensitive GH assay was used [21]. In a study recently reported from Turkey, which investigated 63 acromegaly patients, while the discordance rate between GH and IGF-1 levels was found to be 17.5% when the cut-off value for the nadir GH was taken as 1 ng/mL, the discordance rate increased and reached up to 26.7% when the cut-off value for GH was taken as 0.4 ng/mL [22]. The major reasons for this discordance include failing to provide standardization for GH and IGF-1 level measurements, inadequate GH sampling, age and gender effect, presence of diseases that influence GH and IGF-1 levels such as liver insufficiency, malnutrition, poorly controlled diabetes, and thyroid dysfunction, and usage of certain drugs such as oral estrogen preparations [23]. IGF-binding protein 3 and acid labile subunit measurements, which are performed to determine disease activity in patients with discordant GH and IGF-1 levels, have not provided additional benefit to IGF-1 measurement alone [24, 25]. Although the present study also found serum MMP-2 level to be significantly better than cathepsin B and MMP-9 levels in differentiating active from controlled disease, serum GH and IGF-1 levels were found superior to MMP-2. Evaluation of the cut-off value determined according to the ROC curve revealed that a MMP-2 level of 118.36 ng/mL showed a sensitivity of 75% and a specificity of 77.5% in differentiating active from controlled disease. The risk of active disease was demonstrated to be 3.3 fold higher in the patients with a MMP-2 level of >118 ng/mL as compared to those with a MMP-2 level of <118 ng/mL. Based on these results, serum MMP-2 measurement was not found to be superior to serum GH and IGF-1 measurements in determining disease activity. However, significantly higher serum MMP-2 level in the active acromegaly group suggested that MMP-2 might have a role in GH secretion. Nevertheless, the fact that increased GH secretion might increase MMP-2 level needs to be clarified.
In the present study, serum MMP-2 and MMP-9 levels were not determined to be correlated with tumor size and invasiveness. The levels of these markers were not measured before and after treatment; instead, treated patients were grouped according to their disease activity, which was determined cross-sectionally. If the expressions of these markers, MMP-2 in particular, in the pituitary gland after surgery were studied and their correlations were investigated, a data on the contribution of MMP-2 to the development of pituitary adenoma and to GH secretion could be obtained. Absence of a healthy control group, limited number of active acromegaly patients, and not using gender-specific reference values in measuring serum IGF-1 level could be stated as the other limitations of the study.
In conclusion, the present study is the first to demonstrate higher serum MMP-2 level in active acromegaly patients than in controlled acromegaly patients and to determine a cut-off value to assess the disease activity. Nevertheless, serum MMP-2 level was not found to be superior to GH and IGF-1 level measurements in distinguishing active disease from controlled disease and currently it cannot be recommended to be used instead of GH and IGF-1 level measurements. Moreover, the results need to be confirmed by a study that would be conducted in a larger patient group including a healthy control group. Studying serum levels of MMP-2 and its endogenous inhibitor TIMP-2 and their expression levels in pituitary adenomas would provide more information about the role of MMP system in the pathogenesis and functionality of pituitary adenomas.
References
S. Melmed, Medical progress: acromegaly. N. Eng. J. Med. 355, 2558–2573 (2006)
S. Melmed, A. Colao, A. Barkan, M. Molitch, A.B. Grossman, D. Klienberg, D. Clemmons, P. Chanson, E. Laws, J. Schlechte, M.L. Vance, K. Ho, A. Giustina; Acromegaly Consensus Group., guidelines for acromegaly management: an update. J. Clin. Endocrinol. Metab. 94, 1509–1517 (2009)
E.O. Machado, G.F. Taboada, L.V. Neto, F.R. van Haute, L.L. Corrêa, G.A. Balarini, Y. Shrank, M. Goulart, M.R. Gadelha, Prevalence of discordant GH and IGF-I levels in acromegalics at diagnosis, after surgical treatment and during treatment with octreotide LAR. Growth. Horm. IGF Res. 18, 389–393 (2008)
I.M. Holdaway, M.J. Bolland, G.D. Gamble, A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur. J. Endocrinol 159, 89–95 (2008)
M.R. Farnoud, F. Farhadian, J.L. Samuel, P. Derome, F. Peillon, J.Y. Li, Fibronectin isoforms are differentially expressed in normal and adenomatous human anterior pituitaries. Int. J. Cancer 61, 27–34 (1995)
K. Thapar, K. Kovacs, B.W. Scheithauer, L. Stefaneanu, E. Horvath, P.J. Pernicone, D. Murray, E.R. Laws Jr., Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB-1 antibody. Neurosurgery 38, 99–106 (1996)
A. Page-McCaw, A.J. Ewald, Z. Werb, Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 8, 221–233 (2007)
A. Nagase, R. Visse, G. Murphy, Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 69, 562–573 (2006)
M.J. Duff, The role of proteolytic enzymes in cancer invasion and metastasis. Clin. Exp. Metastasis. 10, 145–155 (1992)
H. Kawamoto, T. Uozomi, K. Kawamoto, K. Arita, Y. Yano, T. Hirohata, Type IV collagenase activity and cavernous sinus invasion in human pituitary adenomas. Acta Neurochir. (Wien). 138, 390–395 (1996)
W. Liu, Y. Matsumoto, M. Okada, K. Miyake, K. Kunishio, N. Kawai, T. Tamiya, S. Nagao, Matrix metalloproteinase 2 and 9 expression correlated with cavernous sinus invasion of pituitary adenomas. J. Med. Invest. 52, 151–158 (2005)
E. Beaulieu, Z. Kachra, N. Mousseau, L. Delbecchi, J. Hardy, R. Béliveau, Matrix metalloproteinases and their inhibitors in human pituitary tumors. Neurosurgery 45, 1432–1440 (1999)
S. Yokoyama, H. Hirano, K. Moraki, M. Goto, S. Imamura, J.I. Kuratsu, Are nonfunctioning pituitary adenomas extending into the cavernous sinus aggressive and/or invasive? Neurosurgery 49, 857–862 (2001)
U.J. Knappe, C. Hagel, B.W. Lisboa, W. Wilczak, D.K. Lüdecke, W. Saeger, Expression of serine proteases and metalloproteinases in human pituitary adenomas and anterior pituitary lobe tissue. Acta Neuropathol. 106, 471–478 (2003)
M. Páez Pereda, M.F. Ledda, V. Goldberg, A. Chervín, G. Carrizo, H. Molina, A. Müller, U. Renner, O. Podhajcer, E. Arzt, G.K. Stalla, High levels of matrix metalloproteinases regulate proliferation and hormone secretion in pituitary cells. J. Clin. Endocrinol. Metab. 85, 263–269 (2000)
J. Daroszewski, M. Bolanowski, M. Kaluzny, M. Siewinski, The imbalance of cathepsin B-like activity in acromegalic patients-preliminary report. Neuro. Endocrinol. Lett. 31, 256–260 (2010)
A. Giustina, P. Chanson, M.D. Bronstein, A. Klibanski, S. Lamberts, F.F. Casanueva, P. Trainer, E. Ghigo, K. Ho, S. Melmed; Acromegaly Consensus Group., A consensus on criteria for cure of acromegaly. J. Clin. Endocrinol. Metab. 95, 3141–3148 (2010)
K. Yoshimura, T. Tsuchida, K. Kawamoto, Expression of cathepsin B and cystatin C in the human adenohypophysis and in pituitary adenomas. Oncol. Rep. 7, 27–31 (2000)
I. Berdowska, Cysteine proteases as disease markers. Clin. Chim. Acta 342, 41–69 (2004)
A.N. Paisley, C.J. O’Callaghan, K.C. Lewandowski, C. Parkinson, M.E. Roberts, W.M. Drake, J.P. Monson, P.J. Trainer, H.S. Randeva, Reductions of circulating matrix metalloproteinase 2 and vascular endothelial growth factor levels after treatment with pegvisomant in subjects with acromegaly. J. Clin. Endocrinol. Metab. 91, 4635–4640 (2006)
G.A. Kanakis, A. Chrisoulidou, A. Bargiota, L. Efstathiadou, A. Papanastasiou, A. Theodoropoulou, S.K. Tigas, D.A. Vassiliadi, S. Tsagarakis, M. Alevizaki, The ongoing challenge of discrepant growth hormone and insulin-like growth factor I results in the evaluation of treated acromegalic patients: a systematic review and meta analysis. Clin. Endocrinol. (Oxf). 85, 681–688 (2016)
E.T. Cerit, K. Ağbaht, Ö. Demir, M. Şahin, V.T. Gedik, C. Özcan, D. Çorapçıoğlu, Discordance between GH and IGF-1 levels in Turkish acromegalic patients. Endocr. Pract. 22, 1422–1428 (2016)
P.U. Freda, Monitoring of acromegaly: what should be performed when GH and IGF-1 levels are discrepant? Clin. Endocrinol. (Oxf). 71, 166–170 (2009)
H.J. Kim, S.H. Kwon, S.W. Kim, D.J. Park, C.S. Shin, K.S. Park, S.Y. Kim, B.Y. Cho, H.K. Lee, Diagnostic value of serum IGF-I and IGFBP-3 in growth hormone disorders in adults. Horm. Res. 56, 117–123 (2001)
M. Arosio, S. Garrone, P. Bruzzi, G. Faglia, F. Minuto, A. Barreca, Diagnostic value of the acid-labile subunit in acromegaly: evaluation in comparison with insulin-like growth factor (IGF) I, and IGF-binding protein 1, -2, -3. J. Clin. Endocrinol. Metab. 86, 1091–1098 (2001)
Acknowledgements
We thank Biochemistry Department for laboratory measurements of research assays. The research was funded by Research Fund of Kocaeli University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Medical Faculty of Kocaeli University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Karci, A.C., Canturk, Z., Tarkun, I. et al. Matrix metalloproteinase 2 (MMP-2) levels are increased in active acromegaly patients. Endocrine 57, 148–155 (2017). https://doi.org/10.1007/s12020-017-1283-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1283-8