Abstract
The literature regarding risk factors for gestational diabetes mellitus (GDM) recurrence is inconsistent. We aimed to assess the effect sizes of risk factors of GDM recurrence. We searched electronic databases (1970–2015) and bibliographies for studies that included women with GDM (index pregnancy) who had a consecutive birth. We compared the risk factors among women with and without GDM recurrence. Differences in variables measured on a continuous scale were estimated using the weighted mean difference (WMD). The standardized mean difference (SMD) was used to rate the pooled effects. For categorical variables, the pooled odds ratio was estimated. Cochran’s Q test of heterogeneity was used to choose the model for estimating the pooled effects. Fourteen cross-sectional cohort studies (63 % with sample size ≥100) were considered. Women with GDM recurrence were older (by 1.32 years; P < 0.0001), heavier (by 1.82 BMI; P = 0.013), had higher 100-g oral glucose tolerance test (OGTT) levels (Fasting: by 8.42 mg/dl, 1-h: by 13.0 mg/dl, 2-h: by 18.2 mg/dl, 3-h: by 11.3 mg/dl; P < 0.0001 for all) and higher weight gain between pregnancies (by 3.24 kg; P = 0.012). The SMD effect sizes were relatively small (between 0.3 and 0.4), but weight gain between pregnancies had a medium-large effect size (SMD = 0.8). Insulin use, multiparity, and fetal macrosomia were all associated with GDM recurrence (OR 6.3 [95 % CI 3.9–10.2], OR 1.88 [95 % CI 1.09–3.24] and OR 1.63 [95 % CI 1.25–2.13], respectively). GDM recurrence is multifactorial. Stronger risk factors include insulin use, BMI, multiparity, macrosomia, and weight gain between pregnancies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) defined as any abnormal glucose tolerance first detected during pregnancy. The pathogenesis involves the combination of insulin resistance and defective insulin secretion [1]. Depending on the population studied and the diagnostic tests employed, the prevalence of gestational diabetes mellitus varies widely. The prevalence can range from 1 to 14 % of all pregnancies [2]. GDM recurrence also varies. Past systematic reviews and meta-analysis revealed that the GDM recurrence ranged from 30 to 80 % [3, 4] and that the pooled GDM recurrence was 48 % [95 % CI 41–54 %]. Studies that included non-Hispanic white and primiparous women had substantially lower GDM recurrence rates, which contributes to the variability between studies [5].
Understanding the predictors/risk factors for recurrent GDM can help guide clinical care for future pregnancies in order to prevent recurrence, and also affords a unique opportunity to explore GDM etiology through the study of heterogeneity in risk factors [6]. Kim et al. [4] systematic review was published in 2007 and they discussed the risk factors for GDM recurrence. They presented general information regarding the risk factors: maternal age, body mass index (BMI), weight gain, insulin use, parity, oral glucose tolerance test (OGTT) levels, macrosomia (i.e., risk factors at the index pregnancy), inter-pregnancy interval (IPI) and weight gain between the pregnancies (i.e., risk factors measured at the subsequence pregnancy). They concluded that besides ethnicity, no other risk factors were consistently associated with recurrence of GDM across studies. They also presented a table with each study conclusion regarding each risk factor (whether it was statistically significant or not). No meta-analyses were performed in order to present the risk factors pooled effects and significance. Since about half of the studies that were included in Kim et al. [4] review had sample size <100 women, it may be misleading to only mention whether or not a risk factor was found significant, since smaller studies have limited statistical power. Moreover, since Kim et al. [4] systematic review was published, four new large (two of them with sample size >1000 women) high-quality studies were added to the literature.
Objectives
In this systematic review, we aimed to explore the literature regarding the predictors/risk factors for GDM recurrence and performed meta-analyses in order to estimate their variability and pooled effects. We hypothesize that all of the examined risk factors will be found significant; however, the risk factors that represent the glycemic control during the index pregnancy (e.g., insulin use, OGTT levels etc.) will have the largest effect.
Methods
Sources
The electronic search strategy included the medical literature databases: The Cochrane Library 2015, PubMed and Ovid [1970–2015]. Google Scholar was also searched until August 2015. We used two sets of keywords combinations: (1) “Gestational diabetes” AND “recurrence”; (2) “Gestational diabetes” AND “previous” AND subsequent pregnancy.” The letter keywords combination was performed in order to find studies that did not use the explicit word “recurrence.” All reference lists from the main reports and relevant reviews were hand-searched for additional eligible studies.
Study selection
Studies were included if they reported a specified GDM criteria. For the purpose of this review, we included only studies that examined risk factors for GDM recurrence. The studies’ populations consisted of women with GDM who had a consecutive birth afterwards. The studies’ samples were then divided into two groups: women with GDM recurrence and women without GDM recurrence. The outcome variable was GDM recurrence status (yes/no); the risk factor variables were maternal age, body mass index (BMI), parity, OGTT levels, neonatal birth weight (at the index pregnancy), weight gain between pregnancies, IPI (continuous scale), and insulin use, obstetric history (multiparous compared to primiparous), and macrosomia at the index pregnancy (discrete). The credentials of the investigators are indicated in the authors’ list.
Two independent reviewers (NS and ZN) checked each full-text report for eligibility, and extracted and tabulated all relevant data. Disagreement was settled by consensus among all authors. All procedures conformed to the guidelines for systematic review and meta-analysis of observational studies in epidemiology—MOOSE checklist [7]. Quality scores based on the STROBE checklist [8] were evaluated for all of the studies. A possible maximum score was 22 and was based on the 22 items (partial points given for partial reporting).
Statistical analysis
The statistical analysis and graphical presentation were performed using Stata version 12.1 (Stata Corp., College Station, TX). For continuous variables (maternal age, BMI, OGTT levels, neonatal birthweight, weight gain between pregnancies, and IPI), we used the means and standard deviation of the two groups: GDM recurrence and no GDM recurrence. The effect sizes were calculated using Weighted Mean Difference (WMD). Standardized Mean Difference (SMD) was also calculated in order to quantify the risk factors on the same scale. Most of the studies that examined the OGTT results as risk factors for GDM recurrence reported the glucose levels in mg/dl and as a result, we converted all the studies’ results to mg/dl and performed the meta-analysis. For IPI, most of the studies reported the time period in months and as a result, we converted all the studies’ results to months. The weight gain between pregnancies and the neonatal birth weight were reported in kilograms and in pounds; as a result, we converted all the studies’ results to kilograms and performed the meta-analyses.
According to the guidelines suggested by Cohen [9], we consider a SMD of 0.2 as a small effect size, a SMD of 0.5 as a medium effect size, and SMD of 0.8 and higher as a large effect size. Since the SMD is a slightly upwardly bias measurement on small samples, a correction was made using Hedges and Olkin’s technique [10]. For the discrete variables (insulin use and obstetric history (primiparous/multiparous)), we used the odds ratio as the measure of association.
Heterogeneity of the studies was explored using Cochrane’s Q test of heterogeneity (P < 0.1 considered statistically significant). Inconsistency in the studies’ results was assessed by I 2 which describes the percentage of total variation across studies that is due to heterogeneity rather than chance. When I 2 ≥ 50 %, we assumed that there was more than moderate inconsistency. Random effects model (DerSimonian and Laird) was chosen if Cochrane’s Q test P < 0.1 or I 2 ≥ 50 %. Otherwise, the fixed effects model (inverse variance methods) was chosen. The funnel plot and the Egger test were used to examine publication bias (P < 0.1 considered statistically asymmetric funnel plot). In addition, the Fail Safe N was calculated for each risk factor analysis using Orwin’s method [11]. Quality analyses were performed by examining the association between the risk factors effect sizes and the quality score using meta-regression.
Results
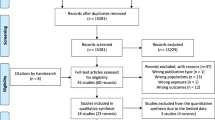
Figure 1 shows the study selection process. Of 181 abstracts identified, 155 (86 %) were excluded due to irrelevance. After a full review, 14 studies were deemed eligible and were considered in the meta-analysis [12–25]. The studies’ characteristics are shown in Table 1 and their examined risk factors are presented in Table 2. Figure 2 shows the studies quality score along with the sample size.
In addition to the risk factors that are presented in Table 2, studies examined other risk factors. For example, “gestational week at GDM diagnosis” [13, 18, 19] that was presented as a risk factor according to two studies (of three), and “gestational week at delivery” [14, 16, 18, 19] that only one study found it to be statistically significant. With regard to glucose control during the GDM pregnancy, Spong et al. [18] reported that the “mean glucose levels during the third trimester” and “admission for diabetic control” were significant risk factors for GDM recurrence, while the “mean glucose levels during the second trimester” and the “hemoglobin A1c” were not significant. Nohira et al. [21] study did find “hemoglobin A1c” to be a significant and Dong et al. [15] found “Severe GDM” (if the OGTT 1-h glucose value was 10.0 mmol/l or above in addition to a 2-h value of 7.8 mmol/l or above, after a 50 g glucose load) to be significant as well.
Meta-analyses were performed for each continuous risk factor. A summary of the results is presented in Table 3 and in Figs. 3 and 4. Figures 3 and 4 presents the forest plots of the risk factors standardized mean differences between women with and without GDM recurrence. A positive value indicates that women with GDM recurrence had a greater value compared with women without GDM recurrence (negative value represent the opposite conclusion). If the confidence interval included the “0” value, the difference was insignificant. Although the pooled WMD of maternal age revealed significant difference where women with GDM recurrence are older, the estimated difference was 1.32 years, which is rather small.
Eight studies examined the OGTT levels [13–17, 21–23]. Of them, six studies [13–15, 17, 22, 23] presented the mean and standard deviation of the fasting measurement for each group (Table 3). Four studies used the 1979 national diabetes data group (NDDG) GDM criteria where a 100-g glucose load is used [13, 14, 22, 23], three studies used a 75-g load [16, 17, 21], and one study used a 50-g load [15]. Since we are using the glucose levels’ means and standard deviations, we have chosen the four studies that used the 1979 NDDG GDM criteria to analyze the post-glucose load measurements. We performed four separate meta-analysis estimations for the different measurement timings and only the fasting measurement included all six studies. Overall, the OGTT results were significant and the first three measurements had the largest impact (SMD = 0.41, SMD = 0.32 and SMD = 0.41, respectively), although their effect sizes were not large (Fig. 3).
Seven studies examined the IPI [14, 19–24]; of them, six studies [14, 19, 21–24] published the mean and standard deviation of the IPI for each group. Overall, IPI was insignificant (SMD = 0.04; P = 0.639). Conversely, the weight gain between pregnancies had a large effect size (SMD = 0.78; P = 0.015). Six studies examined the use of insulin therapy as a predictor for GDM recurrence [13, 14, 16–18, 21]. The fixed effects model was used [heterogeneity P = 0.14]. Pooled odds ratio was 6.3 [95 % CI 3.9–10.2], P < 0.0001.
Four studies examined whether the odds for GDM recurrence is higher for multiparous compared to primiparous women [13, 14, 19, 21]. The fixed effects model was used [heterogeneity P = 0.13]. Pooled odds ratio was 1.88 [95 % CI 1.09–3.24], P = 0.02. Six studies examined whether fetal macrosomia (birth weight >4 kg or birth weight percentile ≥90th) at the index pregnancy, increases that odds for GDM recurrence [14, 15, 18, 20, 24, 25]. The random effects model was used [heterogeneity P = 0.035]. Pooled odds ratio was 1.63 [95 % CI 1.25–2.13], P < 0.0001. No significant associations between the risk factors effect sizes and the quality score were found.
Discussion
Our study was performed in order to examine the significance and importance of the published risk factors for GDM recurrence. Our results indicate that the risk factors that represent the level of glucose intolerance during pregnancy (e.g., insulin use, and neonatal birthweight) and the BMI and weight gain between the pregnancies were found to be the main predictors for GDM recurrence. This meta-analysis included mostly studies who used the 1979 NDDG criteria for GDM diagnosis, while currently the criteria most commonly used are the Carpenter and Coustan criteria and recently the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria [26]. A recent study reported that by adopting the IADPSG criteria, the number of GDM diagnosed women will be doubled [27]. As a result, we can speculate that the number of women with mild GDM will increase and the effect size of the risk factors that represent the level of glucose intolerance during pregnancy will decrease.
Although the IPI was not significant, in the literature we reviewed, we detected contradictions. The studies of Major et al. [19] and Nohira et al. [21] found a significantly shorter IPI among the GDM recurrence group, while the studies of Holmes et al. [23] and Khambalia et al. [24] found a significantly longer IPI in the GDM recurrence group. The studies of Holmes et al. [23] and Khambalia et al. [24] included only primiparous women, which means that their first pregnancy was complicated by GDM. The studies of Major et al. [19] and Nohira et al. [21] included a mix of primiparous and multiparous women which could indicate that the obstetric history may be an effect modifier of the relationship between IPI and GDM recurrence.
During the end of the second trimester, a progressive insulin resistance occurs along with hyperinsulinemia and a mild postprandial hyperglycemia. Most women are able to increase their insulin secretion to compensate for this insulin-resistant state and those women requiring the hypersecretion of insulin to compensate for pregnancy-induced insulin resistance may experience β-cell exhaustion and GDM [1]. Since changes in placental hormones in human pregnancy do not directly correlate with changes in maternal insulin resistance, it is assumed that a synergy with obesity or other pregnancy-related factors may hold the key to understanding how insulin resistance develops during pregnancy [28].
Limitations
Over the past four decades, about 14 studies have examined risk factors/predictors for GDM recurrence. These studies are not without limitations, and as a result, our study suffers from limitations as well. Firstly, most studies did not specify postpartum diabetes screening rates between pregnancies, and it is possible that a significant portion of the subsequent GDM pregnancies are actually affected by preexisting diabetes. As a result, some of the women in the GDM recurrence group actually had type 2 diabetes which could have overestimated the difference between the groups. This is a common problem in this type of study due to universal low compliance to perform the postpartum glucose tolerance test [29]. Secondly, all studies included in the meta-analysis have no adjustment for other exploratory variables and we have synthesized the raw measurements in each study. This limitation ignores the fact that there is dependence between the risk factors; thus, the pooled effects may be biased. For example, the macrosomia pooled effect may be overestimated due to the confounding effect of the weight gain during pregnancy and the women pre-pregnancy BMI. Another technical limitation occurred since in some of studies the maternal age, parity, and IPI data were not presented or the studies used a different type of variable (continuous/discrete) for measuring the predictor.
The Egger test suggested that both maternal age and weight gain between the pregnancies had some tendency towards publication bias. Since each variable was presented as a part of an assembly of risk factors, we assume that theoretically there was no vital reason for publication bias in that matter.
Although there was some missing/unreported information, we were able to conclude whether these deficits would substantially alter our results.
Parity
Four studies examined parity in a discrete scale (primiparous versus multiparous) [13, 14, 19, 21], and another five studies presented the number of children using mean and standard deviation [16–18, 22, 25]. One study did not display any summary statistic for parity but mentioned that it was not significant [12]. As a result, we synthesized women’s obstetric history in a separated meta-analyses. Both analyses found a significant association between obstetric history and GDM recurrence, where women that had GDM recurrence also had more pregnancies. This finding supports Peters et al. [30] conclusion that episodes of insulin resistance may contribute to the decline in β-cell function since each pregnancy is characterized with an episode of insulin resistance. In addition, this finding strengthen Schwartz et al. [5] meta-analysis that found lower GDM recurrence estimates among studies that included primiparous women.
Maternal age
The meta-analysis for maternal age and GDM recurrence did not include three studies; two of them were not included since no summary statistic was published [12, 20]. These two studies reported that there was no significant difference in maternal age between women with and without GDM recurrence. Khambalia et al. [24] was the third study that was not included in this meta-analysis. In the latter study, maternal age was significant where women at the age of 35 or older were at risk for GDM recurrence, compared with women at the age of 25–35 (RR 1.19; 95 % CI 1.02–1.39). In light of these findings, we assume that our conclusion would not have been much changed. It is possible that the maternal age effect size was overestimated. Schwartz et al. [5] found no significant trend between the mean maternal age and the prevalence of GDM recurrence, which strengthen our assumption that the maternal age was overestimated.
IPI
IPI was found to be an insignificant predictor for GDM recurrence. The analysis did not include the study of MacNeill et al. [20], which found no difference in IPI between women with and without GDM recurrence, and did not publish the summary statistics. Since IPI was not significant, we assume that the meta-analysis result would not have changed.
Conclusions
Characterization of women with major risk factors for GDM recurrence is the first step in the acquisition of knowledge regarding the possibilities of intervention and prevention of the GDM recurrence phenomenon. Our study emphasizes the importance of insulin use, BMI, and weight gain between the pregnancies as risk factors for GDM recurrence. Physicians may emphasize the importance of reducing the weight between the pregnancies, as it is a strong modifiable risk factor. Further studies that will explore the IPI along with parity should be considered as well as studies that will include the IADPSG criteria.
References
T.A. Buchanan, A.H. Xiang, Gestational diabetes mellitus. J. Clin. Invest. 115, 485–491 (2005)
Gestational diabetes mellitus, Diabet. Care. 26 (suppl 1), s1035 (2003)
J.N. Bottalico, Recurrent gestational diabetes: risk factors, diagnosis, management, and implications. Semin. Perinatol. 31, 176–184 (2007)
C. Kim, D.K. Berger, S. Chamany, Recurrence of gestational diabetes mellitus: a systematic review. Diabet. Care. 30, 1314–1319 (2007)
N. Schwartz, Z. Nachum, M.S. Green, The prevalence of gestational diabetes mellitus recurrence - effect of ethnicity and parity: a meta-analysis. Am. J. Obstet. Gynecol. 213(3), 310–317 (2015)
C.V. Ananth, Epidemiologic approaches for studying recurrent pregnancy outcomes: challenges and implications for research. Semin. Perinatol. 31, 196–201 (2007)
D.F. Stroup, J.A. Berlin, S.C. Morton, I. Olkin, G.D. Williamson, D. Rennie, D. Moher, B.J. Becker, T.A. Sipe, S.B. Thacker, Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. J. Am. Med. Assoc. 283, 2008–2012 (2000)
J.P. Vandenbroucke, E. von Elm, D.G. Altman, P.C. Gøtzsche, C.D. Mulrow, S.J. Pocock, C. Poole, J.J. Schesselman, M. Egger, Strengthening the reporting of observational studies in epidemiology (strobe): explanation and elaboration. PLoS. Med. 4, e297 (2007)
J. Cohen, Statistical Power Analysis for the Behavioral Sciences, 2nd edn. (Routledge 1988)
L.V. Hedges, I. Olkin, Statistical Methods for Meta-Analysis (Academic Press, New York, 1985)
R.G. Orwin, A Fail-safe n for effect size in meta-analysis. J. Educ. Stat. 8, 157–159 (1983)
H.J.T.C. Bennink, Recurrence of gestational diabetes. Eur. J. Obstet. Gynecol. Reprod. Biol. 7, 359–363 (1977)
E.H. Philipson, D.M. Super, Gestational diabetes mellitus: does it recur in subsequent pregnancy? Am. J. Obstet. Gynecol. 160, 1324–1329 (1989)
F.L. Gaudier, J.C. Hauth, M. Poist, D. Corbett, S.P. Cliver, Recurrence of gestational diabetes mellitus. Obstet. Gynecol. 80, 755–758 (1992)
Z.G. Dong, N.A. Beischer, P. Wein, M.T. Sheedy, Value of early glucose tolerance testing in women who had gestational diabetes in their previous pregnancy. Aust. N. Z. J. Obstet. Gynaecol. 33, 350–357 (1993)
R.G. Moses, The recurrence rate of gestational diabetes in subsequent pregnancies. Diabet. Care. 19, 1348–1350 (1996)
K.A. Foster-Powell, N.W. Cheung, Recurrence of gestational diabetes. Aust. N. Z. J. Obstet. Gynaecol. 38, 384–387 (1998)
C.Y. Spong, L. Guillermo, J. Kuboshige, T. Cabalum, Recurrence of gestational diabetes mellitus: identification of risk factors. Am. J. Perinatol. 15, 29–33 (1998)
C.A. Major, M. deVeciana, J. Weeks, M.A. Morgan, Recurrence of gestational diabetes: who is at risk? Am. J. Obstet. Gynecol. 179, 1038–1042 (1998)
S. MacNeill, L. Dodds, D.C. Hamilton, B.A. Armson, M. VandenHof, Rates and risk factors for recurrence of gestational diabetes. Diabet. Care. 24, 659–662 (2001)
T. Nohira, S. Kim, H. Nakai, K. Okabe, T. Nohira, K. Yoneyama, Recurrence of gestational diabetes mellitus: rates and risk factors from initial GDM and one abnormal GTT value. Diabet. Res. Clin. Pract. 71, 75–81 (2006)
S.H. Kwak, H.S. Kim, S.H. Choi, S. Lim, Y.M. Cho, K.S. Park, H.C. Jang, M.Y. Kim, N.H. Cho, B.E. Merzger, Subsequent pregnancy after gestational diabetes mellitus. Diabet. Care. 31, 1867–1871 (2008)
H.J. Holmes, J.Y. Lo, D.D. McIntire, B.M. Casey, Prediction of diabetes recurrence in women with class A1 (diet-treated) gestational diabetes. Am. J. Perinatol. 27, 47–52 (2010)
A.Z. Khambalia, J.B. Ford, N. Nassar, A.W. Shand, A. McElduff, C.L. Roberts, Occurrence and recurrence of diabetes in pregnancy. Diabet. Med. J. Br. Diabet. Assoc 30, 452–456 (2013)
N.S. Boghossian, E. Yeung, P.S. Albert, P. Mendola, S.K. Laughon, S.N. Hinkle, C. Zhang, Changes in diabetes status between pregnancies and impact on subsequent newborn outcomes. Am. J. Obstet. Gynecol 210(5), 431 e1–431 e14 (2014)
International Association of Diabetes and Pregnancy Study Groups, Recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabet. Care 33(3), 676–682 (2010)
E.A. Ryan, Diagnosing gestational diabetes. Diabetologia 54(3), 480–486 (2011)
L.A. Barbour, C.E. McCurdy, T.L. Hernandez, J.P. Kirwan, P.M. Catalano, J.E. Friedman, Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabet. Care. 302((Supplement 2)), S112–S119 (2007)
M.A. Russell, M.G. Phipps, C.L. Olson, H.G. Welch, M.W. Carpenter, Rates of postpartum glucose testing after gestational diabetes mellitus. Obstet. Gynecol. 108, 1456–1462 (2006)
R.K. Peters, S.L. Kjos, A. Xiang, T.A. Buchanan, Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet 347, 227–230 (1996)
Acknowledgments
We thank Emek Medical Center, Afula, Israel, for the technical support in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Schwartz, N., Nachum, Z. & Green, M.S. Risk factors of gestational diabetes mellitus recurrence: a meta-analysis. Endocrine 53, 662–671 (2016). https://doi.org/10.1007/s12020-016-0922-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0922-9